Abstract
Background
Jian-Pi-Zhi-Dong Decoction (JPZDD) is a dedicated treatment of Tourette syndrome (TS). The balance of neurotransmitters in the cortico-striato-pallido-thalamo-cortical network is crucial to the occurrence of TS and related to its severity. This study evaluated the effect of JPZDD on glutamate (Glu) and γ-aminobutyric acid (GABA) and their receptors in a TS rat model.
Materials and methods
Rats were divided into four groups (n=12 each). TS was induced in three of the groups by injecting them with 3,3′-iminodipropionitrile for 7 consecutive days. Two model groups were treated with tiapride (Tia) or JPZDD, while the control and the remaining model group were gavaged with saline. Behavior was assessed by stereotypic score and autonomic activity. Striatal Glu and GABA contents were detected using microdialysis. Expressions of N-methyl-D-aspartate receptor 1 and GABAA receptor (GABAAR) were observed using Western blot and real-time polymerase chain reaction.
Results
Tia and JPZDD groups had decreased stereotypy compared with model rats; however, the JPZDD group showed a larger decrease in stereotypy than the Tia group at a 4-week time point. In a spontaneous activity test, the total distance of the JPZDD and Tia groups was significantly decreased compared with the model group. The Glu levels of the model group were higher than the control group and decreased with Tia or JPZDD treatment. The GABA level was higher in the model group than the control group. Expressions of GABAAR protein in the model group were higher than in the control group. Treatment with Tia or JPZDD reduced the expression of GABAAR protein. In the case of the mRNA expression, only Tia reduced the expression of N-methyl-D-aspartate receptor 1, compared with the model group.
Conclusion
JPZDD could alleviate impairments in behavior and dysfunctional signaling by downregulating GABAAR in the striatum. We suggest that this acts to maintain the balance of Glu and GABA.
Introduction
Tourette syndrome (TS) is a neuropsychiatric disorder with childhood onset. Tics, a symptom of TS, are sudden, rapid, recurrent, nonrhythmic motor movements or vocalizations, and typically follow a waxing and waning pattern.Citation1 Common tics are either motor (eye blinking, head jerking, grimacing, and jaw, neck, shoulder, or limb movements) or phonic (sniffing, throat clearing, grunting, and chirping). Motor tics often appear before vocal tics and have a typical age of onset of 5–7 years.Citation2 The male-to-female ratio of TS is 4.4:1.Citation3
Stress, anxiety, and fatigue can exacerbate the symptoms, while concentration can reduce symptoms. TS often has a variety of comorbid conditions, including attention-deficit hyperactivity disorder, obsessive–compulsive disorder, learning difficulties, sleep abnormalities, anxiety, and other behavioral abnormalities.Citation4
The etiological and pathophysiological mechanism of TS remains unclear. At present, dysfunction of the Cortico-Striatal-Thalamic-Cortical (CSTC) network is suggested as a core pathophysiological mechanism. Most research focuses on neurotransmitters in the CSTC circuit. Dysregulation of dopaminergic neurotransmission in the CSTC circuit may play a role.Citation5 Overactivity of dopaminergic systems is observed in TS.Citation6,Citation7 However, the implicated neurotransmitters are involved in various neurological diseases in addition to TS.Citation8,Citation9
Excitatory and inhibitory neurotransmitters are in a dynamic balance. The dynamic characterization of amino acids is especially useful for understanding the pathogenesis of psychiatric disorders, including TS.Citation10 Glutamate (Glu) and γ-aminobutyric acid (GABA) are the main excitatory and inhibitory neurotransmitter, respectively. TS may have underlying glutamatergic dysfunction; targeting selective subunits of the N-methyl-D-aspartate receptor (NMDAR) or release-modulating Glu autoreceptors is a promising strategy for modulating dysfunctional Glu neurotransmission in TS patients.Citation11 GABA receptors are also key targets for drug design to treat various tics, and GABAA receptor (GABAAR) systems may play a major role in the pathophysiology of TS.Citation12 Although there is both clinical and experimental evidence that these amino acids are involved in TS, the role of their receptors remains unclear.
Improved treatments for TS are needed. Recent study has reported the anti-tic properties of traditional Chinese medicines.Citation13,Citation14 Jian-Pi-Zhi-Dong Decoction (JPZDD) is a specific treatment for TS that has displayed curative effects in a clinical study of TS treatment.Citation15 However, the ability of JPZDD to modulate the balance of excitatory and inhibitory neurotransmission is still not understood. TS affects striatal circuitry, so we used microdialysis to measure basal striatal dialysate levels of Glu and GABA in a TS rat model. We used Western blot and real-time polymerase chain reaction (PCR) to measure the expression of the GABAAR and NMDAR1. In addition, we assessed the efficacy of JPZDD in the treatment of TS.
