Abstract
Introduction
In this article, the COMT gene val158met polymorphism and attention-deficit hyperactivity disorder (ADHD)-related differences in diffusion-tensor-imaging-measured white matter (WM) structure in children with ADHD and controls were investigated.
Patients and methods
A total of 71 children diagnosed with ADHD and 24 controls aged 8–15 years were recruited. Using diffusion tensor imaging, COMT polymorphism and ADHD-related WM alterations were investigated, and any interaction effect between the COMT polymorphism and ADHD was also examined. The effects of age, sex, and estimated total IQ were controlled by multivariate analysis of covariance (MANCOVA).
Results
First, an interaction between the COMT val158met polymorphism and ADHD in the right (R) cingulum (cingulate gyrus) (CGC) was found. According to this, valine (val) homozygote ADHD-diagnosed children had significantly lower fractional anisotropy (FA) and higher radial diffusivity (RD) in the R-CGC than ADHD-diagnosed methionine (met) carriers, and val homozygote controls had higher FA and lower RD in the R-CGC than val homozygote ADHD patients. Second, met carriers had higher FA and axial diffusivity in the left (L)-uncinate fasciculus and lower RD in the L-posterior corona radiata and L-posterior thalamic radiation (include optic radiation) than the val homozygotes, independent of ADHD diagnosis. Third, children with ADHD had lower FA in the L-CGC and R-retrolenticular part of the internal capsule than the controls, independent of the COMT polymorphism.
Conclusion
Significant differences reported here may be evidence that the COMT gene val158met polymorphism variants, as well as ADHD, could affect brain development. ADHD and the COMT polymorphism might be interactively affecting WM development in the R-CGC to alter the WM connectivity in children with val homozygote ADHD.
Introduction
Attention-deficit hyperactivity disorder (ADHD), which is characterized by symptoms of inattention, hyperactivity, and impulsivity, is a neurodevelopmental disorder. It has a worldwide pooled prevalence of 5.2%.Citation1 ADHD has been reported to be associated with alterations in the dopaminergic systemCitation2,Citation3 and in the structural architecture of the brain, such as white matter (WM) connectivity.Citation4 Diffusion tensor imaging (DTI) is a neuroimaging method that gives information about WM integrity. It quantifies the pattern of water motion with fractional anisotropy (FA), radial diffusivity (RD), axial diffusivity (AD), and mean diffusivity (MD) parameters.Citation5 DTI has been recently used widely to investigate WM alterations related to ADHD. Altered WM structure was reported in the first DTI study carried out in prepubertal children diagnosed with ADHD who were largely medication free.Citation6 Later, various studies demonstrated findings to support WM disturbances in patients with ADHD.Citation7,Citation8 In these patients, both reduced and elevated FA values in comparison to controls have been reported. It has been suggested that reduced FA values seen in ADHD might be associated with axonal damage or delayed myelination, and elevated FA values might be related to decreased neuronal branching.Citation4 Recently, Witt and Stevens demonstrated a significant linear relationship between FA and RD measures of WM tracts and ADHD symptom severity.Citation9
Although there is substantial support for alterations in WM integrity in patients with ADHD, we know little about whether and how dopaminergic system interferes with WM in ADHD. Because the dopaminergic system has a well-known effect on the etiology and pharmacologic treatment of ADHD, the impact of genes related to dopaminergic system has been investigated.Citation2,Citation3 The catechol-O-methyltransferase (COMT) gene, which codes for an enzyme active in dopamine degradation, is one of the most extensively studied genes related to the dopaminergic system and ADHD. A single-nucleotide polymorphism (SNP) of guanine (G allele) to adenine (A allele) transition at codon 158 of COMT gene results in valine (val) to methionine (met) substitution (val158met SNP, rs4680). Carrying the met/met genotype leads to three- to fourfold reduction in COMT activity, resulting in a higher dopaminergic state.Citation10 COMT genotypes have been reported to be related to alterations in the brain structure through polymorphism-associated variations in dopamine amounts, in both the gray matterCitation11–Citation14 and WM.Citation15–Citation18
Collectively, the literature has provided separate findings that demonstrated ADHD-associated WM disturbances and the dopaminergic-system-related alterations in the brain structure. However, “How dopaminergic system affects WM integrity in relation to ADHD?” is still a question of interest. A very recent study searched the answer to this question and concurrently investigated the impact of ADHD and COMT polymorphism in WM. The authors found that val homozygous children diagnosed with ADHD (the ones with the lower dopaminergic state) had altered WM connectivity compared to those who were met carriers.Citation19
Based on the literature, dopaminergic involvement in the pathophysiology of ADHD makes the COMT gene polymorphism a suspect and a probable underlying cause of WM alterations seen in ADHD. As briefly mentioned above, there are DTI studies conducted to investigate WM abnormalities related to ADHD or COMT polymorphism. However, we have little information about the impact of COMT polymorphisms on the WM integrity in patients with ADHD. In the current study, we examined the COMT gene val158met polymorphism-associated differences in DTI-measured WM structure in children diagnosed with ADHD and the healthy controls. We formed several hypotheses: 1) Children with COMT val homozygote genotype polymorphism (the ones with the lower dopaminergic state) would exhibit impaired WM integrity in comparison to the met carriers. 2) Specific axonal circuits would show variations in DTI measurements according to the COMT genotypes. Because of the role of the dopamine in the cognitive processes, we expected to find alterations in the WM tracts that are known to function in cognitive processing (ie, uncinate fasciculus [UNC], corticothalamic–thalamocortical connections such as posterior thalamic radiation [PTR], and corona radiata). 3) We expected that children with ADHD would have different diffusion properties in comparison to healthy controls, representing abnormalities in the WM structure. Because of the high heterogeneity of previous findings, no specific hypothesis was formed regarding the location of expected effects.Citation4 4) DTI-measured WM microstructure abnormalities and parent- and teacher-rated ADHD symptoms (inattention, hyperactivity/impulsivity) would show a relationship. 5) ADHD and COMT polymorphism would present an interaction in a particular WM region to alter the connectivity. We hypothesized that this interaction would occur in a WM region that is involved in attention processing and executive functioning. In this context, we expected to find such an interaction in the cingulum (cingulate gyrus) (CGC) since this region has been shown to be related to attention processing and executive functioning.Citation20–Citation22
Patients and methods
In this study, 71 children diagnosed with ADHD and 24 control subjects were recruited; genetic materials were assessed to determine the COMT val158met genotypes, and DTI data were acquired. The participants were evaluated at Child and Adolescent Psychiatry Outpatient Clinic of Ege University, between December 2011 and March 2013. Their neuroimaging evaluation was completed in the radiology department of the same institution. Ege and Pamukkale University Ethics Committees approved the study, and the study was performed in accordance with the ethical standards presented in the 1964 Declaration of Helsinki and its later amendments. Written informed consent was acquired from the parents.
