Abstract
Dalfampridine extended release (ER) 10 mg is an oral tablet form of the potassium (K+) channel-blocking compounded dalfampridine, also known as fampridine, and chemically 4-aminopyridine or 4-AP, which received regulatory approval in the United States for the treatment of walking in patients with multiple sclerosis (MS) in January 2010. Two pivotal Phase 3 clinical trials demonstrated significant improvements in walking in patients with the four primary forms of MS following administration of dalfampridine ER tablets 10 mg twice daily. The drug is thought to act by restoring conduction in focally demyelinated axons and by enhancing neurotransmission, thereby leading to improved neurological function. This review describes how dalfampridine represents a new pharmacotherapeutic approach to the clinical management of mobility impairment. It describes the mechanism of action and chemistry of dalfampridine ER, its pharmacokinetics, tolerability, and side effects, and the outcomes of multicenter trials showing its efficacy in improving walking speed. Clinician and patient global assessments, as well as patient self-assessment of the impact of MS on their gait disability, confirm clinically relevant benefit from the therapy. Patients tolerate the drug well and their improvement in terms of household and community ambulation, inferred from analysis of pooled data from several studies, is likely to translate into benefits in the performance of instrumental activities of daily living and a reduction in the neuropsychiatric burden of disease.
Walking impairment in multiple sclerosis
Impaired ability to walk constitutes one of the major disabling neuromuscular deficits associated with multiple sclerosis (MS).Citation1–Citation3 It is often the most visible sign of MS and has been reported to affect 80% of persons within 15 years of disease onset.Citation1,Citation4,Citation5 At this time there is a 40% probability of need for walking assistance and a 25% probability of need for a wheelchair. As a consequence of compromised activities of daily living that involve walking about the home or in the community, impairments of gait lead to loss of independence, decreased sense of self-worth due the external appearance of disablement, lost employment opportunities and reduced quality of life.Citation3,Citation6 The impact of mobility impairment on patient autonomy, caregiver burden, mood, and the costs of care are disproportionately high relative to those associated with other neurologic diseasesCitation2 and compound the already heavy neuropsychiatric burden of the disease.Citation2,Citation7–Citation9 As such, maintaining mobility is ranked as one of the highest priorities among patients with MS, regardless of disease duration or level of disability.Citation3
Conventional approaches to the management of gait impairment primarily involve physical rehabilitation and the functional retraining of gait. This may be coupled with pharmacological management of symptoms that compromise gait, such as spasticity. Gait retraining is usually done by physical therapists and often involves the use of assistive mobility aids such as orthoses, canes, crutches, or walking frames. In advanced stages of the disease power scooters or wheelchairs may be prescribed.Citation10 A recent survey identified the fact that many patients elect not to use these devices; 37% of respondents did not use aids out of embarrassment and a further 36% reported that they did not use their prescribed aids as much as they should.Citation11 Mobility aids do provide supports that constrain the disablement, but do not restore neuromuscular function per se. Similarly, pharmacologic agents for the management of spasticity reduce abnormalities of tone that impair locomotor capacity, but do so without reversing the underlying gait deficit. Antispastic and spasmolytic agents may also lead to unwanted central depressant actions of anergia and increased fatigue, thereby further contributing to the loss of mobility. Pharmacotherapeutics designed to slow progression of the disease, eg, interferons and glatiramer acetate, also provide little or no direct symptomatic relief of gait disorders, and recovery of function occurs only if the disease goes into remission.
Against the backdrop of this conventional management of impaired gait in MS, there has been considerable interest in the development of a new class of pharmacotherapeutics designed to reverse the underlying neurologic deficits associated with demyelination of axons within the CNS and thereby restore neurological function. The first drug in this class to be approved by the US Food and Drug Administration (FDA) is dalfampridine extended release (ER) (Acorda Therapeutics, Inc., New York), marketed under the name Ampyra®. In a series of Phase 2 and Phase 3 randomized clinical trials, dalfampridine ER 10 mg tablets taken twice daily improved patients’ speed on the Timed 25-Foot Walk test (T25FW).Citation12–Citation14 Based on the magnitude of the improvements in gait, and by reference to studies in other populations, the expected clinical benefits would be notable improvements in the patients’ functional capacity to ambulate within their home or in the community.Citation15 Several previous reviews of this drug have elaborated on the underlying pharmacology, the developmental history of the drug, and the outcome of clinical trials up to the time of regulatory approval by the FDA in January 2010.Citation16–Citation21
The US FDA determined that the previously adopted nonproprietary name “fampridine” was too similar to another approved medication and could lead to medical errors. The US Adopted Name Council therefore changed the nonproprietary name to “dalfampridine”. In addition, the FDA determined that the term “extended release” will be used to refer to this formulation rather than the previously used “sustained release”. These conventions have been employed in this manuscript. The drug is referred to by its International Non-proprietary Name of “fampridine” outside the US.
The present review briefly summarizes the pharmacological mechanism of action and pharmacokinetics of dalfampridine ER in individuals with MS. It also describes the quantitative and subjectively reported outcomes of clinical trials demonstrating efficacy for walking, safety, and tolerability. Unique to this review is commentary on the clinical significance of the observed therapeutic efficacy, and the current status of regulatory approval in other parts of the world. The review concludes with some perspective on how future research with dalfampridine might be directed to further explore the impact of the drug on neuropsychiatric burden in a more direct, rather than inferential, manner.
The information used to inform this review was largely derived from PubMed searches using the MeSH terms “4-aminopyridine”, “fampridine”, “dalfampridine”, “ampyra”, “human”, “clinical trials”, “multiple sclerosis”, and identifying publications in English from 1985 to 2011. The search was completed March 15, 2011 and a total of 101 published articles were retrieved. Primary consideration was given to the original reports of Phase 2 and 3 clinical trials with supplemental information derived from subsequent reviews, meta-analyses, editorials, conference proceedings and abstracts. No attempt was made herein to independently conduct a formal systematic review of the quality of evidence emerging from these publications; however precis of both the critical analyses and the rebuttals are described in order to retain impartiality of this review. Information on mechanism of action and pharmacological properties was derived from studies involving animal models of demyelination. The FDA website (www.accessdata.fda.gov) provided access to prescribing information, tolerability, and adverse effects of dalfampridine ER. The Biogen Idec website (www.biogenidec.com) provided information on the regulatory status of the drug in Europe. Biogen Idec has the license to market dalfampridine ER outside the United States.
