Abstract
Objective
The aim of this study was to investigate the fractional anisotropy (FA) and mean diffusivity (MD) using a diffusion tensor imaging technique and whole-brain voxel-based analysis in patients with comitant strabismus.
Patients and methods
A total of 19 (nine males and ten females) patients with comitant strabismus and 19 age-, sex-, and education-matched healthy controls (HCs) underwent magnetic resonance imaging examination. Imaging data were analyzed using two-sample t-tests to identify group differences in FA and MD values. Patients with comitant strabismus were distinguishable from HCs by receiver operating characteristic curves.
Results
Compared with HCs, patients with comitant strabismus exhibited significantly decreased FA values in the brain regions of the left superior temporal gyrus and increased values in the bilateral medial frontal gyrus, right globus pallidus/brainstem, and bilateral precuneus. Meanwhile, MD value was significantly reduced in the brain regions of the bilateral cerebellum posterior lobe and left middle frontal gyrus but increased in the brain regions of the right middle frontal gyrus and left anterior cingulate.
Conclusion
These results suggest significant brain abnormalities in comitant strabismus, which may underlie the pathologic mechanisms of fusion defects and ocular motility disorders in patients with comitant strabismus.
Introduction
Strabismus is a very common eye disease characterized by abnormal eye movements. According to Fu et al,Citation1 the prevalence of strabismus was 108 out of 2,260 (5.0%) eligible students in the Central China. Strabismus can be roughly divided into comitant strabismus and incomitant strabismus. Strabismus is not only a cosmetic disease but also causes severe damages to visual acuity (VA), binocular vision, and stereopsis.Citation2 Surgery is currently the main treatment for strabismus.Citation3
Strabismus demonstrates abnormal eye movements, which is accompanied by dysfunction of the cerebral cortex. A previous study showed that eye monosynaptic interhemisphere connections existed in strabismus cats.Citation4 Other studies reported the abnormalities of visual cortex structure in infantile esotropic macaque monkeys.Citation5,Citation6 Another study demonstrated visual cortex suppression in patients with strabismus.Citation7
Diffusion tensor imaging (DTI) is a widely used magnetic resonance imaging (MRI) modality that depicts water diffusion directionality as mean diffusivity (MD) and fractional anisotropy (FA).Citation8 The MD value measures the total amount of diffusion within a voxel and provides the overall magnitude of water diffusion, while the FA value is a scalar value between 0 and 1 calculated from the eigenvalues (λ1, λ2, and λ3) of the diffusion tensor. It measures the overall directionality of water diffusion and reflects the complexity of cytoskeleton architecture, which restricts the intra- and extracellular water movement.Citation9 The direction of water diffusion can indicate myelin sheath damage and tissue changes. Consequently, DTI has been applied to study various diseases, such as schizophreniaCitation10 and stroke.Citation11 DTI has also been used to evaluate brain microstructural changes of patients with strabismus amblyopia. Duan et alCitation12 found that MD values were increased not only in optic radiation but also in certain brain regions of strabismus amblyopia patients with the DTI method. However, very few studies used the DTI strategy to investigate the microstructural changes in comitant strabismus. Here, to our knowledge, our study is the first to explore whole-brain microstructural changes in patients with comitant strabismus.
Patients and methods
Patients
A total of 19 patients with comitant strabismus (nine males and ten females; four esotropia; and 15 exotropia) were recruited from the First Affiliated Hospital of University of South China and the Department of Ophthalmology, The First Affiliated Hospital of Nanchang University. The diagnostic criteria for comitant strabismus were as follows: 1) strabismus starting from birth; 2) stereovision defects (no visual fusion); 3) equal binocular corrected VA; and 4) with alternated cover, the experimental and strabismus angle were equal. Patients were excluded if they met any one of the following conditions: 1) acquired strabismus, incomitant strabismus, and concealed oblique; 2) eye diseases (infection, inflammation, and ischemic diseases); 3) history of eye surgeries (extraocular or intraocular surgeries); 4) psychiatric disorders (obsessive–compulsive disorder, anxiety disorder, schizophrenia, depression, etc), diabetes, cardiovascular diseases, and cerebral infarction diseases; and 5) addictions (eg, drugs and alcohol).