Materials and methods
Drugs and reagents
3,3′-Iminodipropionitrile (IDPN) was purchased from Sigma-Aldrich Co. (St Louis, MO, USA), and tiapride (Tia) from Jiangsu Nhwa Pharmaceutical Co., Ltd. (Xuzhou, People’s Republic of China). o-Phthalaldehyde, Glu, GABA, sodium heptane sulfonate, sodium acetate, boric acid, and β-mercaptoethanol were obtained from Sigma-Aldrich Co. High-performance liquid chromatography (HPLC)-grade methyl alcohol was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Ethylene diamine tetraacetic acid and KH2PO4 were obtained from Beijing Chemical Works (Beijing, People’s Republic of China).
The microdialysis system consisted of a CMA/120 system for freely moving animals (table, cage, and balancing arm) together with a CMA/402 microdialysis pump, a 5 mL glass syringe, a CMA/12 microdialysis probe, and a CMA/470 refrigerated fraction collector.
Preparation of JPZDD
Ten different Chinese medicinal herbs were included in the JPZDD. They were purchased from the Pharmaceutical Department of Dongfang Hospital affiliated to the Beijing University of Chinese Medicine. Director Qing-chun Hao identified the components, and the voucher specimens were deposited. JPZDD contains 10 g Pseudostellaria heterophylla, 10 g Atractylodes macrocephala Koidz, 10 g Poria cocos Wolf, 6 g Pinellia ternata Breit, 6 g Citrus reticulata Blanco, 6 g Saposhnikovia divaricate Schischk, 3 g Gentiana scabra Bge, 10 g Angelica sinensis Diels, 6 g Ligusticum chuanxiong Hort, and 10 g Uncaria rhynchopylla Jacks. The granules were dissolved in 50 mL of distilled water, and then stored at 2°C–8°C until use.
Animals
Forty-eight healthy male Sprague Dawley rats with body weights of 50±10 g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, People’s Republic of China; No SCXK 2012-0001). All animal experimental protocols conformed to the Animal Management Rules of the Chinese Ministry of Health, and the study was approved by the Animal Ethics Committee of the Chinese Academy of Medical Sciences. The animals were maintained in a standard 12-hour light/dark cycle at 21°C±1°C and had access to food and water ad libitum, with the relative humidity of 30%–40%. The rats were fed for 1 week before inducing TS. After 1 week, the rats were randomly divided into two groups: the saline group (control group, n=12) and a TS model group (n=36). The saline group received an intraperitoneal injection of 0.9% saline (15 mL kg−1), and the TS model group received an intraperitoneal injection of IDPN (250 mg kg−1) once daily for seven consecutive days. We assessed behavior of the TS model group by evaluating grades of stereotypy.Citation13,Citation16,Citation17 The TS model group was further divided into three groups: model group (n=12), Tia group (n=12), and JPZDD group (n=12). The control and model groups were gavaged with 0.9% saline (10 mL kg−1), the Tia group with Tia (21 mg kg−1),Citation18 and the JPZDD group with JPZDD (16 g kg−1) once a day for 4 consecutive weeks.
Behavioral recording
Stereotypy recording
Stereotypy recording was conducted by two trained observers who were familiar with the measurements but blind to the group condition. For evaluating the stereotypy,Citation13 each animal was observed for 2 minutesCitation16 once every week after IDPN injection and drug administrations. The average score was calculated for each rat.
Autonomic activity test
The autonomic activity test was conducted every week. We connected the animal behavior analysis system with a spontaneous activity video analysis system. The sequence of each group was random. One rat was placed in every autonomic activity box. Before recording, the rat was allowed to adapt to the environment for 5 minutes. Then, the activity of each rat was recorded for 5 minutes. We chose the total distance as the objective indicator to judge the autonomic activity of the rat.Citation19 The box was kept in shade, and the environment was quiet.
Microdialysis
At the end of the treatment, the rats (n=6) were anesthetized with 10% chloral hydrate (0.035 mL kg−1, intraperitoneal) and transferred to the brain stereotaxic apparatus (STRONG 8003). A CMA/12 microdialysis guide cannula was inserted into the left striatum with the following coordinates relative to Bregma: anterior–posterior +0.2 mm, medial–lateral +3.2 mm, and dorsal–ventral −7.0 mm.Citation20 The microdialysis guide cannula was permanently fixed to the skull using stainless steel screws and methacrylate cement. Animals were allowed to recover for 24 hours before microdialysis experiments began. On the morning of the next day, a microdialysis probe (CMA/12MD, 3 mm long) was inserted into the cannula vertically, while the rat was in a freely moving state. The probes were perfused with compound sodium chloride (0.9%) at 2 µL min−1 for 1 hour to reach stable baseline values. Then, the rat was gavaged. A microdialysis sample was collected every 15 minutes; a total of 24 samples were consecutively collected over the following 360 minutes for each animal. Every other sample collected by microdialysis was analyzed using HPLC with fluorescence detection (FD). The others were analyzed using an ISCUS microdialysis analyzer.