MS, demyelination, and the mechanism of action of dalfampridine
MS is primarily an inflammatory disorder of the central nervous system (CNS) in which focal lymphocytic infiltration leads to damage of myelin and axons.Citation22 Demyelination of axons within the CNS is the most diagnostic neuropathology of MS in the early stages of the disease, and this has prompted characterization of MS as the archetypical demyelinating disease. Myelin deficits may show up as vacuolation, degeneration of the inner lamellae, focal thinning, disseminated foci of demyelination, or incomplete remyelination. These myelin deficits are thought to arise initially from T cell, macrophage, and antibody-mediated oligodendrogliopathy and later from ischemic or toxic virus, or metabolic processes.Citation23,Citation24 As the disease progresses, there is commensurately more evidence of axonal pathology, with a distribution that varies with clinical phenotype. Axonal pathology accounts for the nonreversible nature of the neurological deficits and some of the neuropsychiatric symptoms in the progressive forms of disease.Citation25–Citation29
Myelin deficits lead to exposure of paranodal, fast, voltage-gated potassium (K+) channels, and the attendant abnormal K+ conductance impairs action potential electrogenesis, repetitive axonal discharge, and propagation.Citation30–Citation32 Axonal conduction deficits that arise from demyelination underlie the initial clinical presentation of each of the four primary classifications of MS: relapsing-remitting, primary-progressive, secondary-progressive, and progressive-relapsing. Prominent among the neurologic deficits are visual disturbances, fatigue, and motor and sensory deficits including weakness, incoordination, and spasticity, all of which contribute to impaired balance and mobility, and compound independently impaired emotional and cognitive states.
Dalfampridine is a broad spectrum potassium (K+) channel blocking agent, with the capacity to improve conduction across demyelinated internodes in axons of the central nervous system. It has dose-dependent specificity for different types of K+ channels.Citation33 Dalfampridine prolongs the duration of the Na++ action current at the internode; this increases the safety factor for conduction (ie, the ratio of action current generated by an impulse to the minimum amount of action current needed to maintain conduction) and thus can reverse conduction block due to focal demyelination. This mode of action has been established in various ex vivo and in vivo axonal preparations and animal models of demyelination.Citation34–Citation38 K+ channel blocking compounds also enhance neuroneuronal and neuromuscular transmission by increasing presynaptic and end-terminal calcium (Ca++) influx.Citation39,Citation40 Collectively these properties are thought to underlie the restored axonal conduction and neurological function reported in patients with MS and other demyelinating disorders.
Chemistry of dalfampridine
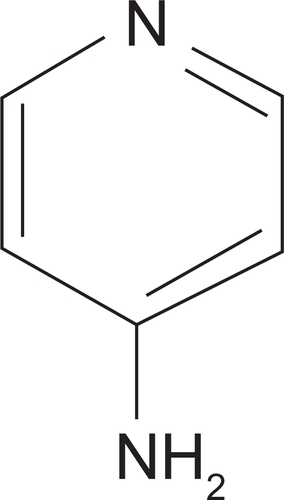
Dalfampridine is also known by its chemical name, 4-aminopyridine, and is a member of a family of mono-amino and di-amino derivatives of pyridine, with a molecular structure of C5H6N2. The pyridine ring has an amino substitution in the 4 position as shown in . The molecular weight of dalfampridine is 94.12.Citation41 An important feature of dalfampridine ER that distinguishes it from the compounded, immediate-release formulations of 4-aminopyridine is its prolonged pharmacokinetic half-life. The extended-release technology, developed by Élan Pharma International Ltd. (EPIL) and termed MXDAS™, consists of a proprietary polymer matrix that controls release by diffusion resulting in lower peak serum levels and longer duration of action.Citation42
Pharmacokinetics of dalfampridine ER in patients with MS
The extended-release form of dalfampridine was developed commercially to overcome some of the pharmacokinetic and safety concerns associated with immediate-release formulations of the drug which had become available from compounding pharmacies.Citation43,Citation44 Concerns also existed about sustained-release forms of the drug available from compounding pharmacies, as they lacked quality control over manufacturing and distribution processes. Most notably, toxicity was associated with the high peak plasma concentrations of the immediate-release formulationCitation45 and the potency of the sustained-release forms was inconsistent, leading to risks associated with irregular dosing. Commercial development of the extended-release tablet form, with lower peak plasma concentrations, was designed to mitigate these risks and to ensure quality control and bring good manufacturing practices to the formulation and distribution of the drug. Reducing dosing requirements of dalfampridine ER to twice daily, instead of 3 to 5 times per day for immediate-release formulations, would also be expected to enhance patient compliance with oral medication.