Nineteen healthy controls (HCs; nine males and ten females) with similar age, sex, and education status were also recruited from healthy volunteers from citizens of Nanchang, Jiangxi, People’s Republic of China. All HCs met the following requirements: 1) no abnormalities in the brain parenchyma on cranial MRI; 2) no ocular diseases with uncorrected or corrected VA >1.0; 3) no psychiatric diseases (obsessive–compulsive disorder, anxiety disorder, schizophrenia, depression, etc); and 4) able to undergo MRI (eg, no cardiac pacemaker or implanted metal devices). All research methods followed the Declaration of Helsinki and were approved by the First Affiliated Hospital of Nanchang University Ethics Committee. All subjects participated voluntarily and were informed of the purposes, methods, and potential risks before signing an informed consent form.
Data acquisition
MRI scanning was performed using a 3T MR scanner (Trio, Siemens, Munich, Germany). Each subject underwent spin echo single-shot echo planar imaging with the following parameters: repetition time/echo time = 7,200/104 ms, number of excitations = 2, matrix =128×128, field of view =230 ×230 mm, slice number = 49, slice thickness =2.5 mm, axial orientation, 64 nonlinear diffusion-weighting gradient directions with b = 1,000 s/mm2, and additional image without diffusion weighting (b = 0 s/mm2).
Data processing
Diffuse tensor images were analyzed with voxel-based analysis of DTICitation13 and processed with Statistic Parametric Mapping 2 (SPM2; Wellcome Department of Cognitive Neurology, London, UK) and FMRIB Software Library (FSL) (Version 3.3; www.fmrib.ox.ac.uk/fsl) software. The analysis was performed according to a previous study.Citation14
Statistical analysis
Analyses were performed using the SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA) for Windows. Two-sample t-tests were used to compare differences of the FA and MD values between strabismus and HC groups in a voxel-based manner using the analysis of covariance, with age and sex as covariates to control the effect of age and sex. P<0.001 was considered statistically significant.
Clinical data analysis
The cumulative clinical measurements including the duration of the onset of disease and best-corrected VA were recorded.
Results
Clinical data of subjects
There were no obvious differences in weight (P=0.958) and age (P=0.986) between the patients with comitant strabismus and the HCs. The mean duration of strabismus was 27.42±9.04 years. No significant differences were found in the best-corrected VA-right (P=0.161) and the best-corrected VA-left (P=0.750) (clinical data are shown in ).
Table 1 Demographic information and clinical measurements for Stra and HCs
FA differences
Compared with the HC group, the FA values were significantly decreased in the brain regions of the left superior temporal gyrus (P<0.001) but increased in the areas of the bilateral medial frontal gyrus, right globus pallidus/brainstem, and bilateral precuneus in the comitant strabismus group (P<0.001; and ). The mean values of altered FA values between the comitant strabismus and HC group are shown in and .
Figure 1 Significantly altered FA values in patients with Stra compared with HCs.
Abbreviations: FA, fractional anisotropy; HCs, healthy controls; Stra, comitant strabismus.
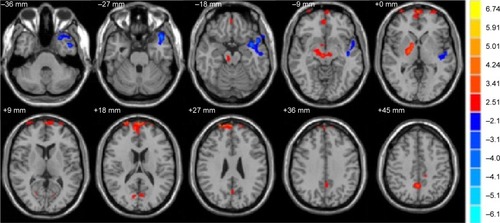
Table 2 Brain regions with significant differences in the FA values between Stra group and HCs
Table 3 Significant differences in the FA values between Stra group and HCs
MD differences
Compared to the HC group, the MD values were significantly decreased in the brain regions of the bilateral cerebellum posterior lobe and left middle frontal gyrus (P<0.01) and increased in the brain regions of the right middle frontal gyrus and left anterior cingulate in the comitant strabismus group (P<0.01; and ).
Figure 2 Significantly altered MD values in patients with Stra compared with HCs.
Abbreviations: HCs, healthy controls; MD, mean diffusivity; Stra, comitant strabismus.
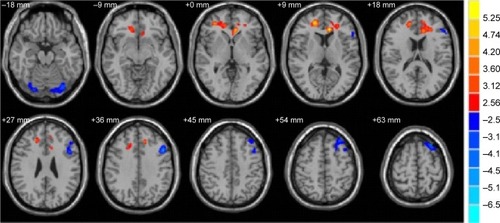
Figure 3 The mean values of altered MD and FA values between the Stra and HCs.