Participants
The ADHD and control groups of subjects were children aged 8–15 years and IQ scores >80. All were living with their families and attending normal schools. None had any history of head injury with unconsciousness and any neurological or other serious medical diseases. The children who were using any constant prescribed medication for medical conditions, who had prior stimulant use history, or the ones who had used psychotropic drugs within the last 6 months were excluded from the study. Among the control and ADHD groups of subjects, participants with any psychiatric disorder were excluded. Only oppositional defiant disorder (ODD) comorbidity was allowed in the ADHD group.
Diagnostic procedures
A “best-estimate procedure”Citation23 was used in the diagnostic evaluation stage. First, the families and teachers completed the Diagnostic and Statistical Manual of Mental Disorders, Fourth edition (DSM-IV) Disruptive Behavior Disorders Rating Scale – teacher and parent forms.Citation24 With the aim to guarantee the inattentive symptoms to be adequately represented in all subjects with ADHD, the ones with 1 SD greater inattentive scores than the age norms for these scales were invited to the diagnostic part of the study. In the diagnostic assessments, a semistructured interview (the Kiddie-Schedule for Affective Disorders and Schizophrenia, Present and Lifetime version) was conducted to the parents and children.Citation25 Second diagnostic interviews were conducted to confirm the presence of ADHD symptoms by two experienced child psychiatrists who were blind to the first diagnostic evaluations.
Twenty-four healthy subjects were recruited from the same community to participate in the control group. The same diagnostic procedures were applied for the assessment of controls. The selected control subjects were those with inattentive scores of 1 SD below the mean for the child’s age on the DSM-IV Disruptive Behavior Disorders Rating Scale. In total, 71 children with ADHD and 24 control subjects were recruited for the study.
Additionally, mental status examinations of each child were performed with the Wechsler Intelligence Scale for Children-Revised vocabulary (verbal IQ) and block design (performance IQ) subtests, and estimated IQs were obtained based on these subtests.Citation26
DNA extraction and genotype determination
The results of the genotypic analyses of the COMT val158met (c.1947 G>A, rs4680) polymorphism are presented in the following sections.
Salting-out procedure was used to extract DNA from saliva.Citation27 The genotypes were determined by a TaqMan™ fluorogenic 5′-nuclease assay using TaqMan probes; primer Express 3.0 (Thermo Fisher Scientific, Waltham, MA, USA) was used to design both the polymerase chain reaction primers and the TaqMan probes. For the COMT gene, the 1947 G>A (Val158Met) rs4680 custom-made primers and probes were used, and the SNP amplification assays were performed according to the manufacturer’s instructions. The amplifications and analyzes were performed with an ABI Prism 7500 Real-Time PCR System (Thermo Fisher Scientific) using the SDS 2.0.6 software for allelic discrimination (Thermo Fisher Scientific).
Imaging acquisition
In the neuroimaging phase, 3.0 T magnetic resonance (MR) imaging scanner (Siemens Allegra, İzmir, Turkey) with a 12-channel head coil, at the Ege University Center of Neuroradiology, was used. All the participants were scanned based on MR scan protocol beginning with T1-weighted anatomical imaging using the magnetization-prepared rapid gradient-echo sequence (repetition time (TR) =1,900 ms; echo time (TE) =2.71 ms; inversion time (TI) =900 ms; 176 sagittal slices, 1 mm ×1 mm ×1 mm isotropic voxels). Diffusion-weighted echoplanar images were acquired along 30 diffusion gradient directions for the acquisition of 45 slices throughout the whole brain (TR =6,300 ms, TE =95 ms, field of view =256 mm2, b-value =1,000 s/m, isotropic voxel dimensions =2 mm3, slice thickness =2 mm). MR-compatible video goggles were used to show movies to the children with ADHD during the scan to enhance compliance with the procedure.
Image processing
The Oxford Center for Functional Magnetic Resonance Imaging of the Brain Statistical Library (FMRIB Software Library [FSL 4.1.4], http://www.fmrib.ox.ac.uk/fsl) was used in the image analyses and tensor calculations.Citation28 The imaging data were processed in the following manner. The diffusion images were corrected for the distortion, induced by gradient coils and simple head motion, using the eddy current correction routine within FSL. First, the FA images were created by fitting a tensor model to the raw diffusion data, and then FSL’s Brain Extraction Tool was used to remove non-brain tissue from the image.Citation29 The diffusion tensor was then calculated using FSL DTIFIT for whole brain volumes, and the resulting FA maps together with the AD (λ1) and RD ([λ2 + λ3]/2) maps were used in subsequent tract-based spatial statistics (TBSS) analysis.
Voxelwise statistical analyses of the FA data were performed using TBSS.Citation30 Each individual FA map was projected onto the common FA skeleton to obtain the individual FA skeleton, on which a voxelwise analysis was performed to examine the population differences. All the subjects’ FA data were then aligned into a common space (ie, normalized into the 1 mm ×1 mm ×1 mm Montreal Neurological Institute MNI152 standardized space) using the FMRIB’s Non-Linear Image Registration Tool,Citation31,Citation32 which uses a b-spline representation of the registration warp field.Citation33 This combined transformation was applied to all the parametric maps (FA, AD, MD, and RD). Next, the mean FA image was created and thinned to create a mean FA skeleton that represents the centers of all tracts common to the group. Each subject’s aligned FA data were then projected onto this skeleton, and the resulting data were fed into voxelwise cross-subject statistics. The FA threshold was then set at 0.2 (TBSS default), in order to confine the analysis to WM. Voxelwise permutation-based nonparametric inferenceCitation34 was performed on skeletonized FA data, using FSL Randomize Version 2.1. We performed a multiple comparison correction using threshold-free cluster enhancement,Citation35 which allowed us to avoid making an arbitrary choice of the cluster-forming threshold, while preserving the sensitivity benefits of clusterwise correction. To compare trace, AD, and RD, we used FSL using the FA images to achieve nonlinear registration and skeletonization stages and also to estimate the projection vectors from each individual subject onto the mean FA skeleton. The nonlinear warps and skeleton projection can then also be applied to other images.
