Abstract
Background
Multiple sclerosis (MS) is the most common chronic autoimmune demyelinating and inflammatory disease of the central nervous system, afflicting both the body and mind. The risk of suffering from MS is 2.5–3.5 times greater in females than in males. While there is extant research on fatigue, depression, and cognitive impairment in patients with MS during its clinical course, there is a lack of research focusing on sleep, psychological functioning, and physical activity (PA) at the point of disease onset. The aims of the present study were therefore, to assess the markers of mental toughness (MT) as a dimension of psychological functioning, sleep disturbances (SD), and PA among patients at the moment of disease onset and to compare these with the corresponding values for healthy adolescents and young adults.
Methods
A total of 23 patients with MS at disease onset (mean age =32.31 years; 91% females), 23 healthy adolescents (mean age =17.43 years; 82% females), and 25 healthy young adults (mean age =20.72 years; 80% females) took part in the study. They completed questionnaires covering sociodemographic data, MT, SD, and PA.
Results
Patients with MS had similar scores for MT traits as those in healthy adolescents and healthy young adults, and equivalent levels of moderate-intensity PA and SD as young adults. MS patients reported lower levels of vigorous PA compared to both healthy adolescents and young adults.
Conclusion
The pattern of the results of the present study suggests that the onset of MS is not associated with poor MT, poor sleep, or reduced moderate-intensity PA. Lower levels of vigorous PA were observed in MS patients. Low levels of vigorous PA may lead to decreased cardiorespiratory fitness in patients with MS and, in the long run, to reduced cardiovascular health and degraded psychological functioning.
Introduction
Neurodegenerative conditions such as dementia, Parkinson’s disease, Huntington’s disease (HD), and multiple sclerosis (MS) are often characterized by significant psychological and physical deficits such as sleep disturbance (SD) and low physical activities that interfere significantly with patients’ abilities and their overall quality of life.Citation1–Citation7 In this context, MS is the most common chronic autoimmune demyelinating and inflammatory disease of the central nervous system. Demyelination in association with axonal damage results in slowing of nerve signals, leading to typical MS symptoms such as feeling tired (fatigue), pain, visual problems, paresthesia, and problems of movement and balance.Citation8 MS is associated with several comorbidities including an increase in depressive symptoms, social withdrawal, reduced sexual drive, increased physical inactivity, and SD.Citation9,Citation10 Further, excessive fatigue severely reducing physical activity (PA) and exercising is experienced by at least two-thirds of MS patients.Citation11
A broad range of medications is available to treat MS during the course of the disease.Citation12–Citation16 More specifically, the past two decades have witnessed remarkable advances in the treatment options for MS. New drugs have been developed on the basis of the knowledge of the pathobiology of MS,Citation17 and as for now, medical treatment is the first-line treatment for MS.Citation12,Citation14–Citation16 Accordingly, several disease-modifying drugs including interferon beta 1a and 1b, glatiramer acetate, natalizumab, mitoxantrone, and fingolimod are licensed worldwide to reduce the frequency of clinical attacks with the hope of slowing disability progression.Citation17–Citation19 Fingolimod is a once-daily oral medication approved for relapsing MS.Citation17 Because interferon beta and glatiramer acetate have favorable long-term safety profiles and minimal monitoring requirements, they remain the common first-line choices despite competition from new oral therapies.Citation17 Medication, though, was not the focus of concern in the present paper; instead, we examined psychological and sleep- and PA-related characteristics at illness onset.
Specifically, although research on the quality of life in MS patients has gained increasing interest, we decided to introduce a further psychological dimension, that is, we asked about patients’ mental toughness (MT). MT refers to an individual’s capacity to be consistently successful in coping with difficult life circumstances.Citation20 Most studies in this field have focused on MT in elite athletes; these studies have shown that mentally tough athletes are able to cope with stress during a competition and to remain more focused and confident in the course of competition.Citation20–Citation23
Other studies have considered the association between MT and stress. For example, Gerber et alCitation24,Citation25 showed that adolescents with higher scores for MT were more resilient against stress. Accordingly, adolescents who were mentally tougher in a stressful situation were at a low risk of burnout compared to peers with lower scores for MT.Citation26,Citation27 The bot tom line appears to be that people with lower levels of MT experience greater stress when dealing with difficult and frustrating situations.