Abbreviations: FA, fractional anisotropy; HCs, healthy controls; MD, mean diffusivity; Stra, comitant strabismus.
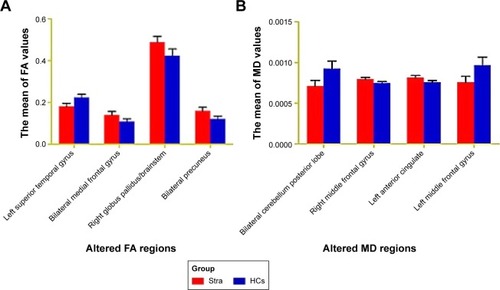
Table 4 Brain regions with significant differences in the MD values between Stra group and HCs
The mean values of altered MD values between the comitant strabismus and HC groups are shown in and .
Table 5 Significant differences in the MD values between Stra group and HCs
Receiver operating characteristic curve
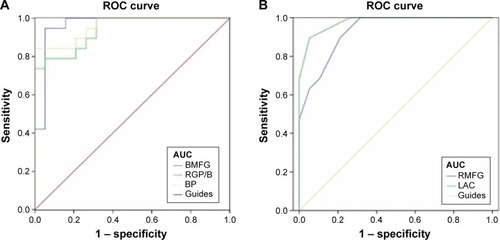
We proposed that the differences of the FA and MD values between the strabismus and HC groups might be useful diagnostic markers. To test this possibility, mean values of the FA and MD of different brain regions were extracted and used to analyze receiver operating characteristic (ROC) curves. The areas under the ROC curve for FA values were as follows: bilateral medial frontal gyrus (0.964), right globus pallidus/brainstem (0.939), and bilateral precuneus (0.958) (). The areas under the ROC curve for MD values were as follows: right middle frontal gyrus (0.931) and left anterior cingulate (0.978) ().
Figure 4 ROC curve analysis of the mean FA and MD values for altered brain regions.
Abbreviations: AUCs, area under the ROC curves; BMFG, bilateral medial frontal gyrus; BP, bilateral precuneus; FA, fractional anisotropy; LAC, left anterior cingulated; RMFG, right middle frontal gyrus; MD, mean diffusivity; ROC, receiver operating characteristic; RGP/B, right globus pallidus/brainstem.
Discussion
Our study is the first to evaluate whole-brain microstructural changes of FA and MD values in patients with comitant strabismus using a DTI approach. The FA is markedly sensitive to microstructural changes of white matter (WM), while the MD may help to better understand how the diffusion tensor is changing. The reduction of the FA values indicates WM neuropathology. Increased tissue water will lead to an increase in the MD values, and the decreased MD value may indicate cell proliferation.Citation15 We found FA values remarkably decreased in the brain regions of the left superior temporal gyrus but increased in the areas of the bilateral medial frontal gyrus, right globus pallidus/brainstem, and bilateral precuneus. Meanwhile, the MD values were significantly decreased in the brain regions of the bilateral cerebellum posterior lobe and left middle frontal gyrus and increased in the brain regions of the right middle frontal gyrus and left anterior cingulate.
The superior temporal gyrus located in the temporal lobe of the human brain is involved in the processing of language.Citation16 A previous study has shown that the superior temporal area controls the representation of three-dimensional structures and shapes.Citation17 It has been well known that patients with strabismus often manifest dysfunction of fusion and stereopsis.Citation18 Yan et alCitation14 found that patients with comitant exotropia had smaller WM volumes in the right inferior temporal gyrus. In agreement with these findings, we found that the FA value was decreased in left superior temporal gyrus in comitant strabismus, which reflected abnormalities of WM fibers in these areas. These results suggest that comitant strabismus may cause dysfunction of the left superior temporal gyrus.
