Abstract
Anhedonia, defined as the state of reduced ability to experience feelings of pleasure, is one of the hallmarks of depression. Hedonic tone is the trait underlying one’s characteristic ability to feel pleasure. Low hedonic tone represents a reduced capacity to experience pleasure, thus increasing the likelihood of experiencing anhedonia. Low hedonic tone has been associated with several psychopathologies, including major depressive disorder (MDD), substance use, and attention-deficit hyperactivity disorder (ADHD). The main neural pathway that modulates emotional affect comprises the limbic–cortical–striatal–pallidal–thalamic circuits. The activity of various components of the limbic–cortical–striatal–pallidal–thalamic pathway is correlated with hedonic tone in healthy individuals and is altered in MDD. Dysfunction of these circuits has also been implicated in the relative ineffectiveness of selective serotonin reuptake inhibitors used to treat anxiety and depression in patients with low hedonic tone. Mood disorders such as MDD, ADHD, and substance abuse share low hedonic tone as well as altered activation of brain regions involved in reward processing and monoamine signaling as their features. Given the common features of these disorders, it is not surprising that they have high levels of comorbidities. The purpose of this article is to review the neurobiology of hedonic tone as it pertains to depression, ADHD, and the potential for substance abuse. We propose that, since low hedonic tone is a shared feature of MDD, ADHD, and substance abuse, evaluation of hedonic tone may become a diagnostic feature used to predict subtypes of MDD, such as treatment-resistant depression, as well as comorbidities of these disorders.
Introduction
Emotions, mood, and affect are the underlying phenomena of psychological states and disorders. Emotions represent complex psychological states that are elicited as a response to one’s external environment and involve the interplay between behavioral and physiological responses. Unlike emotions, which are specific and usually occur in response to a stimulus, mood is a general feeling of one’s psychological state that is longer lasting than specific emotions.Citation1 Core affect is defined as a neurophysiological state that underlies the general feelings of “good” or “bad”, “drowsy” or “energized”.Citation2 It has been suggested that the core affect shapes hedonic valence of one’s experiences.
Anhedonia, defined as the state of reduced ability to experience feelings of pleasure, is one of the hallmarks of depression.Citation3,Citation4 However, the term anhedonia is insufficient to summarize the intricate and multidimensional reward-associated deficits displayed by patients with neuropsychiatric disorders.Citation2,Citation5 Deficits in reward-related processing may present as loss of interest or pleasure and may impede an individual’s ability to engage in goal-directed behavior.Citation2,Citation5 These behaviors may include the lack of anticipation or prediction of expected rewards, lack of ability to evaluate the perceived values and costs associated with anticipated rewards, inability to gauge the amount of effort required to attain rewards, inability to evaluate whether the effort is sufficiently rewarded, and lack of motivation to execute actions required to attain rewards.Citation5
If anhedonia is the state of reduced ability to feel pleasure, then hedonic tone, also referred to as hedonic capacity or hedonic responsiveness, is the trait or genetic predisposition underlying one’s baseline range and lifelong characteristic ability to feel pleasure. Low hedonic tone represents a reduced capacity to experience pleasure at any given time, thus increasing the likelihood of experiencing anhedonia.Citation6,Citation7
Low hedonic tone has been associated with several psychopathologies, including major depressive disorder (MDD), substance use, and schizophrenia,Citation8,Citation9 although variations in hedonic tone can also be observed in healthy individuals.Citation10
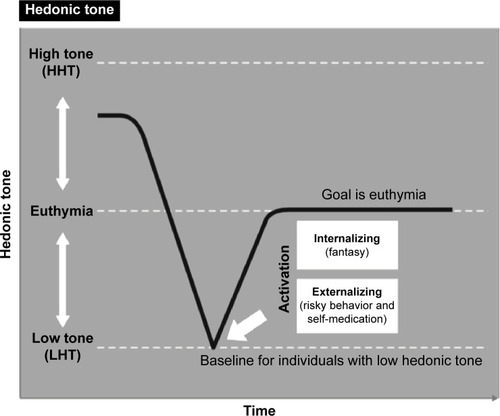
It can be hypothesized that individuals suffering with genetically lower set point, or lower hedonic tone, will need to do more to feel neutral or euthymic, resulting in an increased need for stimulation.Citation11 In part, this may manifest as seeking external stimulation (eg, dangerous or risky behavior and substance abuse) or internal stimulation (eg, fantasy) that will raise their hedonic tone.Citation12 Regardless of the choice to externally or internally raise their hedonic tone, patients with this trait would consistently attempt to cope by finding ways to maximize pleasure and raise mood from their low baseline tone. Furthermore, when stimulation is absent, individuals with low hedonic tone would be hypothesized to experience a shift toward that individual’s more usual lower hedonic tone and therefore suffer a drop in their mood to their more common baseline dysphoric state ().Citation12
Figure 1 Hypothesized results of lower hedonic tone and behaviors to reach euthymia.