The basic pharmacokinetics of an extended release formulation of dalfampridine in MS were initially reported by Bever et al.Citation46 Twelve patients with Extended Disability Status Scale (EDSS) scores of 4 to 7.5 participated in an open-label study in which they received escalating doses (7.5 mg to 25 mg every 12 hours) of dalfampridine. The mean time-to-peak and serum half-life were 5.0 and 5.2 hours, respectively, and maximum tolerable doses varied from 10 mg twice daily to 25 mg twice daily. These parameters were subsequently confirmed and elaborated by Vollmer and HenneyCitation47 in a multicenter, open-label, evaluation of dose-proportionality in 24 patients with clinically definite MS. The pharmacokinetics of single escalating doses of dalfampridine ER (5–20 mg) are summarized in . In a second study, the steady state pharmacokinetic properties of dalfampridine ER 20 mg twice daily were evaluated in the same patients over a 14-day period and compared with their single-dose study results.Citation48 The pharmacokinetic parameters on day 1 replicated those of the single-dose study. The time to maximum plasma concentration (Tmax) values did not differ between days 1, 8, and 15 in the steady-state study, and the values found in the single-dose study (range 3.25–3.78 hours). The maximum plasma concentration (Cmax) values on day 8 and 15 were significantly higher than those on day 1, and in the single-dose study reflected the accumulation of dalfampridine with multiple dosing. With the exception of one case of severe nausea, all adverse events in this study were mild to moderate and there were no significant changes in clinical laboratory values, vital signs, physical examination, or cardiac function (QTc intervals). This study provided support for the use of twice-daily dosing, approximately 12 hours apart, in this population. The absence of harmful effects on cardiac function has been confirmed elsewhere.Citation49
Table 1 Dalfampridine pharmacokinetic parameters
In other studies, dalfampridine ER has been shown to be completely absorbed from the gastrointestinal gut following oral administration and to be almost completely (95.9%) eliminated unchanged via the kidneys (0.5% recovered from feces).Citation50 Small percentages are eliminated as the metabolite 3-hydroxy-4-aminopyridine (4.3%) and 3-hydroxy-4-aminopyridine sulfate (2.6%), neither of which is pharmacologically active at K+ channels.Citation50,Citation51 Dalfampridine ER has a relative bioavailability of 96% compared with an aqueous oral solution. Plasma protein binding is low (1%–3%) and the drug has an apparent volume of distribution of 2.6 L/kg. There are no indications of abnormal liver enzymes associated with the use of dalfampridine ER and the drug does not adversely interact with baclofen (10 mg) or interferon-β-1b (subcutaneous injections of 8 million units), two medications commonly prescribed for use by MS patients.Citation51 Dalfampridine ER kinetics were not affected by coadministration of these drugs.Citation51
With respect to the interaction of dalfampridine ER with other drugs, it is noted in the prescribing informationCitation51 that in vitro data with human liver microsomes showed that dalfampridine ER was not a direct or time-dependent inhibitor of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A4/5. Dalfampridine ER is not likely to affect the pharmacokinetics of drugs that are substrates of these enzymes. Other in vitro studies with cultured human hepatocytes with 0.025 μM, 0.25 μM, 2.5 μM, and 25 μM dalfampridine ER had little or no effect on CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2E1, or CYP3A4/5 enzyme activities. Consequently, the potential for dalfampridine ER to induce human hepatocytes at therapeutic concentrations is remote. In vitro, dalfampridine ER is not a substrate or an inhibitor for the p-glycoprotein transporter. The pharmacokinetics of dalfampridine ER are unlikely to be affected by drugs that inhibit the p-glycoprotein transporter, and dalfampridine ER is not likely to affect the pharmacokinetics of drugs that are substrates of the p-glycoprotein transporter.
In patients with renal impairment, accumulation of drug may occur with repeated administration. The drug should be used cautiously in individuals with mild renal impairment (creatinine clearance [CrCl] 51–80 mL/min) as the risk of seizures in these patients is unknown and the drug is not appropriate for patients with moderate or severely impaired renal function (CrCl ≤ 50 mL/min). Plasma levels of dalfampridine ER in these patients may approach those seen at a dose of 15 mg twice daily, a dose that may be associated with an increased risk of seizures.Citation52 Because patients with renal impairment would require a dose lower than 10 mg twice daily and no strength smaller than 10 mg is available, dalfampridine ER is contraindicated in patients with moderate to severe renal impairment. Elderly patients are more likely to have decreased renal function and it is particularly important to know the estimated CrCl in these patients before initiating treatment with dalfampridine ER. Estimated CrCl should be known before initiating any treatment with dalfampridine ER.
The risks of dalfampridine ER and pregnancy are classified as Pregnancy Category C.Citation51 There are no adequate and well-controlled studies of dalfampridine ER in pregnant women. Administration of dalfampridine ER to animals during pregnancy and lactation resulted in decreased offspring viability and growth at doses similar to the maximum recommended human dose of 20 mg/day. Dalfampridine ER should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.Citation51
Tolerability and adverse events (side effects)
The treatment-emergent adverse events associated with the use of dalfampridine ER are summarized in . The table reports pooled data from one Phase 2 and two Phase 3 studies and includes placebo data for comparison.Citation51 Generally the drug is well tolerated by the majority of individuals within the recommended dosing of 10 mg twice daily; most adverse events are mild and resolve without management within hours or a few days. Mild dizziness, gastrointestinal discomfort, and some agitation or wakefulness are frequently encountered. At higher doses, eg, at 20 to 30 mg twice daily, the risk of more serious and intolerable adverse events increases. The most serious risk is for the induction of epileptiform seizures, eg, focal, tonic-clonic, and complex partial, and individuals who have a history or indicated risk of seizures should not expose themselves to further risk. In one of the Phase 2 trials,Citation13 2 out of a total of 159 patients experienced seizures while taking 20 mg twice daily; 1 of these patients had accidentally overdosed on 2 occasions, and experienced 2 partial complex seizures. There are no reports of recurrent seizures being induced by dalfampridine-ER. In the most recent Phase 3 clinical trial of dalfampridine ER (n = 239), 4 patients in the placebo group and 4 patients in the dalfampridine ER group withdrew because of adverse events.Citation14 The largest proportional differences between treatment and placebo groups were found in adverse events of insomnia, headache, dizziness, nausea, back pain, and balance disorder, as reported using MedDRA terms (Medical Dictionary for Regulatory Activities). The majority of treatment-related events were mild or moderate in intensity, transient, and did not lead to discontinuation from the study.