Instrumentation and chromatographic conditions of HPLC with FD
HPLC was performed using Agilent 1200 HPLC with G1321A FD, and Eclipse AAA column (4.6×150 mm, 5 µm) with an excitation wavelength (λex) of 340 nm and an emission wavelength (λem) of 455 nm, and a column temperature of 40°C. The derivative liquid was compounded as follows: 5 mg o-Phthalaldehyde was dissolved in 120 µL dehydrated ethanol, and then added to 1 mL borate buffer (0.2 mol/L, pH 9.2) and 10 µL β-mercaptoethanol. Mobile phase A consisted of sodium borate buffer, methanol, and tetrahydrofuran (in a ratio of 400:95:5), and mobile phase B consisted of sodium acetate buffer solution (pH 7.2) and methanol (in a ratio of 120:380). The gradient elution procedure was 0–10 minutes, B (0%–63%), 10–12 minutes, B (63%), 12–17 minutes, B (100%), 17–18 minutes, B (100%–0%), and 18–21 minutes, B (0%). The flow rate was 0.8 mL min−1.
Western blot for NMDAR1 and GABAAR
The left striatum (n=6 per group) was homogenized and lysed in radioimmunoprecipitation lysis buffer. The protein concentration was quantified with the Bradford method. The protein was used for Western blot with a GABAAR α4 primary antibody (1:1,000, ab4120; Abcam, Cambridge, UK) and an NMDAR1 primary antibody (1:500, ab109182; Abcam), incubated overnight at 4°C. After incubation, the membranes were washed three times with Tris-buffered saline with Tween 20, and then incubated with the secondary antibody (1:2,000) at room temperature for 1 hour. The blots were visualized with Super ECL Plus Detection Reagent (sc-2048; Santa Cruz Biotechnology Inc., Dallas, TX, USA). The electrochemiluminescence signals were detected with Quantity One software (Bio-Rad Laboratories Inc., Hercules, CA, USA). GAPDH (ab8245; Abcam) was used as an internal control to validate the amount of protein loaded onto the gels.
Real-time PCR for NMDAR and GABAAR
The right dorsolateral striatum (n=6 per group) was dissected and frozen at −80°C. Total RNA from the striatum was isolated with Trizol reagent according to the manufacturer’s protocol (Tiangen Biotech, Beijing, People’s Republic of China). The primers were as follows: NMDAR1 forward primer 5′-CGGTATCAGGAATGCGACTC-3′ and reverse primer 5′-GGAAAATCCCAGCTACGATG-3′; GAPDH (NMDAR1) forward primer 5′- TGGAGTCTACTGGCGTCTT-3′ and reverse primer 5′-TGTCATATTTCTCGTGGTTCA-3′; GABA α4 forward primer 5′-CAGACGGAAGATGGGCTATT-3′ and reverse primer 5′-TCATCGTGAGGACTGTGGTT-3′; and GAPDH (GABA α4) forward primer 5′- CAACTCCCTCAAGATTGTCAGCAA-3′ and reverse primer 5′-GGCATGGACTGTGGTCATGA-3′. The basic protocol for real-time PCR was an initial denaturation at 95°C for 2 minutes, followed by 45 cycles of amplification. For cDNA amplification, the cycles consisted of denaturation at 95°C for 10 minutes, annealing at 95°C for 15 seconds, and elongation at 60°C for 60 seconds. The SYBR green signal was detected using an ABI7500 real-time PCR machine (Thermo Fisher Scientific). PCR products were analyzed using gel electrophoresis and a melting curve analysis to confirm specific amplifications. mRNA expressions were normalized to GAPDH. Transcript levels were quantified using the ΔΔCt value method.
Statistical analyses
The data are presented as the mean ± standard deviation. All data were analyzed with Statistical Package for the Social Sciences version 17.0 software (SPSS Inc., Chicago, IL, USA). The mean values were compared using one-way analysis of variance after normal distribution and homogenous variance, followed by the Student–Newman–Keuls test. In all tests, the criterion for statistical significance was P<0.05.
Results
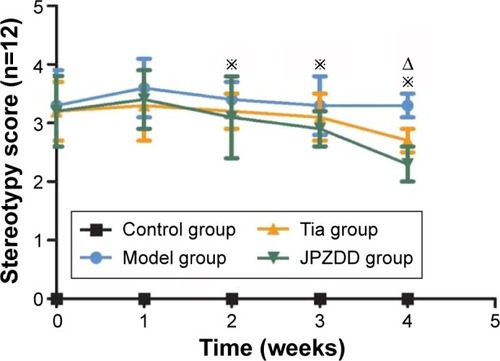
Stereotypy score
Rat model groups with TS induced by IDPN showed abnormal stereotypes in different degrees compared with the control group (P<0.05), but there were no differences between the model groups (P>0.05). Two weeks after treatment, the stereotypy scores for the JPZDD and Tia groups showed a significant decrease compared with the model group (P<0.05). Four weeks after treatment, the JPZDD group showed a significant decrease in the severity of stereotyped behavior compared with the Tia group (P<0.05) ().