Abbreviations: HHT, high hedonic tone; LHT, low hedonic tone.
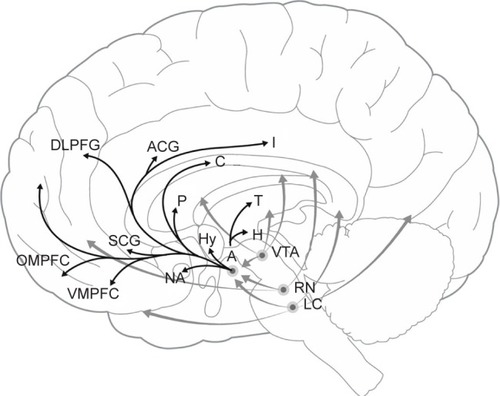
Thus, it is not surprising that a large body of evidence points to the involvement of the reward system, particularly the mesolimbic dopaminergic system, in mediating the degree of anhedonia at any given timeCitation6,Citation7 as well as the risk of developing dysthymia and depression.Citation13,Citation14 Recent research into the neurobiology of anhedonia has focused on neurobiology of reward and motivation and has identified associated dopaminergic circuits as playing a key role in the maintenance of hedonic tone,Citation2,Citation4,Citation15,Citation16 specifically neural circuits that contain bottom-up and top-down projections into the prefrontal cortex (PFC), lateral habenula, and the ventral tegmental area (VTA) dopamine (DA) system.Citation17 In association, specific deficits in a variety of PFC areas have been implicated in anhedonia ().Citation5
Figure 2 Hypothesized regions contributing to modulation of hedonic tone.
Abbreviations: A, amygdala; ACG, anterior cingulate cortex; C, caudate; DLPFG, dorsolateral prefrontal cortex; H, habenula; Hy, hypothalamus; I, insula; LC, locus coeruleus; NA, nucleus accumbens; OMPFC, orbitomedial prefrontal cortex; P, putamen; RN, raphe nucleus; SCG, subgenual cingulate cortex; T, thalamus; VTA, ventral tegmental area; VMPFC, ventromedial prefrontal cortex.
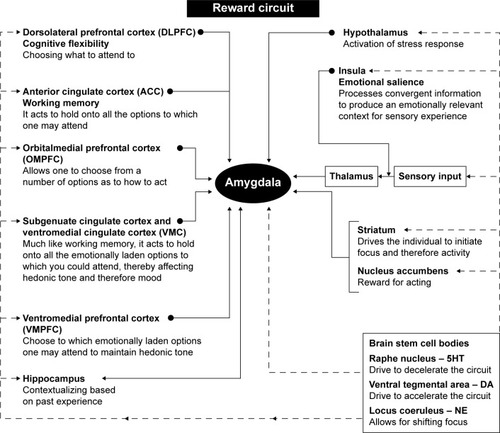
The purpose of this article is to review the neurobiology of hedonic tone as it pertains to depression, attention-deficit hyperactivity disorder (ADHD), and the potential for substance abuse. We propose the hypothesis that the experience of living for these individuals would best be understood through awareness of their low hedonic tone as they endeavor to attain a euthymic state by modulating the neurobiology of these areas. Furthermore, we propose that a dysfunction in the reward circuitry that comprises the ventromedial prefrontal cortex (VMPFC), subgenuate, and the nucleus accumbens (NA) is the underlying biological mechanism to this low hedonic tone and, therefore, the various mood disorders ().
Methods
To identify studies relevant to our review, we performed a literature search on PubMed, PsychNET, and Medline databases until January 2016 using keywords “hedonic tone” AND “mood disorder” AND any of the following terms: “reward pathways”, “dopamine”, “neurobiology”, “depression”, “ADHD”, or “substance abuse”. Inclusion criteria for studies were 1) English language, 2) articles published in peer-reviewed journals, and 3) original research or review articles. Human and nonhuman primate studies were included in the neurobiology of mood and hedonic tone section. All other sections of this review included only human studies.
Neurobiology of mood disorders
Mood and emotional expression are regulated by a complex neural network, which involves the interplay between numerous regions of the central nervous system (CNS). Regions that modulate mood and emotions are closely intertwined with feelings of reward, pleasure, motivation, as well as our internal state and external environment. These regions and the associated neural pathways that regulate these functions span from the cerebral cortex to the brain stem and include the peripheral nervous system via the vagus nerve.