The concept of MT has been recognized recently for its psychological importance not just in coping with stressCitation24 but also for its association with higher PA,Citation28 and for its impact on both,Citation29,Citation30 and objective sleep quality.Citation31 Consequently, there has been growing recognition of the applicability of MT to groups other than non-elite athletes, for example, healthy adolescents;Citation29–Citation31 healthy young adults;Citation24 lower, middle, and senior managers; and clerical/administrative workers in early, middle, and late adulthood.Citation32 However, to the best of our knowledge, MT traits have not so far been assessed in patients with MS, either at illness onset or during the course of the illness. Further aims of the present study were therefore to assess MT traits among patients with MS at disease onset and to compare the results from this group with those from healthy adolescents and young adults.
Overall, the concept of MT has been proven to be associated with a broad variety of cognitive, emotional, and behavioral dimensions. We chose to examine MT in patients with MS for the following reasons: 1) MT consists of four key factors: control (of own life and emotions), commitment, challenge, and confidence (in own abilities and in other people; ), thus covering a range of cognitive–emotional processes closely involved in coping with stress, emotions, unexpected events, and social settings (confidence in other people); 2) MT is related to subjective and objective sleep; 3) MT is related to PA; 4) MT has not previously been assessed in patients with MS; 5) MT offers an excellent basis for both cross-sectional and longitudinal studies; 6) MT reflects a trait rather than a state marker; and 7) given that the Mental Toughness Questionnaire 48 (MTQ48)Citation20 has been validated in different age and professional groups, data from previous studies (samples) are comparable with new samples.
Table 1 Descriptive and inferential statistical overview of mental toughness traits, sleep disturbances, and physical activity of patients with diagnosed MS, healthy adolescents, and healthy young adults
We took all these considerations into account and asked about the MT profile of patients with MS at disease onset. Given that MT is considered a trait marker, we anticipated that scores would not differ from those of healthy controls immediately after a dramatic event such as the diagnosis of MS. Further, we applied MTQ48,Citation20 thus allowing comparison with previous research.
A further core symptom of MS is fatigue, which means increased physical and psychological tiredness. Plausibly, fatigue might be associated with poor sleep though, surprisingly, the association between poor sleep and fatigue has been little studied so far. Only recently, StroberCitation33 reviewed the state-of-the-art and concluded that the association between sleep and fatigue has been understudied and unrecognized, and that poor sleep is a significant contributor to fatigue. In the light of this observation, the first aim of the present study was to assess whether and to what extent SD might be precursors of MS at illness onset and to what extent any SD are comparable to those of healthy adolescents and young adults.
With regard to PA, there are three principal reasons why patients with MS may be less active than healthy people:Citation34,Citation35 fatigue, impairment, and lack of time. Whereas lack of time seems to be a common reason for people not to exercise, impairment and fatigue are potentially MS specific.Citation34–Citation36 With regard to impairment, there is some evidence of a negative feedback loop in that acute PA may lead to immediate impairment together with physical and mental discomfort. However, there is evidence that impairments and discomfort are due to an acute increase in body temperature and not to PA as such (Uhthoff’s phenomenonCitation37). Further, the course of Huntington’s Disease, a neurodegenerative disorder, indicates that a less physically active lifestyle is a preclinical predictor of earlier disease onset.Citation38 Thus, although it is well established that patients with MS report reduced PA during the course of the disease, and although in the case of another neurodegenerative disorder we have learnt that a less active lifestyle is associated with earlier illness onset, to the best of our knowledge, the level of PA at illness onset among patients with MS has yet to be studied. Accordingly, the second aim of the present study was to assess levels of moderate and vigorous PA of patients with MS at disease onset and to compare these levels with those of healthy adolescents and young adults.
Additionally, previous studies have shown that patients with MS reported increased symptoms of depression and anxiety during the course of the disease,Citation5,Citation39 while, more recently, the psychological dimension of quality of life has gained greater attention, thus shifting from a strictly psychiatric perspective to a more comprehensive psychological appraisal.Citation40,Citation41
To summarize, the aims of the present study were to explore to what extent psychological dimensions (here MT), sleep, and PA might be already affected at the onset of MS, thus providing indications of disease onset at psychological, sleep-related, and activity-related levels. To these ends, we assessed patients with MS at illness onset as well as healthy adolescents and healthy young adults. We believe that the pattern of results revealed in this study might be of practical importance, because until now, the only reliable state markers of the onset of MS were neurocognitive assessments to detect neurocognitive impairmentsCitation42–Citation45 and imaging techniques to assess cortical atrophy.Citation46–Citation50 These assessments, however, are time-consuming, expensive, require specialist expertise, and are not a part of annual routine medical checkups. By contrast, changes in sleep quality, PA, and cognitive, emotional, and behavioral frameworks are more easily detected both by individuals affected by MS and by their close family members and friends.