All extracted skeletons were overlaid with the John Hopkins University DTI-based probabilistic tractography atlas. Averaged DTI indices were then calculated for each atlas region.
Regions of interest
The following 21 WM bundles were investigated: the commissural fibers, including the body of the corpus callosum, the genu of the corpus callosum, the splenium of the corpus callosum, and tapetum; the association fibers, including the cingulum (cingulate gyrus) (CGC) R/L, cingulum (hippocampus) (CGH) R/L, external capsule R/L, fornix (column and body) (FX) R/L, fornix (cres) stria terminalis (FX/ST) R/L, superior fronto-occipital fasciculus R/L, superior longitudinal fasciculus (SLF) R/L, sagittal stratum R/L, and the UNC R/L; and the projection fibers, including the anterior corona radiata R/L, anterior limb of internal capsule R/L, posterior corona radiata (PCR) R/L, posterior limb of internal capsule R/L, retrolenticular part of the internal capsule (RLIC) R/L, and the superior corona radiata R/L. Additionally, a tract in the brain stem, including superior cerebellar peduncle R/L and the PTR, including the optic radiation, was also included.
Statistical analysis
For the statistical analysis, the Statistical Package for Social Sciences Version 17.0 (SPSS Inc., Chicago, IL, USA) was used. P-values <0.05 were considered statistically significant. The group differences in the demographic variables were examined with independent sample’s t-test, one-way analyses of variance (one-way ANOVA), and chi-square tests. The three COMT genotypes (val/val, val/met, met/met) were grouped into two groups as val homozygotes (val/val) and met carriers (val/met + met/met) in the analyses.
For the diffusion alterations in the atlas-based tract regions of interest, we performed multivariate analysis of covariance (MANCOVA) to compare the DTI index differences between the COMT val158met genotype (val homozygotes vs met carriers), diagnosis (ADHD vs healthy controls), and interaction between the two (COMT vs diagnosis). We controlled the effects of age, sex, and estimated total IQ. The Bonferroni correction (P=0.05/2=0.025) was used to adjust for possible spurious findings due to multiple testing. Outliers were excluded from the analysis to obtain acceptable fit.
Results
A total of 71 patients with ADHD and 24 healthy controls between the age group of 8 years and 15 years were enrolled in the study. The mean age of the ADHD group was 10.88±1.36 years, and the mean age of the control group was 10.80±2.02 years. There was no statistically significant difference between the study and control groups regarding age (P>0.05). The ADHD group comprised 83% males and ∼17% females. The healthy control group consisted of 75% males and 25% females. The difference between the groups regarding sex was not statistically significant (P>0.05). Again, there was no statistically significant difference between the ADHD and healthy control groups regarding mean estimated IQ (P>0.05; ). Among the ADHD subjects, eleven (15%) were meeting diagnostic criteria for comorbid ODD.
Table 1 Sociodemographic characteristics and estimated WISC-R scores of the diagnostic groups and COMT genotypes
The age, sex, and mean estimated IQ of the study participants were compared according to the COMT gene val158met polymorphism groups. No significant difference was found regarding age, sex, or mean estimated IQ between the genotypes (P>0.05; ). In the genotype distribution, in our samples, no deviation from the Hardy–Weinberg equilibrium was found (P>0.05).
COMT and ADHD effects
MANCOVA was conducted to examine the effects of COMT genotype on the FA, MD, AD, and RD values of the WM tracts by assigning ADHD diagnosis, age, sex, and estimated IQ as covariates. The mean values of the WM tracts that had statistically significantly different DTI measurements between the COMT genotypes (val homozygotes and met carriers) and the ones that had a trend to approximate the significance level (P=0.025) are presented in .
Table 2 The effect of the COMT genotypes on the white matter tracts
Similarly, MANCOVA was carried out to examine the effects of ADHD diagnosis on the FA, MD, AD, and RD values of the WM tracts by assigning COMT genotypes, age, sex and estimated IQ as covariates. The mean values for the WM tracts that had statistically significantly different DTI measurements between the diagnostic groups (patients with ADHD and controls) and the ones that had a trend to approximate the significance level (P=0.025) are presented in .
Table 3 The effect of ADHD on the white matter tracts
Interaction effects
R-CGC FA values
There was a statistically significant interaction between the diagnosis and the COMT genotypes for the R-CGC () FA values (F[1, 84] =8.215, P=0.005, partial η2=0.089), while the effects of sex, age, and estimated IQ were controlled. In other words, the effect of ADHD diagnosis and COMT genotype on the FA values in the R-CGC was dependent on each other. Therefore, an analysis of simple main effects for the diagnosis and COMT genotypes was performed with statistical significance using a Bonferroni adjustment, and the statistical significance was accepted at the P<0.025 level. There was a statistically significant difference in the mean R-CGC FA values between the val homozygotes and met carriers who had ADHD diagnosis (F[1, 84] =12.916, P=0.001, partial η2=0.133). For val homozygotes and met carriers with ADHD diagnosis, the mean R-CGC FA value difference was 0.018 (95% CI, 0.008–0.028) points, which was higher for met carriers than for val homozygotes. The simple main effect of COMT genotype on the mean R-CGC FA values for the control group of children was not statistically significant (F[1, 84] =1.268, P=0.263, partial η2=0.015; ).
Figure 1 Axial R-CGC bundle 3D, coronal, sagittal, and axial images.
Figure 2 Interaction effects- R-CGC FA values.
Abbreviations: ADHD, attention-deficit hyperactive disorder; CGC, cingulum (cingulate gyrus); FA, fractional anisotropy; met, methionine; R, right; val, valine.