Table 2 Adverse reactions occurring in ≥2% of patients treated with dalfampridine ER tablets and more frequently than placebo-treated patients in clinical trials
Patient education about the dangers of using immediate-or extended-release forms of dalfampridine, in a nonregulated or uncontrolled manner, or with accidental overdose, is clearly warranted as serious adverse events have been documented.Citation43,Citation45,Citation53,Citation54 The same applies to the risk of self-dosing dalfampridine ER above the prescribed limits particularly in the circumstance when some evidence of efficacy is present within the prescribed dose and individuals seek to gain greater benefit. Attention to the prescribing information for dalfampridine ER, medication labeling, and directions for use is essential.Citation51 The multilevel Risk Evaluation and Mitigation Strategy (REMS) that Acorda Therapeutics, Inc. has implemented, involving medication guide and communication plan, is described on the FDA website.Citation55
Randomized clinical trials showing efficacy in improving walking ability
A series of uncontrolled Phase 1 and controlled Phase 2 clinical trials of dalfampridine and dalfampridine ER, conducted over a 20-year span, established some indications of efficacy for a variety of neurological impairments associated with MS and generally supported the safety and tolerability of the drug.Citation12,Citation56–Citation63
In 2008, Goodman et alCitation12 reported a Phase 2 parallel-group dose-comparison trial in which subjects were randomly assigned to receive dalfampridine-ER 10 mg twice daily (n = 52), 15 mg (n = 50), 20 mg (n = 57) or placebo (n = 47). A 2-week dose-escalation period was followed by a 12-week stable-dose phase and a 1-week dose-reduction phase. The percentage change in the T25FW test was the primary outcome. The average increase in walking speed was 23.5% (10 mg), 26.0% (15 mg), 15.8% (20 mg), and 12.8% (placebo). The dalfampridine ER treatments did not yield significant improvement over placebo. A subsequent “responder” analysis revealed that the percentage of subjects exhibiting an a priori specified improvement of >20% were higher in the dalfampridine ER-treated groups (35.3% [10 mg], 36.0% [15 mg], and 38.6% [20 mg], respectively) compared with the 8.5% responding to placebo. As with the primary outcome these results did not attain statistical significance, and there was no indication of a dose-response, but they did engender some optimism that the drug may be helpful to a subset of individuals with mobility impairment. The only endpoint attaining significance was the change from baseline in lower extremity strength, which showed an improvement of roughly 15% over placebo in both the 10- and 15-mg dose groups.
The responder type analyses prompted KryscioCitation64 to observe that the investigators had discovered an “innovative method for identifying consistent responders to the drug, providing clinicians with a promising tool for treating a subset of MS patients”. KryscioCitation64 also questioned whether this was a true positive finding or an example of data massaging. While acknowledging that responder analyses are acceptable within the context of regulatory approval by the FDA, KryscioCitation64 also noted that the failure to detect statistically significant outcomes may have been due to a lack of statistical power. A major disadvantage of the responder analysis approach is reduced power relative to that based on group averages.Citation65 In this particular study, to make the responder analysis the primary endpoint and meet FDA requirements on comparing multiple doses with placebo, the investigators would have needed to accrue 195 patients per group to show a doubling of responders with equivalent power (80% power with an adjusted type I error rate of 0.016).
Two pivotal Phase 3 trials were then designed to rigorously evaluate the efficacy of dalfampridine ER in enhancing performance on the T25FW in patients with clinically definite MS. The responder analysis was selected as the analytic method to be employed. In order to directly assess the clinical relevance of the outcomes, the 12-Item MS Walking Scale (MSWS-12) was also administered.Citation66 This scale has been shown to be a reliable and valid patient-based measure of the impact of MS on walking and has been shown to be more responsive than other walking-based scales.Citation66 These trials were conducted under the auspices of FDA Special Protocol Assessments.
The first Phase 3 trial (MS-F203)Citation13 was a double-blind, placebo-controlled, parallel-group study conducted in 33 centers in the United States and Canada. Participants received dalfampridine ER 10 mg twice daily or placebo, over a 14-week treatment period, with each arm of the study preceded by a 2-week placebo run-in. All participants were followed for 4 weeks after completion of the double-blind phase of the trial. A total of 301 patients was randomized into the study, on a 3:1 ratio, to receive treatment drug or placebo, and of these 283 completed all study visits. The primary outcome measure, the T25FW, was evaluated 4 times prior to treatment, 4 times during treatment, and twice in the follow-up period. Patients were allowed to use an assistive device while walking, as long as the same device was used consistently across visits. The a priori defined responder analysis was then employed to determine the number of patients who showed a consistent improvement in walking speed during the double-blind treatment period. The primary efficacy endpoint was calculated using a modified intention-to-treat analysis. Secondary outcomes of efficacy related to neuromuscular benefits included measures of lower extremity muscle strength (Lower Extremity Manual Muscle Test or LEMMT) and spasticity (Ashworth Score). The Subject Global Impression (SGI), Clinician Global Impression (CGI), and MSWS-12 were also used as secondary outcomes to validate the clinical meaningfulness of the response criterion of consistent improvement on the T25FW.
The primary result of MS-F203 was that the proportion of timed walk responders in the dalfampridine ER-treated group (34.8%) was significantly greater than in the group given placebo (8.3%). Responders to treatment had an average increase in walking speed of 25.2% during the course of treatment whereas the placebo group showed a 4.7% improvement. The increase in walking speed was maintained throughout the double-blind treatment period. The responders reported greater improvement in the self-assessment of walking capacity (MSWS-12) than nonresponders. A significant increase in leg strength was observed in dalfampridine ER responders compared with subjects in the placebo group. The responder group also showed greater improvement in MSWS-12 scores than nonresponders. These results supported the a priori FDA-approved efficacy criterion that treatment with dalfampridine ER 10 mg twice daily would significantly increase the chance of having a positive response of improved walking speed that is consistent over time and perceived subjectively as a meaningful improvement in ambulatory deficits.
The second Phase 3 trial (MS-F204) was also a double-blind, placebo-controlled, parallel group study in patients with clinically definite MS. This was conducted across 39 centers in the United States and Canada and involved 239 patients who were randomized to treatment with dalfampridine ER 10 mg twice daily or placebo on a 1:1 ratio. For inclusion, participants had to have a T25FW time between 8 and 45 seconds, and the T25FW was again used as the primary outcome measure. The treatment duration was shortened to 9 weeks because of the previously demonstrated stability of beneficial changes in walking speed over a 3-month period.