Figure 1 Effect of JPZDD on stereotypy score in TS rats.
Abbreviations: JPZDD, Jian-Pi-Zhi-Dong Decoction; TS, Tourette syndrome; Tia, tiapride.
Autonomic activity test
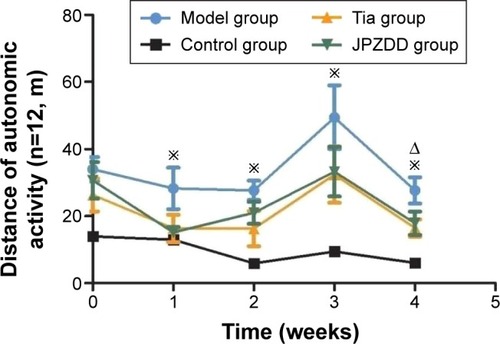
During the treatment, the total distance of the TS model, JPZDD, and Tia groups increased significantly compared with the control group (P<0.05). At 4 weeks after treatment, the total distance of the JPZDD and Tia groups was significantly decreased compared with the model group (P<0.05), but there was no statistical significance between the treatments (P>0.05) ().
Figure 2 Effect of JPZDD on autonomic activity in TS rats.
Abbreviations: JPZDD, Jian-Pi-Zhi-Dong Decoction; TS, Tourette syndrome; Tia, tiapride.
Method validation
The system suitability test confirmed the validity of the analytical procedure as well as the resolution between different peaks of interest. The amino acids were well separated with peak symmetry within 40 minutes (). Glu, aspartate, GABA, glycine, and taurine were separated clearly. Thus, we assumed that this method was specific for the five amino acids. The values of the standard curves for Glu and GABA are shown in .
Figure 3 HPLC-FD analysis of five kinds of amino acids.
Abbreviations: HPLC-FD, high-performance liquid chromatography with fluorometric detection; Asp, aspartate; Glu, glutamate; Gly, glycine; Tau, taurine; GABA, γ-aminobutyric acid; min, minutes.
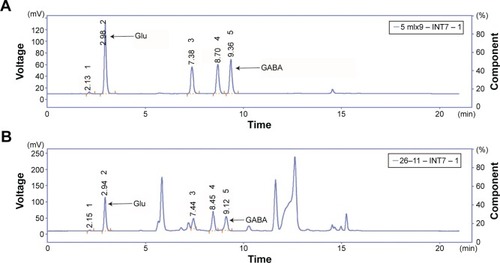
Table 1 The values of the standard curve and linear range of Glu and GABA
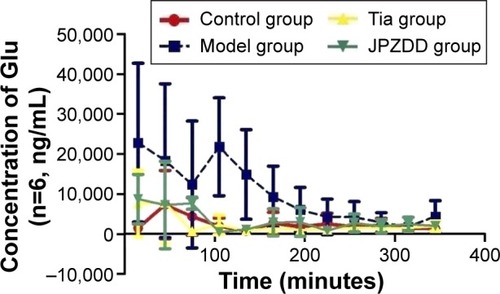
Changes in Glu contents
Every other sample collected from the four groups was evaluated as described earlier (). Glu levels were elevated in the model group and reached a maximum concentration at 105 minutes and then decreased slowly. Glu levels in the model group (22,840.52±19,846.5 ng/mL, 21,780.58±12,219.60 ng/mL, 14,868.27±11,183.42 ng/mL) were higher than the control group (1,469.40±712.67 ng/mL, 1,943.44±2,021.19 ng/mL, 761.87±109.74 ng/mL) at 15, 105, and 135 minutes (P<0.05 for all time points). Compared with the model group, Glu levels decreased in the Tia and JPZDD groups at 15 (7,621.34±8,292.23 ng/mL, 8,709.02±6,186.24 ng/mL) (P<0.05), 105 (2,282.35±2,426.52 ng/mL, 483.6345±287.84 ng/mL) (P<0.01), 135 (854.37±445.26 ng/mL, 1,148.15±712.36 ng/mL) (P<0.01), and 165 minutes (1,443.26±883.83 ng/mL, 2,875.93±3,419.85 ng/mL) (P<0.05), as well as at 195 minutes in the Tia group (987.19±604.77 ng/mL) (P<0.05).
