Abstract
The aim of this study was to describe the effects of varenicline, a smoking cessation aid that acts as a nicotinic agonist, on cognitive function in patients with early clinical Huntington’s disease (HD) who were current smokers. Three gene-positive patients transitioning to symptomatic HD were evaluated using the Unified Huntington’s Disease Rating Scale part I and III (motor and behavioral subscales) at baseline and after 4 weeks of treatment. Cognitive function was assessed using a touch screen computer-based neurocognitive test battery (IntegNeuro®). Varenicline (1 mg twice daily) significantly improved performance in executive function and emotional recognition tasks. Our case reports describe no clinically significant adverse effects and suggest that varenicline improves aspects of cognitive function in patients with early HD. A randomized controlled study is now underway.
Introduction
Huntington’s disease (HD) is a fatal, inherited neurodegenerative disorder characterized by progressive movement characterized by progressive motor, cognitive, and psychiatric symptoms. While clinical diagnosis typically depends on motor abnormalities, predictive genetic testing of patients with HD has shown that very early changes in cognitive function occur in gene carriers before any other clinical symptoms are apparent.Citation1,Citation2 Cognitive dysfunction includes impairments in executive function, perception, visuospatial and psychomotor skills, memory loss, and problems learning new skills. These cognitive deficits ultimately lead to frontal and subcortical dementia.Citation3,Citation4
Patients with HD also have a significant lifetime prevalence of psychiatric disorders.Citation5 Frequently reported psychiatric symptoms include depression, anxiety, irritability, apathy, obsessions and compulsions, and psychoses.Citation6 For many patients and their families, neuropsychiatric symptoms constitute the most distressing aspect of HD. Combined with the progressive decline in cognitive function, these behavioral symptoms have a significant impact on disease coping, interpersonal relationships, judgment, and the ability to live independently.Citation7
The cognitive deficits associated with normal aging and age-related neurodegenerative diseases are proposed to be caused by impairments in cholinergic neurotransmission, in part due to a reduction in the number of nicotinic acetylcholine receptors (nAChRs).Citation8,Citation9 However, postmortem analysis of the HD brain shows no change in the number of nAChRs, rather a significant loss of the neurotransmitter acetylcholine, its key synthetic enzyme (choline acetyl transferase), and transporter (vesicular acetylcholine transporter).Citation10–Citation13 These neurochemical changes within the brain correlate with cognitive defectsCitation3 and support the hypothesis that dysfunctional cholinergic transmission, through a loss of agonist, rather than receptors, may be a significant contributing factor in the cognitive decline observed in HD.
This presents an opportunity to investigate whether pharmacological replacement with an exogenous agonist can improve cognitive function in HD. Indeed, small-scale clinical studies investigating the procholinergic effects of rivastigmine and donepezil in patients with HD indicated a trend for improved cognitive performance.Citation14–Citation16 Further, the nicotine analog varenicline (Champix®) used for smoking cessationCitation17,Citation18 has been shown to improve symptoms in rodent models and in patients with ataxic neurodegenerative disordersCitation19–Citation21 and to display antidepressant activity.Citation22
While recent evidence suggests no increased risk of neuropsychiatric events,Citation23 we performed an open-label study to determine the tolerability of varenicline, a high-affinity agonist at α7 and partial agonist at α4β2 nAChR subtypes,Citation24,Citation25 and its effects on cognitive function in three patients with early HD who smoked.
Case reports
Clinical assessment
Varenicline (Champix®) was administered according to the standard regimen for smoking cessation (initiated at 0.5 mg/d and escalated over a 2-week period to 2 mg/d). The frequency, intensity, and burden of side effects were recorded weekly, in addition to self-reported cigarette use. Disease symptom severity was assessed at baseline and after 4-week treatment with varenicline using parts I (motor) and III (behavior) of the Unified Huntington’s Disease Rating Scale (UHDRS). Categories within part I were analyzed separately:Citation26 oculomotor function (ocular pursuit, saccade initiation, saccade velocity), hyperkinesia (chorea, dystonia, tongue protrusion, dysarthria), fine motor tasks (finger taps, hand pronation/supination, Luria), Parkinsonism (rigidity, bradykinesia), and gait (gait, tandem walking, retropulsion). Categories in part III were analyzed in component parts (depressed mood, apathy, low self-esteem/guilt, suicidal thoughts, anxiety, irritable behavior, aggressive behavior, obsessional thinking, compulsive behavior, delusion, and hallucinations). Higher scores for both UHDRS subscales reflect greater impairment.