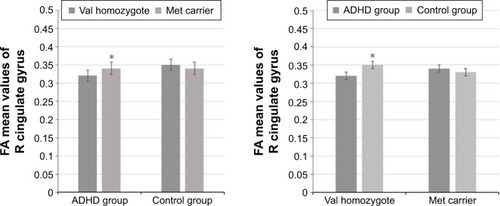
There was also a statistically significant difference in the mean R-CGC FA values between patients with ADHD and controls who were val homozygous (F[1, 84] =12,278, P=0.001, partial η2=0.128). For patients with ADHD and controls with val/val genotype, the mean R-CGC FA value difference was 0.024 (95% CI, 0.011–0.038) points, which was higher for controls than for patients with ADHD. The simple main effect of the diagnosis on the mean R-CGC FA values for the met carriers was not statistically significant (F[1, 84] =0.180, P=0.672, partial η2=0.002; ).
R-CGC RD values
Similar to FA values, we also found a statistically significant interaction between the diagnosis and the COMT genotypes for RD values of the R-CGC (F[1, 86] =7.924, P=0.006, partial η2=0.084). Again, the effects of sex, age, and estimated IQ were controlled. The assessment of the simple main effects revealed that there was a statistically significant difference in the mean CGC RD values between the val homozygotes and met carriers who had ADHD diagnosis (F[1, 86] =6.105, P=0.015, partial η2=0.066). ADHD-diagnosed met-carrier children had statistically significantly lower mean R-CGC RD value than the val homozygotes (0.018 [95% CI, 0.004–0.033]). The simple main effect of COMT genotype on the mean R-CGC RD values for the control group of children was not statistically significant (F[1, 86] =3.025, P=0.085, partial η2=0.034; ).
Figure 3 Interaction effects- R-CGC RD values.
Abbreviations: ADHD, attention-deficit hyperactive disorder; CGC, cingulum (cingulate gyrus); met, methionine; R, right; RD, radial diffusivity; val, valine.
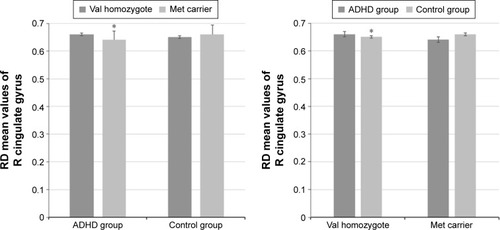
The simple main effect of the diagnosis on the mean R-CGC RD values for the val homozygotes was statistically significant (F[1, 86] =5.348, P=0.023, partial η2=0.059). For patients with val homozygote ADHD and controls, the mean R-CGC RD value difference was 0.023 (95% CI, 0.003–0.043) points, which was lower for controls than for patients with ADHD. The simple main effect of the diagnosis on the mean R-CGC RD values for the met carriers was not statistically significant (F[1, 86] =2.607, P=0.110, partial η2=0.029; )
Clinical symptom correlations
Pearson correlation analysis was performed to investigate the relationship between the DTI measures within each WM bundle and the teacher- and parent-rated inattention and hyperactivity scores assessed by Turgay DSM-IV Disruptive Behaviors Rating Scale. Only the WM bundles that were found to be statistically significantly differentiated between the ADHD and control groups were analyzed. According to this, the FA value of the FX was found to be negatively correlated with parent’s attention-deficit score (r=−0.23; P=0.021). Second, the AD value of the R-FX/ST was found to be negatively correlated with parent’s attention-deficit score (r=−0.21; P=0.035) and parent’s total attention-deficit hyperactivity score (r=−0.23; P=0.023).
Discussion
The first finding of the current study was the interaction effect between the COMT polymorphism and the ADHD in the R-CGC. Accordingly, ADHD-diagnosed val homozygote children had statistically significantly lower mean R-CGC FA and higher RD values than ADHD-diagnosed met carriers, and val homozygote controls had higher mean FA and lower RD values in the R-CGC than patients with val homozygote ADHD. Second, as a main effect, we found evidence for altered WM connectivity according to the COMT polymorphism groups independent of the ADHD effect. Similarly, the met carriers had higher FA and AD values in the L-UNC and lower RD values in the L-PCR and L-PTR (including optic radiation) than the val homozygotes. Another main effect as a third finding of the study was the altered WM connectivity detected in some WM tracts among children with ADHD in comparison to controls, independent of the effect of the COMT genotypes. Children diagnosed with ADHD had lower FA values in the L-CGC and the R-RLIC than the controls. All these results were recorded independent of age, sex, and estimated IQ of the participants. Reduced FA is accepted as an indicator of WM damage or demyelination; increased RD is believed to be related to increased space between fibers, indicating demyelination;Citation36 and decreased AD may suggest axonal injury.Citation37
Interaction between the COMT polymorphism and ADHD in the R-CGC
The cingulum bundle connects the cingulate cortex to the prefrontal cortex, premotor regions, cortical association areas in the parietal and occipital lobes, thalamus, and hippocampus.Citation22,Citation38,Citation39 It can be divided into two segments. The first is the upper part along the main cingulate gyrus (cingulum in the cingulate gyrus) and the second is the lower segment along the ventral side of the hippocampus (cingulum adjoining the hippocampus).Citation40 The cingulate cortex is considered to have a significant role in the complex cognitive processing.Citation41 Thus, this region is critical to the functions that are believed to be impaired in ADHD.Citation22,Citation42 Makris et al compared the cingulum bundle (together with SLF) of the adults who have a childhood history of ADHD with the controls. The authors found that the adults with childhood ADHD had significantly smaller FA values in these tracts relative to controls.Citation22 It has been suggested that especially cingulum bundle (and SLF) is involved in the attention processing and executive functioning.Citation20–Citation22 Therefore, lower FA value may be an indicator of an alteration in these attention and executive function networks in adults with childhood ADHD.Citation22 Similarly, in another study, WM differences as FA reductions were reported in the anterior cingulum of schizophrenia patients, indicating an impairment of the connections between the anterior cingulate and the prefrontal cortex. This finding was suggested to be related to observed deficits in executive functioning in schizophrenia.Citation43 Kasparek et al have recently reviewed the pattern of morphological and functional brain changes in children and adults diagnosed with ADHD.Citation44 They demonstrated some consistent alterations such as reduced gray matter volume, disrupted WM integrity, decreased activity, and functional connectivity in the anterior cingulum of both adult and child patients.Citation44
These findings from the literature emphasize the importance of the CGC for the executive functions and attention in the context of ADHD. Thus, we confirmed our hypothesis regarding the interaction expectation in the CGC. The ADHD and COMT polymorphism interaction that we report in the R-CGC may be explained with probable genetic vulnerability to the disorder. In children who are at genetic risk, decreased FA and increased RA may take a role in the development of the disorder. In other words, an interaction mechanism may drive the association between ADHD and the val risk variant of COMT polymorphism, causing a disruption in the WM integrity. For example, the val/val genotype of COMT polymorphism could cause reduced myelination in the R-cingulum (cingulate cortex) if the child is vulnerable to ADHD, or the children with ADHD may develop WM alterations in this particular region if they are homozygote for the val allele. Konrad and Eickhoff mentioned that both genetic and environmental factors might contribute to disruptions in connections between different brain regions.Citation45 In a similar way, van Ewijk et al discussed various factors underlying separate mechanisms such as gene–environment associations that result in the pathology.Citation46 We consider that the interaction of the COMT risk variant with the ADHD diagnosis in the R-cingulum (cingulate cortex) may be significant from this perspective of view.