In the absence of existing evidence on patients with MS at illness onset, the following research questions were formulated in lieu of hypotheses: first, do the MT traits of patients with MS at illness onset differ significantly from those of healthy adolescents or healthy young adults? Second, are patients with MS at illness onset more likely to report SD than healthy adolescents and healthy young adults? Third, do patients with MS at illness onset report less moderate or less vigorous PA than healthy adolescents and healthy young adults?
Methods
Procedure
Three different populations were assessed and compared in the present study: patients with MS at disease onset, healthy adolescents, and healthy young adults. All the participants were informed about the voluntary character of participation and assured that all the data were gathered anonymously. Written informed consent was obtained from the participants or from their legal representatives in case they were minors (<18 years). All the participants completed a series of questionnaires covering sociodemographic data, MT, SD, and PA (see “Materials” section). Completion of the questionnaire took 30–40 minutes. The local ethics committee of the University of Basel (Basel, Switzerland) approved the study, which was performed in accordance with the principles laid down in the Declaration of Helsinki.
Samples
Patients with diagnosed MS
A total of 23 patients with diagnosed MS (mean age =32.31 years; standard deviation =7.04; 91.3% females) took part in the study. A trained neurologist not otherwise involved in the study diagnosed the patients as having MS 1–30 days before they were enrolled in the study. In other words, the diagnosis of MS was recent; these patients were new cases of MS. The patients completed the questionnaires individually during a routine checkup. A trained psychologist assisted the patients in case of questions.
Healthy adolescents
Data from 23 adolescents (mean age =17.43 years; standard deviation =1.91; 82.61% females) were derived from a larger sample, as already described by Brand et al.Citation28 The participants were young adolescents with regular schedules such as attending school fulltime, individual sports activities and leisure time activities such as playing music, attending music events, and similar. Of the total sample of 1,475 adolescents, data of 23 participants were randomly selected to build a subgroup for the present study (data set) and to present a representative of the pattern of results of the total sample (for more details, refer the study by Brand et al). The participants filled in the questionnaires during a regular school lesson.
Healthy young adults
Data from 25 healthy young adults (mean age =20.72 years; standard deviation =2.53; 80% females) were derived from a larger sample, as already described in another study.Citation24 The participants were young adult students from University of Basel (Switzerland), currently attending university classes fulltime. Of the total sample of 140 young adult students, the data of 25 participants were randomly selected to build a subgroup for the present study (data set) and to present a representative of the pattern of results for the sample as a whole. The participants completed the questionnaires during a regular lecture.
Materials
The participants completed a series of standardized questionnaires related to sociodemographic data, MT, SD, and PA.
Sociodemographic background
The participants reported their sex and age; additionally, patients with diagnosed MS reported time since onset of disease (in days).
Mental toughness
MT was assessed with MTQ48.Citation20 It consists of 48 items, which are aggregated to the following dimensions: challenge (eg, “Challenges usually bring out the best in me”), commitment (eg, “I don’t usually give up under pressure”), emotional (eg, “Even when under considerable pressure I usually remain calm”) and life control (eg, “I generally feel in control”), interpersonal confidence (eg, “I usually take charge of a situation when I feel it is appropriate”), and confidence in ability (eg, “I am generally confident in my own abilities”). The factorial validity of MTQ48 has been proved in previous studies.Citation24,Citation32 Further, MTQ48 has a high test–retest reliability and a high internal consistency. Items are anchored on 5-point Likert scales from 1 (= strongly disagree) to 5 (= strongly agree), with higher scores reflecting greater MT. Additionally, responses across items were summed to obtain an overall MT index (Cronbach’s alpha =0.84–0.91).