The results of this trial established that the proportion of T25FW responders was higher in the dalfampridine ER group (42.9%) compared with the placebo group (9.3%) and the average improvement in walking speed for responders was 24.7% or 0.51 ft/s. The increase in walking speed among dalfampridine ER-treated responders was maintained across the double-blind treatment period and was reversed with treatment discontinuation. The average changes from baseline in MSWS-12 score during the double-blind treatment period were −6.04 for responders compared with 0.85 for nonresponders, indicating a significant reduction in self-assessed ambulatory disability among responders. SGI, and CGI scores both confirmed significant benefit for responders. This study thus provided class 1 evidence that dalfampridine ER produced clinically meaningful improvement in walking ability in a subset of people with MS.
Subsequent to completion of these two Phase 3 studies, pooled analyses have been undertaken combining the results of one of the Phase 2 trials, with comparable design, and the two Phase 3 trials. The conclusions drawn were that the efficacy of dalfampridine ER, as defined by the timed-walk responder rate, was independent of demographics, disease characteristics, and use of immunomodulatory drugs.Citation67 Moreover, the benefits of dalfampridine ER were seen across a wide range of baseline deficits in walking ability.Citation15 Using a scale derived from the post-stroke population, which characterized functional walking into household ambulation (<1.3 ft/s), limited community ambulation (1.3– 2.6 ft/sec), or full community ambulation (>2.6 ft/s), Edwards et alCitation15 reported that a substantial proportion of responders in the three studies sufficiently improved their walking speed to represent a change from less to more function within a class and in many cases individuals would be expected to move from one ambulation class to another.
The role of dalfampridine therapy for gait disorders in MS
The primary indication for dalfampridine ER is for walking in MS patients regardless of the severity of deficit and disease characteristics. Pharmacotherapy would be done in conjunction with the use of mobility aids and would be complementary to symptomatic management strategies such as the use of antispastic agents. The use of dalfampridine ER concurrently with baclofen has been shown to be safe in the course of the clinical studies, which did not exclude concurrent treatment; indeed the reported antispastic properties of dalfampridine ER reported in some of the Phase 2 and 3 trials, and evidenced by improved scores on the Ashworth scale, may indicate the possibility of reducing the dosing requirements of anti-spastic medications that are being used, though this has not been established by clinical studies. The demonstrated efficacy and safety of dalfampridine ER in trials in which concurrent use of immunomodulatory, disease-modifying agents were allowed suggests that parallel pharmacotherapeutic strategies may be employed.
The reported clinical benefits of dalfampridine ER extend beyond those for which class 1 evidence exists. There is persuasive experimental evidence of improvements in leg strength and reductions in spasticity, both of which may enable greater independence with respect to transfers and activities of daily living. Indeed, KachuckCitation18 has reported on a number of benefits revealed on clinical examination and by clinical experience but not necessarily amenable to, or captured in, formal clinical trial protocols. These include improvements in endurance or fatigability, visual acuity, bladder/bowel/sexual dysfunction. Improvements in mental sharpness, problem solving and reading comprehension have also been reported anecdotally. Some experimental support for these findings has been noted.Citation56–Citation62 Clearly, clinical judgement will prevail as to how such benefits to these symptoms, if observed, influence concurrent management approaches.
Challenges to the purported clinical benefit of dalfampridine
There have been two recently documented challenges to the acceptance of dalfampridine ER as a therapeutic agent with meaningful clinical benefit. In the first, an editorial published in Annals of Neurology in 2010, by Hauser and Johnston,Citation68 the authors maintained that the drug “improved the 25 foot walk time by an average of less than one second; 0.88 seconds to be exact in the first trial and 0.5 seconds in the second. Although statistically significant, the benefit appears modest for a treatment that costs $13,000 annually”. The authors also noted the continued risk of seizures and implied there was little gain from this medication over the immediate-release formulations available through compounding pharmacies at one-fiftieth of the cost. A rebuttal by Cohen and Blight,Citation69 on behalf of Acorda Therapeutics, and published in January 2011, focused on some inaccuracies in the editorial concerning the development of the drug, highlighted the risks of serious dosing errors associated with compounded formulations of 4-AP,Citation43,Citation44 and addressed what they considered to be an inaccurate reflection of the average change in walking times experienced by the patients. They noted that the results reported in the editorial “appear to have been derived by an unusually complex, post hoc manipulation of a subset of the trial data”. The a priori specified analysis of the study data focused on reproducible improvements in walking speed, acknowledging the day-to-variability in functional capacity experienced by individuals with MS. They noted that in the 37.6% of responders to dalfampridine ER there was an average 25% improvement in walking speed compared to 6.5% in placebo-treated patients and 7.0% in dalfampridine ER-treated nonresponders. The clinical meaningfulness of this improvement in the responder group was reflected by a significant reduction in self-assessed walking disability, evident in the MSWS-12.
In the second challenge, which occurred about the same time as the rebuttal by Cohen and Blight, the Committee for Medicinal Products for Human Use (CHMP), the European agency responsible for conducting the initial assessment of medicines for which an EU-wide marketing authorization is sought, rejected the application for dalfampridine ER (submitted by Biogen Idec under the name Fampyra™).Citation70 CHMP was also not convinced that Fampyra’s effect on the walking speed was a meaningful benefit for patients. Moreover, they argued that the effect on walking speed could not be linked to meaningful improvements such as better coordination, balance, or stamina or increased range of motion. The Committee was not persuaded that the medicine’s benefits outweighed its side effects. The Committee also noted the lack of adequate long-term data on the medicine’s benefits and safety as well as data on some groups of patients, such as the elderly and patients with epilepsy or heart problems. Biogen Idec is appealing this decision at the time of this review going to press.