The main neural pathway that modulates emotional affect comprises the limbic–cortical–striatal–pallidal–thalamic (LCSPT) circuits, which consist of connections between the orbital and medial prefrontal cortex (OMPFC), ventromedial striatum, ventral pallidum, hippocampal subiculum, mediodorsal and midline thalamic nuclei, and amygdala.Citation18 Through reciprocal connections with cortical regions that control higher cognitive functions as well as regions involved in the regulation of autonomic functions, including the periaqueductal gray and the hypothalamus, these circuits integrate higher cognitive functions with visceral information and external environmental conditions to affect mood and emotional states.Citation19 Furthermore, the connections between the OMPFC and the dorsolateral prefrontal cortex (DLPFC) connect mood dysregulation with deficits in working memory and cognitive flexibility features that are dysfunctional in mood disorders ().Citation20–Citation22
Figure 3 Hypothesized regulation of hedonic tone.
Abbreviations: ACC, anterior cingulate cortex; DA, dopamine; 5HT, 5-hydroxytryptamine; NE, norepinephrine.
The OMPFC includes a significant portion of the cerebral cortex and is specifically involved in decision making and emotional- and reward-driven behaviors.Citation18 Functional studies have identified two prefrontal networks within the OMPFC:Citation23,Citation24 the orbital network having been implicated in sensing food-related information and the anticipation of rewardCitation24 and the medial network projecting to visceral control centers in the hypothalamus and the periaqueductal grayCitation25,Citation26 and modulating visceral activity in response to affective stimuli.Citation24 Thus, the orbital network is responsible for associating stimuli with a reward and specifically with the reward value of the stimulus.Citation27 On the other hand, the medial network is involved with visceral responses to emotions, such as increased respiratory and heart rate, changes in blood pressure, and changes in the digestive activities ().Citation24
As well, the OMPFC has also been implicated in the inhibition of impulsive behavior,Citation28 with reductions in dopaminergic input to the OMPFC having been shown to diminish the ability to inhibit impulsive behavior.Citation29 Since impulsive behavior is one of the features of several mood disorders, dysfunction in dopaminergic inputs to OMPFC may represent a precursor to developing these conditions. Furthermore, in healthy individuals, activation of the medial network is associated with an increase in galvanic skin conductance, which represents an indirect measure of emotional arousal through increased activity in the sympathetic nervous system,Citation30–Citation32 further supporting the regions’ role in the regulation of emotional affect ().
The amygdala as a part of the limbic system plays a crucial role in various forms of emotional learning, including fear conditioning,Citation33 and mediates emotional responses to stress.Citation34 Functional imaging studies have shown that the amygdala is activated by fear conditioning,Citation35 and patients with lesions to the amygdala are unable to recognize fearful stimuli.Citation36 The amygdala receives information about the external environment via the thalamus and the sensory cortex and is reciprocally connected with the OMPFC, the hippocampus, and sensory association areas. The amygdala also sends efferent projections to the striatum, both the dorsal (caudate nucleus and the putamen) and the ventral striatum, and the NA.Citation36 Through projections to brainstem regions that control visceral functions, such as cardiovascular and respiratory functions, the amygdala plays a role in coordinating higher cognitive functions and emotions with the physiological state, including heart rate and respiratory rate observed during stress. Furthermore, increased reactivity of the amygdala to stressful stimuli has been implicated as a precursor to depression in adolescents.Citation37
The amygdala, particularly the central nucleus, coordinates the fear response via connections to autonomic and cortical regions. Sensory stimuli, such as visual, auditory, and other stimuli, are perceived by the visual regions of the thalamus, namely, the lateral geniculate, which sends these messages to the central nucleus of the amygdala. Activation of this nucleus, in turn, coordinates the autonomic response to fear, which includes an increase in the respiratory rate via connections to the parabrachial nucleus, increased arousal via connections to the lateral hypothalamus, increased autonomic activation via projections to the paraventricular nucleus of the hypothalamus, increased catecholamine release via projections to the locus coeruleus, as well as activation of defensive behaviors via connections to the periaqueductal gray.Citation38 In addition, the amygdala also has reciprocal connections with cortical regions involved in the processing and evaluation of sensory information. These regions integrate sensory information and decide whether fear is warranted.Citation38 For example, the hippocampus is involved in contextualizing fear and can either increase or decrease fear reaction based on prior experiences and memories, whereas the medial PFC is involved in fear extinction.Citation39,Citation40 In individuals who have been conditioned to fear, or those with panic disorders, dysfunction in this circuitry leads to an inability to extinguish or contextualize fear.Citation41 Furthermore, the central nucleus of the amygdala is connected to the insular cortex, which is involved in contextualizing sensory inputs of various modalities, including nociceptive, auditory, and visual.Citation42–Citation44 This notion is supported by the findings that lesions in the insular cortex are associated a reduced sensitivity to the pain of others ().Citation45