Physical activity
PA was assessed with the short version of the International Physical Activity Questionnaire (IPAQ). IPAQ was developed by a working group initiated by the World Health Organization and the Centers for Disease Control and Prevention. Based on the results from 12 countries, reliability and validity of IPAQ were comparable to other self-reported measures of PA.Citation51 The short form (self-administered, seven-item) of IPAQ was administered, asking about time spent in PA over the last 7 days. Minutes of sitting and walking as well as moderate-intensity (walking not included) and vigorous-intensity activities were calculated for the past week. The IPAQ questionnaires (short and long versions), including definitions of moderate and vigorous activity, are available at http://www.ipaq.ki.se.
Sleep disturbances
SD were assessed with the Insomnia Severity Index.Citation52 This questionnaire is a seven-item screening measure for insomnia and an outcome measure for use in treatment research. The items, answered on 5-point rating scales ranging from 0 (= not at all) to 4 (= very much), refer in part to the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition)Citation53 criteria for insomnia by assessing difficulty falling asleep, difficulty remaining asleep, early morning awakenings, impaired daytime performance, low satisfaction with sleep, and worry about sleep. The higher the overall score, the more the participant is assumed to suffer from insomnia (Cronbach’s alpha =0.87).
Statistical analysis
A chi-square test was performed to compare sex distributions between the three groups. Next, a series of univariate analysis of variance (ANOVA) was performed with group (MS patients, healthy adolescents, and healthy young adults) as an independent factor and age, MT, SD, and PA as dependent variables. The nominal level of significance was set at alpha P<0.05. All statistical computations were performed with SPSS® 22.0 (IBM Corporation, Armonk NY, USA) for Apple Mac®.
Results
Group differences in sociodemographic background
All descriptive and inferential statistical indices are reported in . Statistically significant group differences were found with regard to age, with MS patients having the highest age, followed by healthy young adults and healthy adolescents. Sex distribution did not differ between the three groups, P>0.05.
Group differences in MT and SD
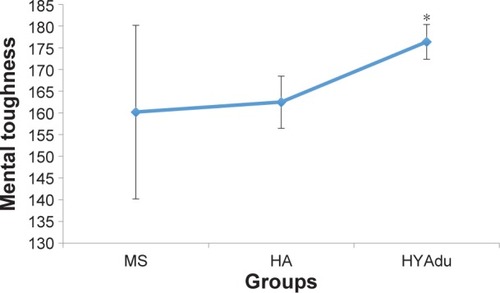
With regard to MT, all descriptive and inferential statistics for patients with diagnosed MS, healthy adolescents, and healthy young adults are shown in . As can be seen from the ANOVAs, there were significant group effects for challenge, commitment, life control, confidence in own abilities, confi-dence total score, and MTQ48 total score. A nonsignificant trend (P<0.1) was observed for control total score. Post hoc analyses after Bonferroni–Holm corrections for P-values showed that healthy young adults had significantly higher scores for challenge, confidence in own abilities, confidence total score, and MTQ48 total score, compared to patients with MS and healthy adolescents (). No significant group differences were observed for control of emotions or interpersonal confidence. Of note, no significant mean differences were observed between healthy adolescents and patients with MS.
Figure 1 Mental toughness.
Abbreviations: MS, multiple sclerosis; HA, healthy adolescents; HYAdu, healthy young adults.
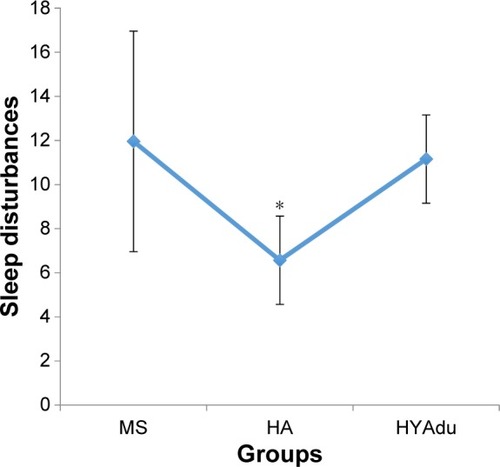
With regard to SD, there was a significant group effect (). Post hoc analyses after Bonferroni–Holm correction for P-values showed that healthy adolescents had the lowest level of SD, whereas SD did not differ between patients with MS and healthy young adults ().
Figure 2 Sleep disturbances.
Abbreviations: MS, multiple sclerosis; HA, healthy adolescents; HYAdu, healthy young adults.