Impact of dalfampridine ER on the neuropsychiatric burden of diseases in MS
The neuropsychiatric burden of MS is multifaceted and complex.Citation7–Citation9 It includes neuropathology-related impairments of emotional control and cognitive processes brought about by demyelination and/or axonal pathology in various locations within the brain sub-serving these functions. Benedict et al,Citation28 for example, have reported that measures of tissue atrophy including whole-brain and central atrophy are especially well correlated with and predictive of cognitive impairment. The burden also involves disability-related, “reactive” processes such as mood disorders associated with diagnosis, experienced disability and its social consequences, and concern for the future because of the progressive nature of the disease and the interaction with the normal functional decline of aging; ie, “accelerated degradation”.Citation2,Citation71 Compounding the burden on the patient is the huge impact of their needs on caregivers,Citation72 both contracted caregivers and loved ones, and the economics of managing their disability, often when their employment has been compromised.Citation2
How does dalfampridine ER impact these states? There is no empirical evidence from controlled trials to directly address these issues. Inference drawn from the Phase 3 trials and earlier studies does suggest that improved mobility may mitigate the dependence on others for some activities of daily living, including personal care and homemaking tasks, and may enable greater community involvement or extended employment. Such benefits would be expected to translate to fewer feelings of dependence and burden of the stigma associated with disability. The extent to which this becomes clinically and personally meaningful will likely depend on the specific disease state and circumstances of each patient.
The neurobiological mechanism of action of dalfampridine ER in restoring conduction in demyelinated axons is unlikely to be confined to the sensory and motor pathways involved in gait, even if there exist subtle differences in K+ channel typology and distribution in different parts of the CNS.Citation33 Indeed one could reasonably project that disseminated foci of demyelination throughout the CNS all represent viable targets for the drug action. In this case, one might expect to find that dalfampridine ER is capable of reversing demyelination-based cognitive and emotional deficits and thereby attenuating the neuropsychiatric burden directly. Smits et al,Citation73 in a small (n = 20) randomized, double-blind, crossover trial, examined the effects of 2 weeks treatment with immediate-release oral 4-aminopyridine (dalfampridine) on cognitive function in patients with definite MS. Although there was a trend for improved performance with the study medication for two of the neuropsychological tests (10/36 delayed recall and the Paced Auditory Serial Addition Test of attention), the study failed to demonstrate significant group effects of dalfampridine on cognitive function. The extent to which medication-induced improvements in performance will be feasible and able to yield clinical benefit, however, must be weighed in the context of the evolving awareness of distinct patterns and regional distribution of gray matter damage that are associated with cognitive impairment in MS patients.Citation27 The correspondence between lesion formation and gray matter atrophy distribution varies in the different forms of MS. Thus the degree to which dalfampridine may be useful will depend on the degree to which demyelination, as distinct from axonal pathology, contributes to the cognitive dysfunction and this may vary among individuals and among clinical phenotypes.
Implications for further research
Two obvious lines of enquiry emerge from the above discussion of the impact of dalfampridine ER on walking ability and the corollary of attenuating the neuropsychiatric burden. The first is to more directly examine the impact of drug-induced improvements in walking on instrumental activities of daily living, employment, or other rehabilitation outcomes. Although challenging because of the multivariate nature of factors influencing performance and outcome, it is through empirically demonstrating benefit in these areas that some of the reactive burden may be lifted. Accompanying those forms of benefit would likely be demonstrable enhancements in the quality of life.Citation74 The second line of enquiry would be to directly evaluate the efficacy of the drug in influencing biomarkers or other indices of cognitive or neuropsychiatric impairment. These types of studies may provide focus for further Phase 2 or 3 studies, or postmarketing Phase 4 investigations that will enable new indications to be identified.
As a first-generation pharmaceutical designed to reverse demyelination-induced conduction deficits, dalfampridine ER is likely to spawn further developments with novel K+ channel blocking agents with similar or more refined mechanisms of action. New insights are emerging about the large family of voltage-gated K+ ion channels.Citation75 Elaboration of the properties of these channels and their relationship to disease and neurological dysfunction is helping to define new therapeutic targets.Citation76
Summary
Dalfampridine ER is the first of a new class of pharmacotherapeutics designed to restore neurological function compromised by central demyelination. It has received approval from the FDA in the United States for the improvement of walking in people with MS and is currently pending regulatory approval in Canada. The FDA has required additional studies investigating the efficacy of lower doses of dalfampridine ER, notably 5 mg tablets. In Europe the drug (under the name Fampyra) has been denied approval for marketing by the CHMP because of what was considered small benefit relative to risk, a decision currently under appeal by Biogen Idec. The improvements in gait brought about by the drug are evident as increased walking speed on a timed walking test (T25FW), positive global assessments by clinician and patient, and reduced walking disability as reflected in the patient self-assessed MSWS-12. Since mobility impairments contribute greatly to the patient burden of disease with respect to activities of daily living, employment, self-image, and mood, improvements in gait would be projected to mitigate some of the reactive neuropsychiatric burden of disease. Whether or not there are direct effects of the drug in reversing demyelination-induced axonal conduction deficits underlying emotional and cognitive impairment remains to be determined by further research.
Disclosure
K C Hayes PhD received and exercised stock options for consulting with Acorda Therapeutics, Inc., New York, the company that sponsored the clinical trials of Fampridine-SR (dalfampridine extended release) and is now marketing the product in the USA under the name Ampyra™.