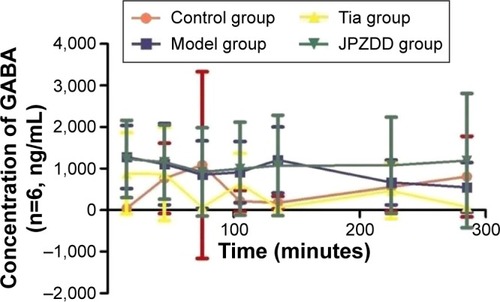
Changes in GABA contents
The GABA levels were elevated in the model group and reached a maximum concentration at 135 minutes and then decreased slowly. Compared with the control group (179.45±153.58 ng/mL), the model group (1,208.37±794.89 ng/mL) had significantly higher levels of GABA at 135 minutes (P<0.05) (). There were no statistically significant differences at other points.
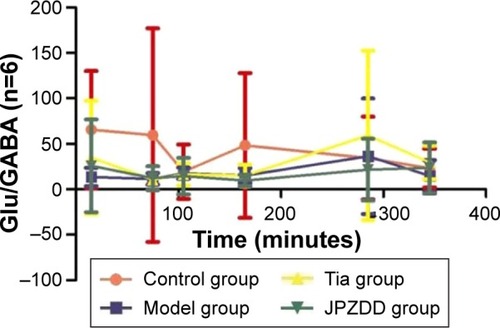
Changes in Glu/GABA
There were no statistically significant differences between the groups in Glu/GABA levels at all points ().
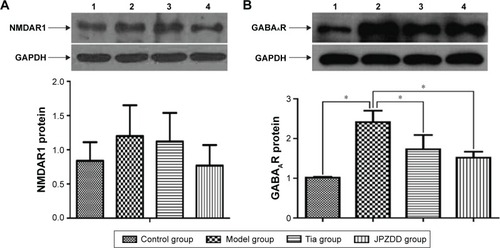
Western blot expression of NMDAR1 and GABAAR
The protein expression trends of NMDAR1 and GABAAR in the striatum were confirmed by Western blot analysis. Normalized with the GAPDH expression level, the expression of GABAAR protein in the model group (2.42±0.29) was higher than that in the control group (1.02±0.02) (P<0.01). After treatment, the expression of GABAAR protein in the Tia group (1.73±0.36) and JPZDD group (1.52±0.15) was lower than that in the model group (P<0.01). There were no significant differences between the two treatment groups (P>0.05) (). No statistically significant differences were present among the groups for the expression of NMDAR1.
Figure 7 Effect of JPZDD on protein expressions of NMDAR1 and GABAAR in TS rats.
Abbreviations: JPZDD, Jian-Pi-Zhi-Dong Decoction; NMDAR1, N-methyl-D-aspartate receptor 1; GABAAR, GABAA receptor; GABA, gamma-aminobutyric acid; TS, Tourette syndrome; Tia, tiapride.
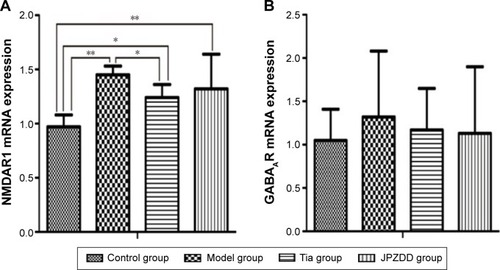
Real-time PCR analysis of NMDAR1 and GABAAR expression
Real-time PCR was used to measure the level of NMDAR1 and GABAAR mRNA in the striatum. A standard curve was drawn for α4-GABAAR, NMDAR1, and GAPDH genes. Melting curve analysis confirmed that there were no primer dimers in the PCR products. The same trend was seen with Western blot (). The mRNA expression of GABAAR in the model group was higher than the control treatment groups, but the differences were not statistically significant. The expression of NMDAR1 in the model group (1.45±0.08) was higher than in the control group (0.97±0.11) (P<0.01). The level of NMDAR1 in both the Tia group (1.24±0.12) and JPZDD group (1.32±0.32) was decreased compared with the model group, but there was a statistically significant difference only between the Tia group and the model group (P<0.01).
Figure 8 Effect of JPZDD on mRNA expressions of NMDAR1 and GABAAR in TS rats. Notes: (A) NMDAR1 mRNA expression. (B) GABAAR mRNA expression. *P<0.05. **P<0.01.
Discussion
The aim of this study was to explore the effect of JPZDD on amino acid neurotransmitters and their receptors in rats with TS induced by IDPN injection. Animal models of TS, as an experimental tool, may assist in the identification of new therapeutic targets, and IDPN is commonly used to develop TS animal model.Citation21 We found that JPZDD can alleviate the symptoms, both stereotypy and autonomic activity, in a rat model of TS. Our findings suggest that treatment with JPZDD compensates for the increased levels of Glu and GABA mainly by downregulating GABAAR expression.