Altered WM connectivity according to the COMT val158met polymorphism genotypes
We reported that COMT polymorphism was related to alterations in the integrity of WM in the L-UNC, L-PCR, and L-PTR (including optic radiation) independent of the ADHD-related effects. The val homozygotes had unfavorable DTI measurements such as reduced FA and AD values or increased RD values. In line with this, two conclusions can be drawn. The first is that the COMT genotype affects certain regions of the brain WM, regardless of whether the child has ADHD; the second is that the lower dopaminergic state may have an impairment effect on the WM connectivity. These findings that we report may be an indicator of gene-related neurodevelopmental changes associated with the COMT genotypes. As it was stated in the introduction section, met variant of the COMT enzyme has up to fourfold reduced activity which leads to a higher dopaminergic state in the cerebral areas, especially in the prefrontal cortex.Citation10,Citation47 Although conflicting literature findings exist, higher dopaminergic levels are found to be associated with the improved performance on the cognitive tasks, in healthy adults and children,Citation48–Citation50 as well as adults with ADHD.Citation51 The COMT val158met polymorphism influences the cognition probably by affecting dopamine regulation in the brain.Citation13 The literature provides evidence for the neurotrophic effects of dopamine on the neuronal growth, differentiation, and survival.Citation52–Citation55 The lower FA and AD values in the val homozygotes that we found may be related to an impaired axonal integrity or delayed myelination and maturation.Citation37,Citation56 Zinkstok et alCitation13 investigated the COMT val158met polymorphism and the brain morphometry in the young adults and reported age-related variations in the gray and WM densities among the val carriers. The authors suggested that the low synaptic dopamine levels due to the high-activity val allele may be associated with a relative delay in the brain maturation (a relative delay in the gray matter loss and/or WM increase).Citation13 In another study, Honea et alCitation12 reported that the val risk variant of COMT val158met polymorphism affects the hippocampal and dorsolateral prefrontal gray matter volume. The authors discussed that the complex genetic variation in COMT might impact the gray matter volume, possibly through neurotrophic or neurotoxic effects of varying levels of dopamine.Citation12 In our study group of children, it is possible that the low dopamine levels in the val homozygotes might have an unfavorable impact on the neuronal growth and maturation, concerning the essential neurotrophic effects of dopamine. Thus, the maturation of the WM tracts might have been delayed as suggested by Zinkstok et al.Citation13 Consequently, our initial expectation was met by the worse DTI measurements that we recorded for the val homozygotes in comparison to the met carriers.
DTI technique is a relatively new method which gives an opportunity to investigate the WM structure and brain networks. In the literature, there is a limited number of studies that examined WM-related alterations according to COMT polymorphism groups. One study, conducted in healthy children and adolescents, examined the COMT genotype-related alterations in the prefrontal WM pathways.Citation16 In contrast to our findings, the authors reported that the val allele was associated with significantly elevated FA and reduced AD and RD values. They concluded that high levels of brain dopamine were associated with reduced brain myelination that might antecede to alterations in cognitive and affective processing that have been found to differ among COMT genotypes. Two other studies investigated for an association of WM integrity with COMT val158met polymorphism by using DTI and again reported worse results regarding DTI parameters in the met carriers. Accordingly, one study showed a reduction in the FA values in the prefrontal cortex of the met carrier substance users,Citation18 and the other one demonstrated decreased FA and AD values in the temporal lobe of met-carrier major depression patients.Citation17 In another study, Liu et al investigated the effects of COMT haplotypes on the WM integrity in the bilateral prefrontal lobes and IQ. They found that the effect is nonlinear and fit an inverted U-model, which may indicate that intelligence-related brain WM integrity is optimal only within a narrow range of dopamine activity, with either too little or too much dopamine having a relatively deleterious effect.Citation57 The authors also added that the val homozygous subjects had worse WM integrity than the met carriers (in the R-corticospinal tract), similar to that we reported for the val homozygotes.Citation57 These results that seem conflicting at first glance may be related to the need for an optimum range of extracellular dopamine for the neuronal structural integrity implying both over- and under-stimulation with dopamine may result in impaired neuronal survival and growth.Citation12 It should be noted that, although the optimum levels of dopamine are beneficial, the research data also support the neurotoxic effects of excess extracellular dopamine.Citation58–Citation60
Because the cognitive functions are related to the dopaminergic system, we hypothesized that COMT polymorphism would be affecting WM tracts that are involved in the cognitive processes. The UNC connects the frontal and temporal lobes,Citation38 and is known to be involved in some cognitive functions (eg, verbal memory and immediate recall of word pairs).Citation61–Citation63 The integrity of UNC has been reported to be disturbed in Alzheimer disease,Citation64 and the severity of cognitive functions was found to be related to the impairment in the UNC as measured by DTI.Citation63 The corona radiata and the PTR include corticothalamic and thalamocortical fibers.Citation38 The contribution of the thalamus to cognitive processes, including attention, speed of information processing, and memory, has been evident,Citation65,Citation66 and the functions of the thalamus are brought about by widely distributed thalamocortical connections.Citation67 Our hypothesis has been supported by the statistical significance that we found in the L-UNC, L-PCR, and L-PTR.
Altered WM connectivity in children with ADHD in comparison to the controls
Regardless of their genotype, children with ADHD exhibited lower FA values in the R-RLIC and the L-CGC than the control group of children. This finding of reduced FA values in the ADHD group was an expected result, since FA is accepted as an index of axonal integrityCitation36,Citation56 although there were inconsistent results in the literature as either decreased or increased measures for FA.Citation4 We initially hypothesized that children with ADHD would have different diffusion properties in comparison to healthy controls, and these findings have confirmed our hypothesis.