References
- BaumHMRothschildBBMultiple sclerosis and mobility restrictionArch Phys Med Rehabil1983645915966661022
- DunnJImpact of mobility impairment on the burden of caregiving in individuals with multiple sclerosisExpert Rev Pharmacoeconomics Outcome Res201010410.1586/ERP.10.34
- SutliffMHContribution of impaired mobility to patient burden in multiple sclerosisCurr Med Res Opin20102610911919916707
- MyhrKMRiiseTVedelerCDisability and prognosis in multiple sclerosis: demographic and clinical variables important for the ability to walk and awarding of disability pensionMult Scler20017596511321195
- Whetten-GoldsteinKSloanFAGoldsteinLBA comprehensive assessment of the cost of multiple sclerosis in the United statesMult Scler199844194259839302
- SalterARCutterGRTyryTImpact of loss of mobility on instrumental activities of daily living and socioeconomic status in patients with MSCurr Med Res Opin20102649350020014979
- PinkstonJBAlekseevaNNeuropsychiatric manifestations of multiple sclerosisNeurol Res20062828429016687055
- GhaffarOFeinsteinAThe neuropsychiatry of multiple sclerosis: a review of recent developmentsCurr Opin Psychiatry20072027828517415083
- PaparrigopoulosTFerentinosPKouzoupisAThe neuropsychiatry of multiple sclerosis: focus on disorders of mood, affect and behaviourInt Rev Psychiatry201022142120233111
- SouzaAKelleherACooperRMultiple sclerosis and mobility-related technology: sytematic review of literatureJ Rehab Res Dev201047201212
- Harris InteractiveKey findings from two new multiple sclerosis surveys [internet]New York (NY)National MS Society and Acorda Therapeutics2008 http:/www.nationalmssociety.org/news/newsdetail/download.aspx?id=1018.
- GoodmanADBrownTRCohenJAFampridine MS-F202 Study GroupDose comparison trial of sustained-release fampridine in multiple sclerosisNeurology2008711134114118672472
- GoodmanADBrownTRCohenJASustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trialLancet200937373273819249634
- GoodmanADBrownTREdwardsKRA phase 3 trial of extended release oral dalfampridine in multiple sclerosisAnn Neurol20106849450220976768
- EdwardsKBrownTSchapiroADDalfampridine extended release tablets improve walking speed across a wide range of baseline deficits: pooled data from three placebo-controlled studies in patients with multiple sclerosis [abstract]62nd Annual Meeting of the American Academy of Neurology2010 Apr 10–17Toronto
- HayesKCFampridine-SR for multiple sclerosis and spinal cord injuryExp Rev Neurother20077453461
- KorenkeARRiveyMPAllingtonDRSustained-release fampridine for symptomatic treatment of multiple sclerosisAnn Pharmacol20084214851465
- KachuckNJSustained release oral fampridine in the treatment of multiple sclerosisExpert Opin Pharmacother2009102025203519586420
- BeverCTJudgeSISustained-release fampridine for multiple sclerosisExpert Opin Investig Drugs20091810131024
- ChwiedukCMKeatingGMDalfampridine extended release in multiple sclerosisCNS Drugs20102488389120839898
- GoodmanADHylandMDalfampridine in multiple sclerosisDrugs Today (Barc)20104663563920967295
- CompstonAColesAMultiple sclerosisLancet20083721502151718970977
- BartenLJAllingtonDRProcacciKARiveyMPNew approaches to the management of multiple sclerosisDrug Des Dev Ther201024343366
- KleinschnitzCMEuthSGKiesseirBCWiendlHImmunotherapeutic approaches in MS: update on pathophysiology and emerging agents 2006Endocr Metab Immune Disord Drug Targets20077356317346203
- DuttaRTrappBDMechanisms of neuronal dysfunction and degeneration in multiple sclerosisProg Neurobiol20101012 [Epub ahead of print].
- SiffrinVVogtJRadbruchHNitschRZippFMultiple sclerosis – candidate mechanisms underlying CNS atrophyTrends Neuroscience201033202210
- RiccitelliGRoccaMAPaganiECognitive impairment in multiple sclerosis is associated to different patterns of gray matter atrophy according to clinical phenotypeHum Brain Mapp2010 [Epub ahead of print].
- BenedictRHCaroneDABakshiRCorrelating brain atrophy with cognitive dysfunction, mood disturbances, and personality disorder in multiple sclerosisJ Neuroimaging2004143 Suppl36S45S15228758
- RoccaMARiccitelliGRodegherMFunctional MR imaging correlates of neuropsychological impairment in primary-progressive multiple sclerosisAm J Neuroradiol2010311240124620299439
- McDonaldWISearsTAThe effects of experimental demyelination on conduction in the central nervous systemBrain1970935835984319185
- WaxmanSGConduction in myelinated, unmyelinated, and demyelinated fibersArch Neurol197734585589907529
- WaxmanSGMembranes, myelin, and the pathophysiology of multiple sclerosisN Engl J Med1982306152915337043271
- JudgeSIBeverCTJrPotassium channel blockers in multiple sclerosis: Neuronal Kv channels and effects of symptomatic treatmentPharmacol Ther200611122425916472864
- BostockHSherrattRMSearsTAOvercoming conduction failure in de-myelinated nerve fibres by prolonging action potentialsNature1978274385387209331
- SherrattRMBostockHSearsTAEffects of 4-aminopyiridine on normal and demyelinated mammalian nerve fibresNature19802835705727354839
- BostockHSearsTASherrattRMThe effects of 4-aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibresJ Physiol19813133013157277221
- BoweCMKocsisJDTargEFWaxmanSGPhysiological effects of 4-aminopyridine on demyelinated mammalian motor and sensory fibersAnn Neurol1987222642682821876
- TargEFKocsisJD4-aminopyridine leads to restoration of conduction in demyelinated rat sciatic nerveBrain Res19853283583612985185
- JankowskaELundbergARudominPSykovaEEffects of 4-aminopyridine on synaptic transmission in the cat spinal cordBrain Res19822401171296284313
- LundhHEffects of 4-aminopyridine on neuromuscular transmissionBrain Res1978153307318210882
- UgesDRAHuizingaT4-aminopyridine; analysis of the substance and a method for the preparation of a solution for injection in manPharm Acta Helv1981561581627255503
- United States Food and Drug Administration: Medication Guide for Ampyra™ (dalfampridine) Extended Release Tablets http://www.fda.gov/downloads/Drugs/DrugSafety/UCM199168.pdf. Accessed March 24, 2011.