The present study showed that the stereotypy score increased significantly for the model rats compared with the control group at 1 week after treatment. In the 2- to 4-week time points, the stereotypy score of both the JPZDD group and Tia group decreased compared with the model group. The score of the JPZDD group decreased compared with the Tia group at the 4-week time point. The total distance assessed during autonomic activity test increased at all time points for the model group compared with the control group. The distance decreased in both the JPZDD group and the Tia group at the 4-week time point. The same trend was seen for the behavioral test. Thus, we infer that JPZDD could improve tic-like behaviors.
The occurrence of TS is postulated to involve dysfunction of the CSTC circuit, which is supported by metabolic imaging studies.Citation22 CSTC circuit dysfunction is associated with hyperexcitability within cortical motor areas and altered intracortical inhibition.Citation23–Citation25 Most studies in the CSTC circuit have focused on monoamine neurotransmitters and their metabolites. TS has been associated with dysfunctional signaling by the neuromodulator dopamine,Citation26 which is strongly linked to mechanisms of reinforcement learning. Other biochemical pathways, including histaminergic neurotransmission and amino acid neurotransmission, are likely to be involved but have received relatively little attention until recently.Citation27 It was proposed that a disrupted excitatory/inhibitory balance in key circuits might underlie many neurodevelopmental disorders including TS.Citation28–Citation30 Previous study has shown that JPZDD is a specific treatment for TS patients, reducing the frequency of spontaneous hyperkinesias and decreasing the content of dopamine, norepinephrine, and Glu.Citation15 In our previous study, we found that JPZDD was an effective formula for treating TS by upregulating dopamine transporter (DAT) expression in the striatum in a TS mouse model.Citation13 We also showed that JPZDD could decrease excessive GABA production in the striatum by downregulating GABAAR expression in a TS mouse model, suggesting that therapeutic strategies targeting the GABAergic system could be effective in treating TS.Citation14 This study, as well as most previous studies, however, determined the average amino acid content in brain tissue but not dynamics. Brain microdialysis sampling can monitor the dynamics of neurotransmitters in the extracellular fluid in humans and rats.Citation31 Microdialysis is commonly used to study neurophysiology, and the dynamic characterization of amino acids is especially useful for understanding pathogenesis of psychiatric disorders.Citation10 Thus, in the present study, we coupled brain microdialysis with HPLC to analyze extracellular fluid samples. We found elevated Glu levels in the model group, which reached a maximum concentration at 105 minutes and then decreased slowly. The Glu levels of the model group were higher than the control group at 15, 105, and 135 minutes. The Glu levels increased in the model group drastically and then declined. It may reflect a slight overcompensatory negative feedback response to the decreased ability of the striatum to clear released Glu when the corticostriatal pathway is activated.Citation32 Compared with the IDPN model group, the level of Glu decreased in the Tia and JPZDD treatment groups at 15, 105, 135, and 165 minutes. The GABA levels were elevated in the model group and reached a maximum concentration at 135 minutes, and then decreased slowly. The results were not in full accord with our previous study.Citation14 This may be because the HPLC was carried after the mice were euthanized, while the microdialysis was done when the rats were in a waking state. The dynamic microdialysis indicated that the anti-tic effect of JPZDD was more pronounced at 105–165 minutes after giving the drug.
Motor abnormalities can result from manipulation of the Glu system in the striatum. TS is associated with a hyper- or hypo-glutamatergic state. TS could be induced by excessive forebrain Glu output.Citation33 Modulation of the Glu system could provide a valuable new pharmacological approach in the treatment of tics associated with TS. A previous study suggests that GABA is decreased rather than increased in TS.Citation34 Postmortem investigations show that approximately 50% of the GABAergic interneurons number within the striatum decreased in the individuals with TS.Citation35,Citation36 In contrast, our result showed that the concentration of GABA increased. A recent study of the primary and secondary motor areas in adolescent TS patients showed that concentrations of GABA were significantly increased in the supplementary motor area in TS.Citation37 Thus, increased GABA may indicate increased inhibitory tone of the supplementary motor area as a consequence of having tics. GABA concentrations could reflect tonic extrasynaptic inhibition. Increases in GABA contribute to tonic inhibition of motor excitability in TS.Citation38 Our data showed that the relative increase in Glu levels exerted a neurotoxic effect of excitatory state, suggesting that the content of GABA increased along with the increase of Glu to maintain the excitatory/inhibitory balance.