Group differences in PA
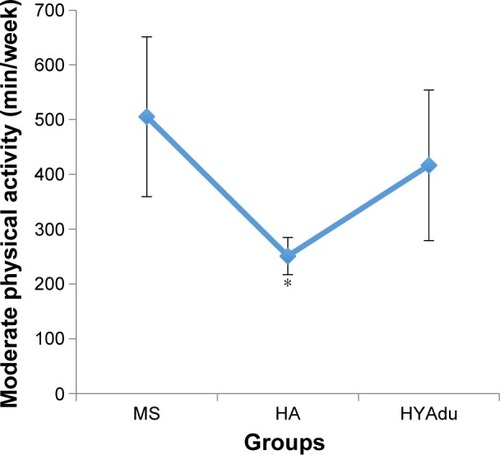
also reports all descriptive and inferential statistical indices related to PA. ANOVAs showed significant effects of group for both moderate and vigorous PA. Post hoc analyses after Bonferroni–Holm corrections for P-values showed that healthy young adults and healthy adolescents reported significantly higher levels of vigorous PA than patients with MS. Furthermore, healthy adolescents also reported more vigorous PA than healthy young adults (). Finally, patients with MS and young adults reported higher levels of moderate PA than did healthy adolescents, whereas no significant differences were found between patients with MS and health young adults ().
Figure 3 Vigorous physical activity.
Abbreviations: MS, multiple sclerosis; HA, healthy adolescents; HYAdu, healthy young adults.
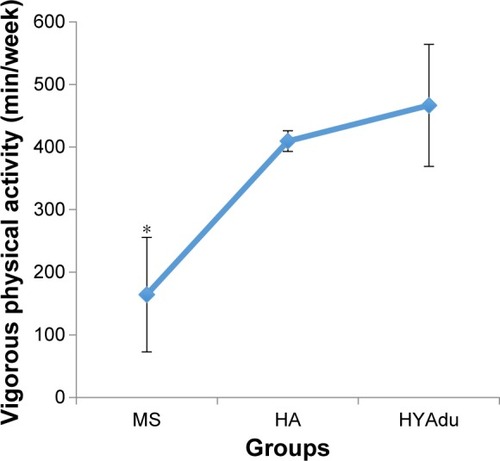
Figure 4 Moderate physical activity.
Abbreviations: MS, multiple sclerosis; HA, healthy adolescents; HYAdu, healthy young adults.
Discussion
The key findings of the present study were that MT levels on all dimensions of patients with MS at illness onset were similar to those of healthy adolescents and healthy young adults; patients with MS also had equal amounts of moderate PA and degrees of SD as young adults. However, patients reported lower levels of vigorous PA than either healthy adolescents or young adults. The present pattern of results adds to the current literature in showing that patients with MS at illness onset did not differ from healthy adolescents and healthy young adults in MT traits, SD, or moderate PA.
Three research questions were formulated and each of these is considered now in turn.
With the first research question, we focused on possible differences in MT traits between patients with MS at illness onset and healthy adolescents and young adults. The pattern of results in patients with MS showed a difference in overall MT score compared to healthy young adults, but not compared to healthy adolescents. Further inspection () revealed that patients with MS at illness onset did not differ statistically from healthy young adults with regard to commitment, life control, emotion control, control overall score, or interpersonal confidence. Next, to further contextualize the present results, it should be noted that the sample of healthy young adults included sports students who, almost by definition, report higher levels of MTCitation54 along with greater PA.
Overall, we believe that the present data add to the existing literature on MT in that this is the very first study on MT traits among patients with MS at illness onset. The study also adds to the current literature on MS in suggesting that at illness onset, MT levels of patients with MS are not different from those of healthy adolescents and young adults. Further and importantly, the present findings are at odds with those studies reporting increased symptoms of depression and anxietyCitation5,Citation39 and a lower quality of life.Citation40,Citation41
Our second research question concerned whether patients with MS at illness onset reported more SD than healthy adolescents or healthy young adults, and the answer was no. Therefore, again, we hold that the current pattern of results adds to the literature in showing that sleep at illness onset in patients with MS is not impaired compared to healthy young adults. Had this been the case, it then would have been possible that either alterations in the neuronal centers responsible for sleep-wake-generation and coordinationCitation55 or psychological issues (refer the study by Riemann et alCitation56 for the hyperarousal model of SD) or both were responsible for poorer sleep. Further, the present results are at odds with those studiesCitation33,Citation57,Citation58 reporting impaired sleep patterns in patients with MS.