Cognitive function was assessed using the fully validated IntegNeuro™ computerized neurocognitive test battery (Brain Resource Company Ltd). The computerized tests show a significant degree of overlap with traditional paper and pencil tests examining the same cognitive construct.Citation27 Domains assessed were manual dexterity (motor tapping), impulsivity and attention (Go–No–Go), switching attention, verbal interference, executive maze, and basic emotion recognition, in addition to a measure of premorbid intelligence (spot the real word). IntegNeuro uses different task versions for repeat testing sessions to minimize the effect of familiarization and practice effects, and test–retest reliability measures have shown a high level of consistency (on average 0.70).Citation28 Raw data from the neuropsychological tests were converted to z-scores normalized for age, sex, and years of education based on a database of >5,000 control subjects provided by the Brain Resource Company.Citation29 All z-scores were computed so that positive scores indicated better performance. Subjects were considered to show a significant deficit in a test if the z-score for any performance measure of that test was beyond two standard deviations below the mean of the age, sex, and education-matched controls in the Brain Resource Company database. Performance measure scores within each test were averaged to produce a domain-specific z-score for each participant.Citation30 Formal statistical analysis to determine group differences in domain-specific z-scores pre and post treatment was not performed due to the small number of cases.
Baseline assessment and follow-up (after 4 weeks of treatment) were carried out by the same clinicians (GF and JD) and are reported in and . The study design was reviewed and approved by the Southern X Regional Ethics Committee (Health and Disability Ethics Committee, New Zealand). Written informed consent was obtained from all participants after the study had been fully explained. All patients were deemed by their treating physician as having capacity to give informed consent.
Table 1 UHDRS part I (motor) scores at baseline and 4 weeks after varenicline treatment
Table 2 UHDRS part III (behavior) scores at baseline and 4 weeks after varenicline treatment
Case 1
A 45-year-old female patient (smoked 15 cigarettes/d) was diagnosed with HD in 2013. Neurological examination showed subtle chorea and dystonic posturing of the arms but no other motor features. Baseline cognitive testing demonstrated significant deficits in motor tapping (number of taps, nondominant), maze navigation (number of trials completed, overrun errors), and emotional identification (accuracy of fear and disgust recognition) compared to age, sex, and years of education-matched controls. No deficits were observed in any of the other cognitive domains tested.
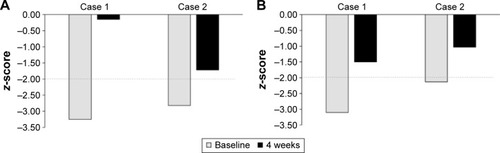
At the time of the study, she was on no medication but had taken venlafaxine to manage depression in the past. She indicated a desire to stop smoking and had previous failed attempts. The patient successfully stopped smoking during week 2. The patient reported a single aggressive outburst that was out of character during week 2 but experienced no other side effects. After 4 weeks of varenicline treatment, UHDRS total motor score decreased from 16 to 9 with notable improvement in oculomotor function and hyperkinesia scores (). Her UHDRS behavioral subscale score was increased from 2 to 8 and could be attributed to the episode of increased irritability/aggression (). The patient self-reported an improvement in her thinking, being able to think more clearly and having a better memory. Cognitive testing showed significant improvements in maze navigation (domain-specific z-score: −3.25 vs −0.15) and facial emotion recognition (domain-specific z-score: −3.10 vs −1.50) following varenicline treatment (). Motor-tapping deficits were not affected by varenicline treatment. She continues to be a nonsmoker and has had no further irritable outbursts.