A recent meta-analysis study compared the DTI findings of patients with ADHD with healthy controls to further reveal the neurobiological underpinnings of the disorder.Citation4 They reported WM alterations most consistently in the bilateral internal capsule, L-cerebellum, R-anterior corona radiata, and R-forceps minor. We also found the RLIC (R side) among the brain regions which exhibited ADHD-related alterations. This part of the internal capsule, being a projection fiber, constitutes mainly the PTR (corticothalamic and thalamocortical fibers, including the optic radiation) and can also include the parieto-, occipito-, and temporopontine fibers.Citation38 Thus, it plays a significant role in the thalamo-cortical information processing. The thalamo-cortico-striatal networks are blamed in the etiopathogenesis of ADHD and reward system-related ADHD behaviors.Citation68,Citation69 In this context, the impaired WM connectivity that we report here may be a noteworthy finding.
In addition to the gene–disorder interaction effect that we found in the R-CGC, we also showed a simple main effect of ADHD on the L side of the CGC. CGC, being a potential target region for a gene–disorder interaction, may be an intersection area in the etiopathogenesis of the disorder.
One of our study hypotheses was concerning the expectation to find a correlation between DTI-measured WM microstructure abnormalities and parent- and teacher-rated ADHD symptoms (inattention, hyperactivity/impulsivity). In line with our expectations, FA of the FX was found to be negatively correlated with the parent-rated inattention score, and AD of the R-FX/ST was found to be negatively correlated with the parent-rated inattention score and parent-rated total inattention–hyperactivity score. The fornix and the cingulum bundles are the most prominent WM fiber tracts within the limbic system.Citation43
The limbic system, which is related to motivation, emotion, and reward, is regulated by higher cortical centers such as the prefrontal cortex. It appears to have a major role in the pathophysiology and pharmacotherapy of ADHD.Citation70 Although it was not at a statistical significance level, patients with ADHD had worse WM integrity (lower FA or AD values) in the FX and FX/ST that approximated to the statistical significance. This trend for a significant disturbance in the fornix may be noteworthy if we consider the role of the fornix in the limbic system, and the relation of the limbic system with ADHD pathophysiology, regarding the motivation-related behavior, emotions, and reward-related circuits. Additionally, the fornix carries association fibers to the memory-related structures such as the mammillary bodies and the hippocampus, and it has been known to play a role in the memory, especially recall memory.Citation71,Citation72 The negative correlation of FA and AD values of the fornix with the parent-rated inattention and total ADHD (inattention and hyperactivity) symptom scores supports the WM alteration trend in the area from a clinical perspective, as well. Compatibly, Davenport et al compared 10–20 year-old patients with ADHD with the healthy controls and demonstrated lower FA values in the L-posterior fornix of patients with ADHD.Citation73 Similarly, Abdul-Rahman et al found significant reduction in the mean R-fornix FA values based on the region-of-interest analysis and a trend for a decreased FA and increased RD along the R-fornix bundle by tract-based analysis in patients with schizophrenia.Citation43
Limitations and conclusion
The findings of the current study revealed that the COMT gene val158met polymorphism variants might affect the brain development independent of the effects of ADHD. Some regions of the WM matter were altered in patients with ADHD in comparison to controls, as well. In addition to these simple main effects, we showed an interaction effect in the R-CGC, revealing that the effects of COMT genotype and the ADHD diagnosis are linked to each other. ADHD-diagnosed val-homozygote children had altered WM connectivity in this region. When interpreting these results, some important limitations should be kept in mind. First of all, we should note a limitation related to the DTI technique and the measured parameters. DTI has been found to be a precious tool for providing specific indices of neuropathology,Citation74 and diffusion parameters are quite sensitive to tissue properties such as myelination, axonal density, and orientation. However, no DTI measure is specifically sensitive to a given property.Citation46,Citation75 It should also be added that the interpretation of the abnormal DTI parameters in psychiatric disorders is somewhat speculative.Citation46,Citation74,Citation75 As van Ewijk et al mentioned, reduced FA values may be an indication of disrupted WM integrity such as demyelination; in another region with crossing fibers, it may represent neuronal branching.Citation4,Citation46 Currently, the inconsistencies between the studies and the lack of straightforward interpretation of the DTI parameters create a challenge in the DTI research. This problem is especially prominent in the ADHD, as a broad-spectrum disorder with phenotypic heterogeneity.Citation4,Citation46,Citation76 Second, limitations related to our study sample characteristics and study methods should be noted. In the ADHD group, ODD comorbidity was allowed, although all the other psychiatric and medical conditions were excluded. Another limitation was related to the subject recruitment stage. The healthy control subjects and the patients were not matched in a case-to-case design regarding age, sex, and IQ. Nevertheless, the group comparisons of these parameters yielded no statistically significant differences. Finally, our study did not examine the cerebellar tracts. Because of the potential role of the cerebellum in the etiopathogenesis of ADHD, future studies should include a more focused examination of the cerebellar pathways.
Conclusively, the findings of the current study contribute to our understanding in terms of possible genetic underpinnings of WM abnormalities, which is still an under-investigated area. Future studies that will be conducted to investigate the neurotransmitter system affecting genes, such as COMT, can provide insight into the neurochemical modulation of the development of WM. More investigations are needed to be carried out to clarify the relationship between the structure of WM pathways and COMT genotype in the context of ADHD. In addition, longitudinal studies will provide extra information regarding the COMT gene and ADHD-related WM abnormalities in time.
Disclosure
Eyup Sabri Ercan is on the advisory board of Eli Lilly and Company (Indianapolis, IN, USA) and Janssen Pharmaceutica (Beerse, Belgium). The other authors report no conflicts of interest in this work.