Third, we asked whether patients with MS at illness onset would report lower moderate, or vigorous PA, as compared to healthy adolescents and healthy young adults, and the answer was no. Specifically, they reported higher levels of moderate PA than healthy adolescents, and, descriptively, than healthy young adults, though these patients also reported lower levels of vigorous activity. Again, we hold that the present pattern of results adds to the current literature in showing that, at illness onset, PA does not appear to be impaired or reduced in patients with MS. Likewise, and in contrast to the findings from patients with Huntington’s Disease,Citation38 PA patterns are not good predictors of preclinical symptoms. Further, the present pattern of results is at odds with those studies showing a decrease in PA among patients with MS.Citation1,Citation34,Citation35
The novelty of the results should be balanced against the limitations of the study, which preclude overgeneralization of the findings. First, the sample sizes were small, though statistically significant mean differences were observed. Second, female participants predominated in the samples, though we note that females have a 2.5–3.5 times greater risk of suffering from MS than males. Third, no objective data were collected; objective assessments of PA and objective sleep measures, for example, would have provided a more complete picture and more reliable data on both PA and sleep. Fourth, it would have been interesting and enlightening to compare the present data with neurocognitive performance and imaging data in order to determine to what extent MT traits, SD, and PA are associated with neuronal changes and cognitive performance. Fifth, the present pattern of results might have emerged due to further latent but unassessed dimensions, which might have biased two or more dimensions in similar or opposite directions. Sixth, it would have been interesting to assess daytime sleepiness and fatigue, and to relate these to SD. This would have allowed a more thorough investigation of the association between poor sleep, tiredness, and fatigue, given that up to now there has been so little research on these associations. Seventh, it might be arguable to what extent it was useful to compare data of patients with MS at disease onset with data of healthy adolescents and healthy young adults (students in sport science). However, we followed Roberts et al,Citation59 who showed a continuity of personality growth toward greater maturity from adolescence to young adulthood. Therefore, we wanted to explore to what extent continuity and change in personality would have been reflected in MT traits among these three different samples. In this view, the present data showed that such comparisons were reasonable, as patients with MS at disease onset reported very similar traits as did the healthy adolescents and healthy young adults. Eighth, the topic of MT gets increasingly assessed among nonelite athletes such as lower, middle, and senior managers, and clerical/administrative workers in early, middle, and later adulthood.Citation32 Accordingly, we consider the present study as a contribution to the research on MT as a general set of personality traits covering dimensions such as stress management, self-esteem, confidence in social environment, and coping with changes. Lastly, the cross-sectional character of the study does not shed any light on the illness course; in this regard, it would be intriguing to know whether MT traits, SD, or amount of PA predict the future course of the illness along with quality of life.
Conclusion
The pattern of results suggests that patients with MS at illness onset show patterns of MT traits, SD, and PA comparable to those of healthy adolescents and young adults. We believe that the therapeutic challenge is to keep these features stable over the course of treatment and illness.
Acknowledgments
The authors thank Nick Emler (University of Surrey, Surrey, UK) for proofreading the manuscript. Further, we thank Ludwig Kappos (University of Basel, Basel, Switzerland) for helpful advice.
Disclosure
The authors report no conflicts of interest in this work.