Case 2
A 44-year-old male patient, diagnosed with HD in 2014, showed slight slowing of rapid alternating movements in the upper limbs and mildly slowed finger tapping on the left side on neurological examination. Chorea and behavioral symptoms were treated with haloperidol (0.5 mg/d), quetiapine (200 mg/d), and citalopram (30 mg/d) at the time of the study. The patient displayed significant deficits in switching of attention (completion time), verbal interference (congruent accuracy, congruent and incongruent reaction time), maze navigation (trials completed, overrun errors, total errors), and emotional identification (accuracy of fear, disgust, and happiness identification) tasks at the baseline clinic visit. He had no desire to stop smoking and continued to smoke ~23 cigarettes/d throughout the study. He self-reported that there had been an incident where he became extremely agitated and kicked the door in week 2; however, this passed quickly and he was unable to recall the cause. No other side effects were reported. Following 4 weeks of varenicline treatment, UHDRS total motor score decreased from 11 to 8. While fine motor task and gait scores showed modest improvement, hyperkinesia and Parkinsonism scores were moderately elevated (). His UHDRS behavioral subscale increased from 4 to 8, due to the aggressive outburst (). Cognitive testing at 4 weeks showed significantly improved performance in the switching attention (domain z-score: −4.00 vs −1.7) and verbal interference (domain z-score: −2.95 vs −1.90) tasks. Performance in tests of executive function (domain z-score: −2.82 vs −1.72) and emotional identification (domain z-score: −2.13 vs −1.03) was also significantly improved (). He continues to smoke and has reported no further episodes of aggression or irritability.
Case 3
The patient was a 40-year-old gene-positive male who had required support to manage behavioral symptoms since 2011. Baseline neurological examination showed no motor symptoms (UHDRS total motor score 0) and a behavioral subscale score of 14. He enjoyed smoking (~20 cigarettes/d) and had no plan to give up. At the time of the study, he was on no medication and reported no effects or side effects of the medication at lower doses (0.5 mg/d and 1.0 mg/d). When the daily dosage of varenicline was increased (2 mg/d), he reported significant alterations in his sense of taste and was unable to drink coffee or take any dairy products. As a result, he discontinued the medication and did not attend follow-up appointments. He was reviewed in the community several months later, and both he and his mother reported that no other side effects were noted other than altered taste.
Discussion
To our knowledge, this is the first report describing beneficial effects of the nicotinic agonist varenicline on motor and cognitive symptoms in patients with early HD. Our preliminary investigation indicates that varenicline was generally well tolerated in these patents, and no clinically significant adverse effects were reported during the 4-week treatment period. Two patients (cases 1 and 2) reported single, brief episodes of increased irritability and aggression in the second week of varenicline treatment, which may be related to dose escalation from 1 mg/d to 2 mg/d. The third patient (case 3) did not continue treatment beyond week 2, due to changes in taste.
No studies have directly investigated smoking in HD; however, previous literature suggests that a significant proportion of patients with HD (40%) are moderate or heavy smokers.Citation31 In the first patient we treated, varenicline was an effective smoking cessation aid. The contribution of nicotine withdrawal to the observed increase in irritability in this patient remains unclear.
Approximately 40% of smokers achieve abstinence with varenicline,Citation32,Citation33 and end-of-treatment abstinence rates increase with increasing varenicline exposure.Citation34 Our result of abstinence in one of three patients after a 4-week treatment is in keeping with these observations. Both patients who completed the study were fully adherent; therefore, smoking cessation efficacy may be related to intention to quit or lower baseline smoking status.Citation34
The patients who completed the course of treatment showed modest motor symptoms assessed by UHDRS but no clinically significant anxiety or depression (assessed by the hospital anxiety and depression rating scale)Citation35 at the time of the study. While both patients displayed different profiles with regard to which specific aspects of attention and verbal tasks were impaired at baseline, both showed impairments of comparable magnitude in maze navigation and emotional identification tasks. This is in agreement with previous literature describing early impairments in executive function, perception, and visuospatial and psychomotor skills.Citation3,Citation4
Following 4 weeks of treatment with varenicline, both patients showed significantly improved performance in maze navigation and improved accuracy of fear and disgust recognition. Deficits in emotion recognition, in particular negative emotions, are a key predictor of clinical diagnosis and progression of HD.Citation36–Citation38 Reversal of this disease biomarker after a relatively brief period of administration suggests that varenicline may have effects that justify further investigation. The cognitive assessment battery used in this study displays equivalent levels of test–retest reliability over a 4-week period to the MATRICS cognitive consensus battery, and these improvements are unlikely to be due to practice effects.Citation39
Cholinergic neurotransmission is an important modulator of cognition within the central nervous system. Indeed, disrupted cholinergic neurotransmission is associated with deficits in emotional processingCitation40 and executive function.Citation41,Citation42 We hypothesized that impaired cholinergic neurotransmission through a loss of agonist, rather than nAChRs, may contribute to the cognitive decline in patients with HD. Stimulation of nAChRs with nicotine has been shown to improve working memory, visual memory, information-processing speed, and emotion recognition in subjects with low baseline cognitive function.Citation43 Therefore, it is likely that the cognitive enhancing effects of varenicline are mediated through its effects as a full agonist at α7 and partial agonist at α4β2 nAChRs, the subtypes thought to be most important in cognitive function.Citation44,Citation45
Varenicline has been previously reported to improve balance and coordination and attenuate ataxias induced by L-Dopa via α4β2 nAChRs.Citation46 Partial agonist effects at this subtype may underlie the improvements in motor function observed in this study. It remains unclear whether a larger effect size would have been observed following a longer treatment period with varenicline or whether varenicline has the potential to improve motor deficits in late-stage patients.