The rapid inhibitory and excitatory responses characteristic of amino acid neurotransmission are mediated by their receptors. NMDAR is the main receptor of Glu. The enhancement of neurotransmission at the NMDAR would be beneficial in TS.Citation8 Extrasynaptic GABA contributes to a local control of tic expression. GABAergic transmission is mediated by the activation of GABAARs.Citation39 Dysfunction involving the GABAAR system may play a major role in the pathophysiology of TS.Citation40 A recent positron emission study on GABAergic neurotransmission in TS indicated a complex pattern of both decreased and increased GABAAR binding.Citation12 In the present study, we found that GABAAR protein in the model group was higher than that in the control group. After treatment, the expressions of GABAAR protein in the Tia and JPZDD groups were lower than that in the model group. The same trend was seen with real-time PCR, but there were no significant differences between these groups. This finding is consistent with our previous study in a TS mouse model.Citation14 The expression of NMDAR1 in the model group was higher than that in the control group. After treatment, the level in the Tia group decreased compared with the model group. Our data suggest that JPZDD could improve tic-like symptoms by downregulating GABAAR expression to alter the content of GABA, and thus maintain the balance of Glu and GABA at a higher new level. NMDAR1 expression decreased, but there was no statistically significant difference, suggesting the NMDAR may not be involved in the mechanism of drug action or does not play a key role. The evidence is less supportive for the use of Glu modulators in TS.Citation41
JPZDD is superior to Tia, with the advantage of multi-targeted regulation, as well as the improvement of negative effects.Citation13 Our results in the present study provide a scientific basis for JPZDD as a clinical treatment for TS. We found that JPZDD has beneficial effects on a TS rat model, but the mechanism remains unclear. We conclude that the effect of JPZDD may be related to maintaining the balance of excitatory/inhibitory neurotransmission by regulating receptor expression.
However, the present study has some limitations as follows. First, there are differences between humans and rats, and thus, further experiments performed on TS children can provide more clinical implication for TS treatment. Second, in addition to glutamatergic and GABAergic neurotransmission, other pathways such as histaminergic pathway may play an important role in tic development. Third, the specific mechanism of each Chinese herb in JPZDD should be validated by pharmacokinetics. Our future studies to better understand the mechanism of JPZDD will focus on other neurotransmitters such as histamine, aspartate, glycine, and taurine, as well as their respective receptors or transporters.
Conclusion
TS is linked to alterations in the balance of excitatory and inhibitory influences within key brain networks, particularly the CSTC brain circuit. JPZDD could alleviate impairments in behavior and dysfunctional signaling by downregulating GABAAR to maintain the balance of Glu and GABA. The effect of JPZDD is stronger than Tia, and the mechanism needs further study.
Acknowledgments
This work was funded by grants from the National Natural Science Foundation of China (no 81273799) and grants from the National Natural Science Foundation for Young Scholars of China (nos 81503615 and 81202725).
Disclosure
The authors report no conflicts of interest in this work.
References
- SwainJEScahillLLombrosoPJKingRALeckmanJFTourette syndrome and tic disorders: a decade of progressJ Am Acad Child Adolesc Psychiatry200746894796817667475
- FellingRJSingerHSNeurobiology of Tourette syndrome: current status and need for further investigationJ Neurosci20113135123871239521880899
- FreemanRDTic disorders and ADHD: answers from a world-wide clinical dataset on Tourette syndromeEur Child Adolesc Psychiatry2007161152317665279
- HallettMTourette syndrome: updateBrain Dev201537765165525604739
- NeunerILudolphATic-Störungen und Tourette-Syndrom in der Lebensspanne [Tics and Tourette’s syndrome throughout the life span]Nervenarzt2009801113771387 German19855949
- SegawaMNeurophysiology of Tourette’s syndrome: pathophysiological considerationsBrain Dev200325Suppl 1S62S6914980375
- NomuraYSegawaMNeurology of Tourette’s syndrome (TS) TS as a developmental dopamine disorder: a hypothesisBrain Dev200325Suppl 1S37S4214980371
- SingerHSMorrisCGradosMGlutamatergic modulatory therapy for Tourette syndromeMed Hypotheses201074586286720022434
- TübingJMünchauACortical GABAergic activity: a mediator of tic control?Mov Disord201530333925758999
- FontehAHarringtonRTsaiALiaoPHarringtonMGFree amino acid and dipeptide changes in the body fluids from Alzheimer’s disease subjectsAmino Acids200732221322417031479