References
- RazazianNYavariZFarniaVExercising impacts on fatigue, depression, and paresthesia in female patients with MSMed Sci Sports Exerc201648579680326656775
- BusseMQuinnLDawesHSupporting physical activity engagement in people with Huntington’s disease (ENGAGE-HD): study protocol for a randomized controlled feasibility trialTrials20141548725494622
- IranzoASleep in neurodegenerative diseasesSleep Med Clin201611111826972029
- GarlovskyJKOvertonPGSimpsonJPsychological predictors of anxiety and depression in Parkinson’s disease: a systematic reviewJ Clin Psychol201610.1002/jclp.22308
- FiestKMWalkerJRBernsteinCNSystematic review and meta-analysis of interventions for depression and anxiety in persons with multiple sclerosisMult Scler Relat Disord201651122626856938
- EnriciIAdenzatoMArditoRBEmotion processing in Parkinson’s disease: a three-level study on recognition, representation, and regulationPLoS One2015106e013147026110271
- CavalloMCavannaAEHarciarekMJohnstonHOstacoliLAngillettaC“Keep up the good work”: a case study of the effects of a specific cognitive training in Alzheimer’s diseaseNeurocase201319654255222823908
- NoseworthyJHLucchinettiCRodriguezMWeinshenkerBGMultiple sclerosisN Engl J Med20003431393895211006371
- SvendsenKBJensenTSHansenHJBachFWSensory function and quality of life in patients with multiple sclerosis and painPain2005114347348115777872
- VeauthierCSleep disorders in multiple sclerosis. ReviewCurr Neurol Neurosci Rep20151552125773000
- BrañasPJordanRFry-SmithABurlsAHydeCTreatments for fatigue in multiple sclerosis: a rapid and systematic reviewHealth Technol Assess200042716111074395
- O’ConnorPComiGFreedmanMSLong-term safety and efficacy of teriflunomide: nine-year follow-up of the randomized TEMSO studyNeurology2016861092093026865517
- LublinFMillerDHFreedmanMSOral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trialLancet2016387100231075108426827074
- KapposLWiendlHSelmajKDaclizumab HYP versus interferon beta-1a in relapsing multiple sclerosisN Engl J Med2015373151418142826444729
- KapposLRadueEWChinPRitterSTomicDLublinFOnset of clinical and MRI efficacy occurs early after fingolimod treatment initiation in relapsing multiple sclerosisJ Neurol2016263235436026645392
- KapposLKuhleJMultanenJFactors influencing long-term outcomes in relapsing-remitting multiple sclerosis: PRISMS-15J Neurol Neurosurg Psychiatry201586111202120726374702
- WingerchukDMCarterJLMultiple sclerosis: current and emerging disease-modifying therapies and treatment strategiesMayo Clin Proc201489222524024485135
- LuEWangBWGuimondCSynnesASadovnickDTremlettHDisease-modifying drugs for multiple sclerosis in pregnancy: a systematic reviewNeurology201279111130113522933738
- FreedmanMSHughesBMikolDDEfficacy of disease-modifying therapies in relapsing remitting multiple sclerosis: a systematic comparisonEur Neurol200860111118437041
- CloughPEarleKSewellDMental toughness: the concept and its measurementSolutions Sport Psychol20023243
- MackMGRaganBGDevelopment of the mental, emotional, and bodily toughness inventory in collegiate athletes and nonathletesJ Athl Train200843212513218345336
- CrustLCloughPJRelationship between mental toughness and physical endurancePercept Mot Skills2005100119219415773710
- Antonini PhilippeRSagarSSHauwDGerberMPlayers perceptions of coaches’ contribution to their mental toughnessInt J Sports Sci Coaching2016101317
- GerberMKalakNLemolaSAre adolescents with high mental toughness levels more resilient against stress?Stress Health201329216417122941714
- GerberMBrandSFeldmethAKAdolescents with high mental toughness adapt better to perceived stress: a longitudinal study with Swiss vocational studentsPers Individ Dif201354808814
- GerberMFeldmethAKLangCThe relationship between mental toughness, stress, and burnout among adolescents: a longitudinal study with Swiss vocational studentsPsychol Rep2015117370372326652888
- GerberMFeldmethAKElliotCBrandSHolsboer-TrachslerEPühseUMental health in Swiss vocational students: the moderating role of physical activityJ Res Adolesc2015256374
- BrandSKalakNGerberMDuring early to mid adolescence, moderate to vigorous physical activity is associated with restoring sleep, psychological functioning, mental toughness and male genderJ Sports Sci Epub201641
- BrandSKalakNGerberMDuring early and mid-adolescence, greater mental toughness is related to increased sleep quality and quality of lifeJ Health Psychol20162190591525060987