Conclusion
Our case reports suggest that varenicline may provide multiple benefits by reducing the negative health impact of tobacco smoking, in addition to providing beneficial effects on motor and cognitive symptoms. A randomized control trial is now underway to further investigate the efficacy of varenicline in the symptomatic treatment of patients with HD.
Acknowledgments
This work was supported by the Health Research Council New Zealand (grant number 3620187) and the New Zealand Pharmacy Education Research Trust (grant number 264).
Disclosure
The authors have no commercial relationships and declare no conflicts of interest in this work.
References
- PhillipsWShannonKMBarkerRAThe current clinical management of Huntington’s diseaseMov Disord200823111491150418581443
- YouSCGeschwindMDShaSJExecutive functions in premanifest Huntington’s diseaseMov Disord201429340540924375511
- MontoyaAPriceBHMenearMLepageMBrain imaging and cognitive dysfunctions in Huntington’s diseaseJ Psychiatry Neurosci2006311212916496032
- NovakMJTabriziSJHuntington’s disease: clinical presentation and treatmentInt Rev Neurobiol20119829732321907093
- van DuijnEKingmaEMTimmanRCross-sectional study on prevalences of psychiatric disorders in mutation carriers of Huntington’s disease compared with mutation-negative first-degree relativesJ Clin Psychiatry200869111804181019026253
- van DuijnEKingmaEMvan der MastRCPsychopathology in verified Huntington’s disease gene carriersJ Neuropsychiatry Clin Neurosci200719444144818070848
- Van LiewCGluhmSGoldsteinJCronanTACorey-BloomJThe functional implications of motor, cognitive, psychiatric, and social problem-solving states in Huntington’s diseasePsychiatry201376432333524299091
- ZanardiALeoGBiaginiGZoliMNicotine and neurodegeneration in ageingToxicol Lett20021271–320721512052660
- PerryEKPerryRHSmithCJNicotinic receptor abnormalities in Alzheimer’s and Parkinson’s diseasesJ Neurol Neurosurg Psychiatry19875068068092956364
- BatesGTabriziSJonesLHuntington’s Disease4th edOxford University PressOxford, UK2014
- WaldvogelHJKimEHTippettLJVonsattelJPFaullRLThe neuropathology of Huntington’s diseaseCurr Top Behav Neurosci201522338025300927
- SpokesEGNeurochemical alterations in Huntington’s chorea: a study of post-mortem brain tissueBrain198010311792106102490
- LangeKWJavoy-AgidFAgidYJennerPMarsdenCDBrain muscarinic cholinergic receptors in Huntington’s diseaseJ Neurol199223921031041532417
- FernandezHHFriedmanJHGraceJBeason-HazenSDonepezil for Huntington’s diseaseMov Disord200015117317610634264
- RotUKobalJSeverAPirtosekZMesecARivastigmine in the treatment of Huntington’s diseaseEur J Neurol20029668969012453090
- de TommasoMSpecchioNSciruicchioVDifruscoloOSpecchioLMEffects of rivastigmine on motor and cognitive impairment in Huntington’s diseaseMov Disord200419121516151815390067
- CoeJWBrooksPRVetelinoMGVarenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessationJ Med Chem200548103474347715887955
- TonstadSRollemaHVarenicline in smoking cessationExpert Rev Respir Med20104329129920524911
- ZesiewiczTASullivanKLTreatment of ataxia and imbalance with varenicline (chantix): report of 2 patients with spinocerebellar ataxia (types 3 and 14)Clin Neuropharmacol200831636336519050414
- ZesiewiczTASullivanKLGoochCLLynchDRSubjective improvement in proprioception in 2 patients with atypical Friedreich ataxia treated with varenicline (Chantix)J Clin Neuromuscul Dis200910419119319494730
- ZesiewiczTAGreensteinPESullivanKLA randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3Neurology201278854555022323747
- RollemaHGuanowskyVMineurYSVarenicline has antidepressant-like activity in the forced swim test and augments sertraline’s effectEur J Pharmacol20096051–311411619168054