References
- PolanczykGde LimaMSHortaBLBiedermanJRohdeLAThe worldwide prevalence of ADHD: a systematic review and metaregression analysisAm J Psychiatry2007164694294817541055
- SwansonJMFlodmanPKennedyJDopamine genes and ADHDNeurosci Biobehav Rev2000241212510654656
- FaraoneSVBiedermanJNeurobiology of attention-deficit hyperactivity disorderBiol Psychiatry199844109519589821559
- van EwijkHHeslenfeldDJZwiersMPBuitelaarJKOosterlaanJDiffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysisNeurosci Biobehav Rev20123641093110622305957
- BeaulieuCThe basis of anisotropic water diffusion in the nervous system – a technical reviewNMR Biomed2002157–843545512489094
- NagelBJBathulaDHertingMAltered white matter microstructure in children with attention-deficit/hyperactivity disorderJ Am Acad Child Adolesc Psychiatry201150328329221334568
- ChuangT-CWuM-THuangS-PWengM-JYangPDiffusion tensor imaging study of white matter fiber tracts in adolescent attention-deficit/hyperactivity disorderPsychiatry Res2013211218618723394679
- ChenM-HLiouY-JAripiprazole-associated acute dystonia, akathisia, and parkinsonism in a patient with bipolar I disorderJ Clin Psychopharmacol201333226927023422384
- WittSTStevensMCRelationship between white matter microstructure abnormalities and ADHD symptomatology in adolescentsPsychiatry Res2015232216817425795595
- LachmanHMPapolosDFSaitoTYuYMSzumlanskiCLWeinshilboumRMHuman catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disordersPharmacogenetics1996632432508807664
- CerasaAGioiaMCLabateALiguoriMLanzaPQuattroneAImpact of catechol-O-methyltransferase Val(108/158) Met genotype on hippocampal and prefrontal gray matter volumeNeuroreport200819440540818287936
- HoneaRVerchinskiBAPezawasLImpact of interacting functional variants in COMT on regional gray matter volume in human brainNeuroimage2009451445119071221
- ZinkstokJSchmitzNvan AmelsvoortTThe COMT val158met polymorphism and brain morphometry in healthy young adultsNeurosci Lett20064051–2343916857316
- VillemonteixTDe BritoSASlamaHStructural correlates of COMT Val158Met polymorphism in childhood ADHD: a voxel-based morphometry studyWorld J Biol Psychiatry201516319019925495556
- LiJYuCLiYCOMT val158met modulates association between brain white matter architecture and IQAm J Med Genet B Neuropsychiatr Genet2009150B337538018615479
- ThomasonMEDoughertyRFColichNLCOMT genotype affects prefrontal white matter pathways in children and adolescentsNeuroimage201053392693420083203
- HayashiKYoshimuraRKakedaSCOMT Val158Met, but not BDNF Val66Met, is associated with white matter abnormalities of the temporal lobe in patients with first-episode, treatment-naïve major depressive disorder: a diffusion tensor imaging studyNeuropsychiatr Dis Treat2014101183119025061303
- ZhangXLeeMRSalmeronBJPrefrontal white matter impairment in substance users depends upon the catechol-o-methyl transferase (COMT) val158met polymorphismNeuroimage201369626923219927
- HongS-BZaleskyAParkSCOMT genotype affects brain white matter pathways in attention-deficit/hyperactivity disorderHum Brain Mapp201536136737725201318
- MakrisNPandyaDNNormandinJJQuantitative DT-MRI investigations of the human cingulum bundleCNS Spectr2002707522528
- MakrisNKennedyDNMcInerneySSegmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI studyCereb Cortex200515685486915590909
- MakrisNBukaSLBiedermanJAttention and executive systems abnormalities in adults with childhood ADHD: a DT-MRI study of connectionsCereb Cortex20081851210122017906338
- LeckmanJFSholomskasDThompsonWDBelangerAWeissmanMMBest estimate of lifetime psychiatric diagnosis: a methodological studyArch Gen Psychiatry19823988798837103676
- ErcanESAmadoSSomerOÇıkıoğluSDevelopment of a test battery for the assessment of attention deficit hyperactivity disorderÇocuk ve Gençlik Ruh Sağlığı Derg/Turkish J Child Adolesc Ment Heal200183132144
- GöklerBÜnalFPehlivantürkBKültürEÇAkdemirDTanerYReliability and validity of schedule for affective disorders and schizophrenia for school age children-present and lifetime version-Turkish version (K-SADS-PL-T)Çocuk ve Gençlik Ruh Sağlığı Derg/Turkish J Child Adolesc Ment Heal2004113109116
- KaufmanASFactor analysis of the WISC-R at 11 age levels between 6 1/2 and 16 1/2 yearsJ Consult Clin Psychol1974432135147
- MillerSADykesDDPoleskyHFA simple salting out procedure for extracting DNA from human nucleated cellsNucleic Acids Res198816312153344216
- WoolrichMWJbabdiSPatenaudeBBayesian analysis of neuroimaging data in FSLNeuroimage2009451 supplS173S18619059349
- SmithSMFast robust automated brain extractionHum Brain Mapp200217314315512391568
- SmithSMJenkinsonMJohansen-BergHTract-based spatial statistics: voxelwise analysis of multi-subject diffusion dataNeuroimage20063141487150516624579
- AnderssonJJenkinsonMSmithSwebpage on the InternetNon-Linear OptimisationFMRIB Technical Report TR07JA1Univ Oxford FMRIB Cent OxfordUK2007 Available from: http://www.fmrib.ox.ac.uk/analysis/techrep/Accessed September 16, 2015
- AnderssonJLRJenkinsonMSmithSNon-Linear Registration aka Spatial NormalisationFMRIB Technical Report TR07JA2 In Pract2007622 Available from: http://www.fmrib.ox.ac.uk/analysis/techrep/Accessed September 16, 2015
- RueckertDSonodaLIHayesCHillDLLeachMOHawkesDJNonrigid registration using free-form deformations: application to breast MR imagesIEEE Trans Med Imaging199918871272110534053
- NicholsTEHolmesAPNonparametric permutation tests for functional neuroimaging: a primer with examplesHum Brain Mapp200215112511747097
- SmithSMNicholsTEThreshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inferenceNeuroimage2009441839818501637
- HarsanLAPouletPGuignardBBrain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imagingJ Neurosci Res200683339240216397901
- LazarMWeinsteinDMTsurudaJSWhite matter tractography using diffusion tensor deflectionHum Brain Mapp200318430632112632468
- MoriSOishiKJiangHStereotaxic white matter atlas based on diffusion tensor imaging in an ICBM templateNeuroimage200840257058218255316