- BrandSGerberMKalakN“Sleep well, our tough heroes!” – in adolescence, greater mental toughness is related to better sleep schedulesBehav Sleep Med201412644445424229399
- BrandSGerberMKalakNAdolescents with greater mental toughness show higher sleep efficiency, more deep sleep and fewer awakenings after sleep onsetJ Adolesc Health201454110911323998848
- PerryJLCloughPJCrustLEarleKNichollsARFactorial validity of the mental toughness questionnaire-48Pers Individ Dif2013545587592
- StroberLBFatigue in multiple sclerosis: a look at the role of poor sleepFront Neurol201562125729378
- MotlRWBenefits, safety, and prescription of exercise in persons with multiple sclerosisExpert Rev Neurother201414121429143625413175
- AsanoMDuquettePAndersenRLapierreYMayoNEExercise barriers and preferences among women and men with multiple sclerosisDisabil Rehabil201335535336123347461
- LionettiECastellanetaSFrancavillaRIntroduction of gluten, HLA status, and the risk of celiac disease in childrenN Engl J Med2014371141295130325271602
- WaschbischATallnerAPfeiferKMaurerMMultiple sclerosis and exercise: effects of physical activity on the immune systemNervenarzt200980668869219159912
- TrembathMKHortonZATippettLA retrospective study of the impact of lifestyle on age at onset of Huntington diseaseMov Disord201025101444145020629137
- de CerqueiraACSemionato de AndradePGodoy BarreirosJMTeixeiraALNardiAEPsychiatric disorders in patients with multiple sclerosisCompr Psychiatry201563101426555486
- BaumstarckKBoyerLBoucekineMMichelPPelletierJAuquierPMeasuring the quality of life in patients with multiple sclerosis in clinical practice: a necessary challengeMult Scler Int2013201352489423533758
- BaumstarckKBoucekineMBoyerLQuantification of relevance of quality of life assessment for patients with cognitive impairment: the suitability indicesBMC Neurol2014147824708665
- TiemannLPennerIKHauptsMSchlegelUCalabresePCognitive decline in multiple sclerosis: impact of topographic lesion distribution on differential cognitive deficit patternsMult Scler200915101164117419667010
- LenschEMatzkeMPetereitHFSchererPSchrammSCalabresePIdentification and management of cognitive disorders in multiple sclerosis – a consensus approachJ Neurol2006253Suppl 1I29I3116477483
- CalabresePNeuropsychology of multiple sclerosis – an overviewJ Neurol2006253Suppl 1I10I1516477479
- KalbeECalabresePFenglerSKesslerJDemTect, PANDA, EASY, and MUSIC: cognitive screening tools with age correction and weighting of subtests according to their sensitivity and specificityJ Alzheimers Dis201334481383423313929
- CalabreseMGajofattoAGobbinFLate-onset multiple sclerosis presenting with cognitive dysfunction and severe cortical/infratentorial atrophyMult Scler201521558058925432947
- CalabreseMFavarettoAPorettoVLow degree of cortical pathology is associated with benign course of multiple sclerosisMult Scler201319790491123069877
- CalabreseMFavarettoAMartiniVGalloPGrey matter lesions in MS: from histology to clinical implicationsPrion201371202723093801
- CalabreseMAtzoriMBernardiVCortical atrophy is relevant in multiple sclerosis at clinical onsetJ Neurol200725491212122017361339
- CalabreseMAgostaFRinaldiFCortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosisArch Neurol20096691144115019752305
- CraigCLMarshallALSjöströmMInternational physical activity questionnaire: 12-country reliability and validityMed Sci Sports Exerc2003351381139512900694
- BastienCHVallieresAMorinCMValidation of the Insomnia Severity Index as an outcome measure for insomnia researchSleep Med20012429730711438246
- American Psychiatric AssociationDiagnostic and Statistical manual of Mental Disorders 4th edition: DSM-IV-TRWashington DCAmerican Psychiatric Association2000
- GerberMKalakNLemolaSAdolescents’ exercise and physical activity are associated with mental toughnessMent Health Phys Act2012513542
- CirelliCTononiGMolecular neurobiology of sleepHandb Clin Neurol20119819120321056187
- RiemannDSpiegelhalderKFeigeBThe hyperarousal model of insomnia: a review of the concept and its evidenceSleep Med Rev2010141193119481481
- StroberLBArnettPAAn examination of four models predicting fatigue in multiple sclerosisArch Clin Neuropsychol200520563164615894455
- ArnettPAStroberLBCognitive and neurobehavioral features in multiple sclerosisExpert Rev Neurother201111341142421375446
- RobertsBWCaspiAMoffittTEThe kids are alright: growth and stability in personality development from adolescence to adulthoodJ Per Soc Psychol2001814670683