- ThomasKHMartinRMKnipeDWHigginsJPGunnellDRisk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysisBMJ2015350h110925767129
- ObachRSReed-HagenAEKruegerSSMetabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitroDrug Metab Dispos200634112113016221753
- MihalakKBCarrollFILuetjeCWVarenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptorsMol Pharmacol200670380180516766716
- CiammolaASassoneJColciagoCAripiprazole in the treatment of Huntington’s disease: a case seriesNeuropsychiatr Dis Treat200951419557093
- PaulRHLawrenceJWilliamsLMRichardCCCooperNGordonEPreliminary validity of “integneuro”: a new computerized battery of neurocognitive testsInt J Neurosci2005115111549156716223701
- WilliamsLMSimmsEClarkCRPaulRHRoweDGordonEThe test-retest reliability of a standardized neurocognitive and neurophysiological test battery: “neuromarker”Int J Neurosci2005115121605163016287629
- ClarkCRPaulRHWilliamsLMStandardized assessment of cognitive functioning during development and aging using an automated touchscreen batteryArch Clin Neuropsychol200621544946716904862
- AndersonDJMalyszJGronlienJHStimulation of dopamine release by nicotinic acetylcholine receptor ligands in rat brain slices correlates with the profile of high, but not low, sensitivity alpha4beta2 subunit combinationBiochem Pharmacol200978784485119555668
- EhretJCDayPSWiegandRWojcieszekJChambersRAHuntington disease as a dual diagnosis disorder: data from the national research roster for Huntington disease patients and familiesDrug Alcohol Depend2007862–328328616930866
- BroseLSWestRStapletonJAComparison of the effectiveness of varenicline and combination nicotine replacement therapy for smoking cessation in clinical practiceMayo Clin Proc201388322623323489449
- EbbertJOWyattKDHaysJTKleeEWHurtRDVarenicline for smoking cessation: efficacy, safety, and treatment recommendationsPatient Prefer Adherence2010435536221049087
- RavvaPGastonguayMRFaesselHMLeeTCNiauraRPharmacokinetic-pharmacodynamic modeling of the effect of varenicline on nicotine craving in adult smokersNicotine Tob Res201517110611325145377
- ZigmondASSnaithRPThe hospital anxiety and depression scaleActa Psychiatr Scand19836763613706880820
- BoraEVelakoulisDWalterfangMSocial cognition in Huntington’s disease: a meta-analysisBehav Brain Res201629713114026455876
- LofflerLARadkeSMorawetzCDerntlBEmotional dysfunctions in neurodegenerative diseasesJ Comp Neurol201652481727174326011035
- BaezSHerreraEGershanikOImpairments in negative emotion recognition and empathy for pain in Huntington’s disease familiesNeuropsychologia20156815816725582408
- SilversteinSMJaegerJDonovan-LeporeAMA comparative study of the MATRICS and IntegNeuro cognitive assessment batteriesJ Clin Exp Neuropsychol201032993795220455131
- MasonSLZhangJBegetiFThe role of the amygdala during emotional processing in Huntington’s disease: from pre-manifest to late stage diseaseNeuropsychologia201570808925700742
- WallaceTLBertrandDImportance of the nicotinic acetylcholine receptor system in the prefrontal cortexBiochem Pharmacol201385121713172023628449
- LogueSFGouldTJThe neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibitionPharmacol Biochem Behav2014123455423978501
- NiemegeersPDumontGJQuisenaertsCThe effects of nicotine on cognition are dependent on baseline performanceEur Neuropsychopharmacol20142471015102324766971
- LevinEDBradleyAAddyNSiguraniNHippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memoryNeuroscience2002109475776511927157
- LevinEDalpha7-Nicotinic receptors and cognitionCurr Drug Targets201213560260622300026
- QuikMZhangDPerezXABordiaTRole for the nicotinic cholinergic system in movement disorders; therapeutic implicationsPharmacol Ther20141441505924836728