The medial frontal cortex is involved in the control, monitor, and selection of behaviors. The supplementary eye field located in the medial frontal cortex is involved in the execution of ocular movements.Citation19 A previous study demonstrated that the frontal eye field (FEF) played an important role in controlling eye movements of monkeys.Citation20 Another research reported that the FEF was responsible for rapid or saccadic ocular movements.Citation21 Moreover, the FEF was shown to be in charge of sustained attention.Citation22 Yan et alCitation14 found that patients with comitant exotropia had smaller WM volumes in right frontal lobe/sub-gyral. Additionally, Chan et alCitation23 observed increased gray matter volume in the FEF of adults strabismus. Consistent with these findings, we found that the MD values were significantly increased in the brain regions of the right middle frontal gyrus in patients with comitant strabismus, suggesting the dysfunction of WM fibers in these areas. Furthermore, we found that the mean area of the MD values of the right middle frontal gyrus was 0.931 in the ROC curve. Therefore, we speculated that comitant strabismus possibly caused abnormalities of WM fibers in right middle frontal gyrus, which may explain the impairment of regular eye movements in the patients with comitant strabismus.
Interestingly, we found that the FA values were markedly increased in the area of the bilateral medial frontal gyrus, and the MD values were decreased in the left middle frontal gyrus in patients with comitant strabismus. An increase in the FA values in the bilateral medial frontal gyrus may reflect the WM alterations in these areas. The decreased MD values in the left middle frontal gyrus may indicate cell proliferation defects in these areas. Moreover, the mean area of the FA of the bilateral medial frontal gyrus was 0.964. These changes may suggest a functional reorganization in the FEF in patients with comitant strabismus.
The cerebellum is involved in the control of movementsCitation24 and the execution of cognitionCitation25 and language.Citation26 Moreover, a previous study demonstrated that the cerebellum was involved in the execution of eye and hand movements.Citation27 Another study reported that activation of cerebellar vermis was related to visually guided saccades.Citation28 Joshi and DasCitation29 found that the posterior interposed nucleus in the cerebellum participated in conjugating eye movements in strabismic monkeys. In our study, we observed that the MD values were significantly decreased in the brain regions of the bilateral cerebellum posterior lobe in patients with comitant strabismus, which may reflect abnormalities of microstructural changes in these areas. We speculated that comitant strabismus possibly lead to pathological microstructural changes in the bilateral cerebellum posterior lobe, which manifested in the impairment of regular eye movements in the patients with comitant strabismus.
The cingulate cortex, as a part of the limbic cortex, has many functions, such as pain,Citation30 depression,Citation31 and anxiety disorder.Citation32 Yan et alCitation14 found that WM volumes were decreased in the right cingulate gyrus in patients with adult strabismus. In our study, we observed that the MD values were significantly increased in the brain regions of the left anterior cingulate in patients with comitant strabismus, which suggested abnormal microstructural changes in the left anterior cingulate. Furthermore, the mean area of the MD values of the left anterior cingulate was 0.978 in the ROC curve. Therefore, we surmised that the comitant strabismus might lead to the dysfunction of the left anterior cingulate.
Conclusion
Our study showed that patients with comitant strabismus had microstructural changes of WM in many brain regions and provided important information to understand the underlying neural mechanisms of the fusion defects and ocular motility disorders in patients with comitant strabismus. However, there are some limitations to our study. First of all, the sample size is small, and we did not consider different clinical outcomes of the strabismus, such as exotropia and esotropia. Future research should distinguish between different types of strabismus to more accurately assess brain activities and functional changes.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81400372, 81560285, and 81160118). This was not an industry-supported study.
Disclosure
The authors report no conflicts of interest in this work.