- BurtonJMBellCMO’ConnorPW4-aminopyridine toxicity with unintentional overdose in four patients with multiple sclerosisNeurology2008711833183419029525
- SchwamESevere accidental overdose of 4-aminopyridine due to compounding pharmacy errorJ Emerg Med200910.1016/2009.04.037
- BeverCTJrYoungDAndersonPAThe effects of 4-aminopyridine in multiple sclerosis patients: results of a randomized, placebo-controlled, double-blind, concentration-controlled, crossover trialNeurology199444105410598208399
- BeverCTJrYoungDTierneyDThe pharmacokinetics and tolerability of a slow-release formulation of 4-aminopyridine in multiple sclerosis patientsNeurology199545Suppl 4A351
- VollmerTHenneyHRPharmacokinetics and tolerability of single escalating doses of fampridine sustained-release tablets in patients with multiple sclerosis: a phase 1–11, open-label trialClin Ther2009312206221419922891
- VollmerTBlightARHenneyHRSteady-state pharmacokinetics and tolerability of orally administered fampridine sustained-release 10-mg tablets in patients with multiple sclerosis; a 2-week, open-label, follow-up studyClin Ther2009312215222319922892
- MarchBCardiTAssessment of the cardiac safety of fampridine-SR sustained-release tablets through a thorough QT/QTc evaluation at therapeutic and subtherapeutic doses in healthy individualsExpert Opin Inv Drugs20091818071815
- BlightARHenneyHRPharmacokinetics of 14C-radio-activity after oral intake of a single dose of 14C-labelled fampridine (4-aminopyridine) in healthy volunteersClin Ther20093132833519302905
- United States Food and Drug Administration: Prescribing Information for Ampyra™ (dalfampridine) extended release tablets www.accessdata.fda.gov/drugsatfda_docs/label/.../022250s000lbl.pdf. Accessed March 24, 2011.
- SmithWSwanSMarburyTHenneyH3rdSingle-dose pharmacokinetics of sustained-release fampridine (Fampridine-SR) in healthy volunteers and adults with renal impairmentJ Clin Pharmacol20105015115919966074
- PickettTAEnnsRAtypical presentation of 4-aminopyridine overdoseAnn Emerg Med1996273823858599505
- StorkCMHoffmanRSCharacterization of 4-aminopyridine in overdoseJ Toxicol Clin Toxicol1994325835877932918
- United States Food and Drug Administration: Risk Evaluation and Mitigation Strategy (REMS) for Ampyra (Dalfampridine) 10 mg tablets http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022250s000REMS.pdf. Accessed March 24, 2011.
- DavisFAStefoskiDRushJOrally administered 4-aminopyridine improves clinical signs in multiple sclerosisAnn Neurol1990271861922317014
- StefoskiDDavisFAFautMSchaufCL4-Aminopyridine improves clinical signs in multiple sclerosisAnn Neurol19872171772435223
- StefoskiDDavisFAFitzsimmonsWELuskinSSRushJParkhurstGW4-Aminopyridine in multiple sclerosis: Prolonged administrationNeurology199141134413481891078
- Van DiemenHAPolmanCHvan DongenTMThe effect of 4-aminopyridine on clinical signs in multiple sclerosis: a randomized, placebo-controlled, double-blind, cross-over studyAnn Neurol1992321231301510353
- Van DiemenHAPolmanCHvan DongenMM4-Aminopyridine induces functional improvement in multiple sclerosis patients: a neurophysiological studyJ Neurol Sci19931162202268336169
- PolmanCHBertelsmannFWvan LoenenACKoetsierJC4-aminopyridine in the treatment of patients with multiple sclerosis. Long-term efficacy and safetyArch Neurol1994512922968129642
- SchwidSRPetrieMDMcDermottMPTierneyDSMasonDHGoodmanADQuantitative assessment of sustained release 4-aminopyridine for symptomatic treatment of multiple sclerosisNeurology1997488178219109861
- GoodmanADCohenJACrossAFampridine-SR in multiple sclerosis: a randomized, double-blind, placebo controlled, dose ranging studyMult Scler20071335736817439905
- KryscioRJFampridine for MS responders: Clinically relevant or hypothesis generating?Neurology2008711130113118672471
- AltmanDGRoystonPThe cost of dichotomizing continuous variablesBMJ2006332108016675816
- HobartJCRiaziALampringDLFitzpatrickRThompsonAJMeasuring the impact of MS on walking ability: The 12-Item MS Walking Scale (MSWS-12)Neurology200360313612525714
- BrownTSchapiroREdwardsKResponse to treatment with dalfampridine extended release tablets in patients with multiple sclerosis is independent of baseline patient characteristics and concomitant immunomodular therapy [abstract]62nd Annual Meeting of the American Academy of Neurology2010 Apr 10–17Toronto
- HauserSLJohnstonSC4-Aminopyridine: new life for an old drugAnn Neurol201068A8A9
- CohenRBlightAR4-Aminopyridine: new life for an old drug. ReplyAnn Neurol20116921822021280101
- Biogen Idec website. www.biogenidec.com. Accessed March 24, 2011.
- HayesKCWolfeDLTrujilloSABurkellJAOn the interaction of disability and aging: Accelerated degradation models and their influence of projections of future care needs and costs for personal injury litigationDisabil Rehabil20103242442819968570
- FigvedNMyhrKMLarsenJPAarslandDCaregiver burden in multiple sclerosis: the impact of neuropsychiatric symptomsJ Neurol Neurosurg Psychiatry2007781097110217237144
- SmitsRCEmmenHHBertelsmannFWKuligBMvan LoenenACPolmanCHThe effects of 4-aminopyridine on cognitive function in patients with multiple sclerosis: a pilot studyNeurology199444170117057936300
- ZwibelHLContribution of impaired mobility and general symptoms to the burden of multiple sclerosisAdv Ther2009261043105720082242
- JudgeSISmithPJStewartPEBeverCTJrPotassium channel blockers and openers as CNS neurologic therapeutic agentsRecent Pat CNS Drug Discov2007220022818221232
- WulffHCastleNAPardoLAVoltage-gated potassium channels as therapeutic targetsNat Rev Drug Discov20098982100119949402