- GallowayMPGhoddoussiFNeedlemanRBBrusilowWSGlutamate as a target in Tourette syndrome and other neuropsychiatric disordersJ Neurol Sci2010293112612720409560
- LernerABagicASimmonsJMWidespread abnormality of the gamma-aminobutyric acid-ergic system in Tourette syndromeBrain Res201213519261936
- WangDHLiWLiuXFZhangJMWangSMChinese medicine formula “Jian-Pi-Zhi-Dong Decoction” attenuates Tourette Syndrome via downregulating the expression of dopamine transporter in miceEvid Based Complement Alternat Med2013201338568523431337
- ZhangWYuWJWangDHWeiLiLeeMKWangSMEffect of “Jian-Pi-Zhi-Dong Decoction” on gamma-aminobutyric acid in a mouse model of Tourette syndromeEvid Based Complement Alternat Med2014201440750924812567
- YuWJBaiXZhangWWeiLiShiXMWangSMEffects of Jianpi Zhi dong Decoction on the neurotransmitters of tourette syndrome childrenChina J Trad Chin Med Pharm201530517571761
- DiamondBIReyeMGBorisonRA new animal model for Tourette syndromeAdv Neurol1982352212256959491
- KhanHAAlhomidaASArifIANeurovestibular toxicities of acrylonitrile and iminodipropionitrile in rats: a comparative evaluation of putative mechanisms and target sitesToxicol Sci2009109112413119244277
- MogwitzSBuseJEhrlichSRoessnerVClinical pharmacology of dopamine-modulating agents in Tourette’s syndromeDavideMAndreaECInternational Review of NeurobiologyNew YorkAcademic Press2013281349
- MitchellHAAhernTHLilesLCJavorsMAWeinshenkerDThe effects of norepinephrine transporter inactivation on locomotor activity in miceBiol Psychiatry200660101046105216893531
- ZhuGeQCThe Rat Brain in Stereotaxic Coordinates3rd edBeijingPeople’s Medical Publishing House2005
- GodarSCMosherLJDi GiovanniGBortolatoMAnimal models of tic disorders: a translational perspectiveJ Neurosci Methods2014238546925244952
- JeffriesKJSchoolerCSchoenbachCHerscovitchPChaseTNBraunARThe functional neuroanatomy of Tourette’s syndrome: an FDG PET study III: functional coupling of regional cerebral metabolic ratesNeuropsychopharmacology20022719210412062910
- OrthMRothwellJCMotor cortex excitability and comorbidity in Gilles de la Tourette syndromeJ Neurol Neurosurg Psychiatry2009801293418931001
- OrthMMünchauARothwellJCCorticospinal system excitability at rest is associated with tic severity in Tourette SyndromeBiol Psychiatry200864324825118243162
- HeiseKFStevenBLiuzziGAltered modulation of intracortical excitability during movement preparation in Gilles de la Tourette syndromeBrain2010133Pt 258059020008030
- BuseJSchoenefeldKMünchauARoessnerVNeuromodulation in Tourette syndrome: dopamine and beyondNeurosci Biobehav Rev20133761069108423085211
- CoxJHSeriSCavannaAEHistaminergic modulation in Tourette syndromeExpert Opin Orphan Drugs201642205213
- RubensteinJLRMerzenichMMModel of autism: increased ratio of excitation/inhibition in key neural systemsGenes Brain Behav20032525526714606691
- RamamoorthiKLinYThe contribution of GABAergic dysfunction to neurodevelopmental disordersTrends Mol Med201117845246221514225
- ClarkeRALeeSEapenVPathogenetic model for Tourette syndrome delineates overlap with related neurodevelopmental disorders including autismTransl Psychiatry20122e15822948383
- Jaquins-GerstlAShuZZhangJLiuYWeberSGMichaelACThe effect of dexamethasone on gliosis, ischemia, and dopamine extraction during microdialysis sampling in brain tissueAnal Chem201183207662766721859125
- NicNiocaillBHaraldssonBHanssonOO’ConnorWTBrundinPAltered striatal amino acid neurotransmitter release monitored using microdialysis in R6/1 Huntington transgenic miceEur J Neurosci200113120621011135020
- McGrathMJCampbellKMParksCRBurtonFHGlutamatergic drugs exacerbate symptomatic behavior in a transgenic model of comorbid Tourette’s syndrome and obsessive–compulsive disorderBrain Res20008771233010980239
- McNaughtKSMinkJWAdvances in understanding and treatment of Tourette syndromeNat Rev Neurol201171266767622064610
- KalanithiPSAZhengWKataokaYAltered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndromeProc Natl Acad Sci U S A200510237133071331216131542
- KataokaYKalanithiPSAGrantzHDecreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette SyndromeJ Comp Neurol2010518327729119941350
- DraperAStephensonMCJacksonGMIncreased GABA contributes to enhanced control over motor excitability in Tourette syndromeCurr Biol201424192343234725264251
- JacksonGMDraperADykeKPépésSEJacksonSRInhibition, disinhibition, and the control of action in Tourette syndromeTrends Cogn Sci2015191165566526440120
- GalvanAKuwajimaMSmithYGlutamate and GABA receptors and transporters in the basal ganglia: what does their subsynaptic localization reveal about their function?Neuroscience2006143235137517059868
- DuJCChiuTFLeeKMTourette syndrome in children: an updated reviewPediatr Neonatol201051525526420951354
- GradosMAAtkinsEBKovacikovaGIMcVicarEA selective review of glutamate pharmacological therapy in obsessive–compulsive and related disordersPsychol Res Behav Manage20158115131