References
- FuJLiSMLiuLRPrevalence of amblyopia and strabismus in a population of 7th-grade junior high school students in Central China: the Anyang Childhood Eye Study (ACES)Ophthalmic Epidemiol201421319720324742059
- FengLZhouJChenLHessRFSensory eye balance in surgically corrected intermittent exotropes with normal stereopsisSci Rep201551307526287935
- OgütMSOnalSDemirtasSAdjustable suture surgery for correction of various types of strabismusOphthalmic Surg Lasers Imaging200738319620217552385
- AlekseenkoSVShkorbatovaPYToporovaSNInterhemisphere connections of the visual cortex in cats with bilateral strabismusNeurosci Behav Physiol20063691015101917024341
- TychsenLBurkhalterANeuroanatomic abnormalities of primary visual cortex in macaque monkeys with infantile esotropia: preliminary resultsJ Pediatr Ophthalmol Strabismus19953253233288531039
- WongAMBurkhalterATychsenLSuppression of metabolic activity caused by infantile strabismus and strabismic amblyopia in striate visualcortex of macaque monkeysJ AAPOS200591374715729279
- ChenVJTarczy-HornochKFunctional magnetic resonance imaging of binocular interactions in visual cortex in strabismusJ Pediatr Ophthalmol Strabismus201148636637421117523
- BeaulieuCThe basis of anisotropic water diffusion in the nervous system – a technical reviewNMR Biomed2002157–843545512489094
- PierpaoliCBasserPJToward a quantitative assessment of diffusion anisotropyMagn Reson Med19963668939068946355
- SunYChenYLeeRBezerianosACollinsonSLSimKDisruption of brain anatomical networks in schizophrenia: a longitudinal, diffusion tensor imaging based studySchizophr Res20161711–314915726811255
- JangSHYiJHChoiBYChanges of the corticospinal tract in the unaffected hemisphere in stroke patients: a diffusion tensor imaging studySomatosens Mot Res2016181726891746
- DuanYNorciaAYeatmanJA surveyof the integrity of major white matter tracts in strabismic amblyopiaJ Vis20151512650
- ShuNLiJLiKYuCJiangTAbnormal diffusion of cerebral white matter in early blindnessHum Brain Mapp200930122022718072278
- YanXLinXWangQDorsal visual pathway changes in patients with comitant extropiaPLoS One201056e1093120532166
- AlexanderALLeeJELazarMDiffusion tensor imaging of the brainNeurotherapeutics20074331632917599699
- KovelmanIWagleyNHayJSMultimodal imaging of temporal processing in typical and atypical language developmentAnn N Y Acad Sci2015133771525773611
- MysoreSGVogelsRRaiguelSEToddJTOrbanGAThe selectivity of neurons in the macaque fundus of the superior temporal area for three-dimensional structure from motionJ Neurosci20103046154911550821084605
- FengXZhangXJiaYImprovement in fusion and stereopsis following surgery for intermittent exotropiaJ Pediatr Ophthalmol Strabismus2015521525725643371
- StuphornVThe role of supplementary eye field in goal-directed behaviorJ Physiol Paris20151091–311812825720602
- ThompsonKGHanesDPBichotNPSchallJDPerceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual searchJ Neurophysiol1996766404040558985899
- HanesDPWurtzRHInteraction of the frontal eye field and superior colliculus for saccade generationJ Neurophysiol200185280481511160514
- EstermanMLiuGOkabeHReaganAThaiMDeGutisJFrontal eye field involvement in sustaining visual attention: evidence from transcranial magnetic stimulationNeuroimage201511154254825655445
- ChanSTTangKWLamKCChanLKMendolaJDKwongKKNeuroanatomy of adult strabismus: a voxel-based morphometric analysis of magnetic resonance structural scansNeuroimage200422298699415193630
- PaulinMGThe role of the cerebellum in motor control and perceptionBrain Behav Evol199341139508431754
- StoodleyCJThe cerebellum and cognition: evidence from functional imaging studiesCerebellum201211235236521373864
- MurdochBEThe cerebellum and language: historical perspective and reviewCortex201046785886819828143
- NitschkeMFArpTStavrouGErdmannCHeideWThe cerebellum in the cerebro-cerebellar network for the control of eye and hand movements – an fMRI studyProg Brain Res200514815116415661188
- HayakawaYNakajimaTTakagiMFukuharaNAbeHHuman cerebellar activation in relation to saccadic eye movements: a functional magnetic resonance imaging studyOphthalmologica2002216639940512566881
- JoshiACDasVEMuscimol inactivation of caudal fastigial nucleus and posterior interposed nucleus in monkeys with strabismusJ Neurophysiol201311081882189123883862
- BoccardSGPereiraEAMoirLDeep brain stimulation of the anterior cingulate cortex: targeting the affective component of chronicpainNeuroreport2014252838824100411
- OnodaKYamaguchiSDissociative contributions of the anterior cingulate cortex to apathy and depression: topological evidence from resting-state functional MRINeuropsychologia201577101826235668
- ShinouraNYamadaRTabeiYThe right dorsal anterior cingulate cortex may play a role in anxiety disorder and visual functionNeurol Res2013351657023317801