- MufsonEJPandyaDNSome observations on the course and composition of the cingulum bundle in the rhesus monkeyJ Comp Neurol1984225131436725639
- WakanaSCaprihanAPanzenboeckMMReproducibility of quantitative tractography methods applied to cerebral white matterNeuroimage200736363064417481925
- BushGLuuPPosnerMICognitive and emotional influences in anterior cingulate cortexTrends Cogn Sci20004621522210827444
- BushGCingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorderBiol Psychiatry201169121160116721489409
- Abdul-RahmanMFQiuASimKRegionally specific white matter disruptions of fornix and cingulum in schizophreniaPLoS One201164e1865221533181
- KasparekTTheinerPFilovaANeurobiology of ADHD from childhood to adulthood: findings of imaging methodsJ Atten Disord20132010113
- KonradKEickhoffSBIs the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorderHum Brain Mapp201031690491620496381
- van EwijkHHeslenfeldDJZwiersMPDifferent mechanisms of white matter abnormalities in attention-deficit/hyperactivity disorder: a diffusion tensor imaging studyJ Am Acad Child Adolesc Psychiatry2014537790.e799.e24954828
- Diaz-AsperCMWeinbergerDRGoldbergTECatechol-O-methyltransferase polymorphisms and some implications for cognitive therapeuticsNeuroRx2006319710516490416
- MalhotraAKKestlerLJMazzantiCBatesJAGoldbergTGoldmanDA functional polymorphism in the COMT gene and performance on a test of prefrontal cognitionAm J Psychiatry2002159465265411925305
- BruderGEKeilpJGXuHCatechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operationsBiol Psychiatry2005581190190716043133
- DiamondABriandLFossellaJGehlbachLGenetic and neurochemical modulation of prefrontal cognitive functions in childrenAm J Psychiatry2004161112513214702260
- BoonstraAMKooijJJSBuitelaarJKAn exploratory study of the relationship between four candidate genes and neurocognitive performance in adult ADHDAm J Med Genet B Neuropsychiatr Genet2008147339740217886261
- FishellGvan der KooyDPattern formation in the striatum: developmental changes in the distribution of striatonigral neuronsJ Neurosci198777196919782886562
- KüppersEBeyerCDopamine regulates brain-derived neurotrophic factor (BDNF) expression in cultured embryonic mouse striatal cellsNeuroreport20011261175117911338187
- MaLZhouJDopamine promotes the survival of embryonic striatal cells: involvement of superoxide and endogenous NADPH oxidaseNeurochem Res200631446347116758354
- ZhouQYPalmiterRDDopamine-deficient mice are severely hypoactive, adipsic, and aphagicCell1995837119712098548806
- KubickiMWestinC-FMaierSEDiffusion tensor imaging and its application to neuropsychiatric disordersHarv Rev Psychiatry200210632433612485979
- LiuBLiJYuCHaplotypes of catechol-O-methyltransferase modulate intelligence-related brain white matter integrityNeuroimage201050124324920005296
- SantiagoMMatarredonaERGraneroLCanoJMachadoANeurotoxic relationship between dopamine and iron in the striatal dopaminergic nerve terminalsBrain Res20008581263210700592
- FumagalliFRacagniGColomboERivaMABDNF gene expression is reduced in the frontal cortex of dopamine transporter knockout miceMol Psychiatry200381189889914593425
- ThompsonPMHayashiKMSimonSLStructural abnormalities in the brains of human subjects who use methamphetamineJ Neurosci200424266028603615229250
- SeldenNRGitelmanDRSalamon-MurayamaNParrishTBMesulamMMTrajectories of cholinergic pathways within the cerebral hemispheres of the human brainBrain1998121pt 1224922579874478
- NestorPGKubickiMGurreraRJNeuropsychological correlates of diffusion tensor imaging in schizophreniaNeuropsychology200418462963715506830
- MorikawaMKiuchiKTaokaTNagauchiKKichikawaKKishimotoTUncinate fasciculus-correlated cognition in Alzheimer’s disease: a diffusion tensor imaging study by tractographyPsychogeriatrics2010101152020594282
- TaokaTIwasakiSSakamotoMDiffusion anisotropy and diffusivity of white matter tracts within the temporal stem in Alzheimer disease: evaluation of the “tract of interest” by diffusion tensor tractographyAJNR Am J Neuroradiol20062751040104516687540
- Van Der WerfYDTisserandDJVisserPJThalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging. A magnetic resonance imaging-based volumetric analysisBrain Res Cogn Brain Res200111337738511339987
- FamaRSullivanEVThalamic structures and associated cognitive functions: relations with age and agingNeurosci Biobehav Rev201554293725862940
- YuanRDiXTaylorPAGohelSTsaiY-HBiswalBBFunctional topography of the thalamocortical system in humanBrain Struct Funct Epub2015430
- Henriquez-HenriquezMZamorano-MendietaFRothhammer-EngelFAboitizFNeurocognitive models for attention deficit hyperactivity disorder and their consequences on the searching of endophenotypesRev Neurol201050210911620112219
- StarkRBauerEMerzCJADHD related behaviors are associated with brain activation in the reward systemNeuropsychologia201149342643421163276
- GraceAAPsychostimulant actions on dopamine and limbic system function: relevance to the pathophysiology and treatment of ADHDSolantoMVStimulant Drugs and ADHD: Basic and Clinical NeuroscienceNew York, NYOxford University Press2001134155
- TsivilisDVannSDDenbyCA disproportionate role for the fornix and mammillary bodies in recall versus recognition memoryNat Neurosci200811783484218552840
- VannSDDenbyCLoveSMontaldiDRenowdenSCoakhamHBMemory loss resulting from fornix and septal damage: impaired supra-span recall but preserved recognition over a 24-hour delayNeuropsychology200822565866818763885
- DavenportNDKaratekinCWhiteTLimKODifferential fractional anisotropy abnormalities in adolescents with ADHD or schizophreniaPsychiatry Res2010181319319820153608
- AlexanderALLeeJELazarMFieldASDiffusion tensor imaging of the brainNeurotherapeutics20074331632917599699
- JonesDKKnöscheTRTurnerRWhite matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRINeuroimage20137323925422846632
- WillcuttEGNiggJTPenningtonBFValidity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypesJ Abnorm Psychol20121214991101022612200