Emotional responses to stressful stimuli are partly mediated by the reciprocal connections of the amygdala, the thalamus, and the hippocampus.Citation46 Whereas the dorsal part of the hippocampus is primarily involved in memory consolidation, the ventral hippocampus has been shown to modulate emotions and mood.Citation46,Citation47 Interestingly, neurons in the hippocampus of adults display a high degree of neuroplasticity and neurogenesis and are easily influenced by life experiences and medications.Citation48
The basal ganglia, including the dorsal striatum (eg, caudate nucleus, putamen), the ventral striatum (eg, NA, olfactory tubercle, globus pallidus, ventral pallidum), the subthalamic nucleus, and the substantia nigra, are also closely interconnected with the PFC and the amygdala.Citation49 The basal ganglia are involved in motor control, as well as motivation and reward processing.Citation49 It has been demonstrated that in healthy individuals, volume of the pallidumCitation50 and the caudateCitation51 are correlated with anhedonia scores, suggesting that these structures may be involved in the regulation of hedonic tone. More specifically, the caudate nucleus is thought to be involved in evaluating the magnitude of a reward and in driving appropriate behaviors required to obtain that reward.Citation52,Citation53 Based on these functions, individuals with low activity in the caudate nucleus would have a reduced capacity to estimate the value of a reward or to initiate actions needed to obtain a reward. These are also the characteristics of low hedonic tone.Citation12
The DLPFC circuit is involved in working memory and cognitive flexibility and has been linked to the pathogenesis of mood disorders ().Citation20,Citation21 This circuit originates in the DLPFC and corresponds to Brodmann’s areas 9 and 10. The major efferent connections of DLPFC are composed of pyramidal neurons, which project to the dorsolateral caudate nucleus, which, in turn, projects to the globus pallidus and substantia nigra. These basal ganglia regions project to the ventral anterior and mediodorsal thalamic nuclei, which send afferent projections back to the DLPFC.Citation21 Dysfunction of this circuit has been linked to impaired reasoning ability and cognitive inflexibility.Citation21 Patients with DLPFC syndrome exhibit poor working memory and organizational abilities as well as inability to shift their attention between tasks.Citation21 It has also been shown that depression is associated with hypoactivity in the DLPFC ().Citation22
Neural circuits regulating mood and emotional affect release a wide range of neurotransmitters and are also affected by circulating hormones, particularly those involved in the stress response.Citation19 As is the case throughout the CNS, neuronal activity within LCSPT circuits is predominantly glutamatergic and is locally modulated through the gamma-aminobutyric acid (GABA) system.Citation54 Glutamate is the main excitatory neurotransmitter in the CNS and activates all regions of the LCSPT circuits. Furthermore, disruptions in glutamate signaling have been implicated in disorders of reward processing, including substance abuse.Citation55 On the other hand, GABA acts as the main inhibitory neurotransmitter/modulator of the glutaminergic synapses and is present in interneurons situated throughout and acts to specifically inhibit neuronal activity.Citation56 In corticostriatal circuits, GABA neurons are responsible for the selection and inhibition of motivated behaviors.Citation57 Although glutamate and GABA are the predominant neurotransmitters in the LCSPT circuits, their activity can be modulated by a variety of other neurotransmitters and neuromodulators. For example, endocannabinoids have been shown to inhibit the release of both glutamate and GABA in the VTA.Citation58 Similarly, glutamate and GABA release can be inhibited by opioids in various regions of the CNS, including the regions involved in reward processing, such as the VTA, amygdala, and the hippocampus.Citation58–Citation60 Furthermore, the activity of glutamate and GABA-containing neurons can also be modulated by monoamines. In fact, serotonergic modulation of glutamate release has been implicated as one of the strategies for treating MDD.Citation61
With respect to mood regulation, monoamine neurotransmitters (including serotonin, DA, norepinephrine (NE), and epinephrine) have been the main focus of research and have been the primary targets in pharmaceutical treatments of depression.Citation19 The monoamine hypothesis of depression postulates that depression is caused by decreased modulating function of serotonin, NE, or DA in the brain.Citation62 Nevertheless, this theory does not explain the apparent lack of efficacy in a relatively large subset of patients with treatment-resistant depression (TRD) who continue to suffer with residual symptoms, including anhedonia. Nonetheless, the extent to which monoamines influence neuronal activity is dependent on the expression level of the neurotransmitter itself as well as on the number of available receptors and transporters on the postsynaptic membrane. For example, serotonin can bind to 14 different receptor types,Citation63–Citation65 and the effect of serotonin on neurons can be region-specific.Citation61 As such, one can imagine that this variability may explain the individual differential response to the various antidepressants. For example, it has been proposed that vortioxetine, which is described as a mixed multimodal antidepressant, likely acts as an atypical serotonin modulator, enhancing GABA activity in the striatum but reducing GABA activity in the PFC.Citation61 Vortioxetine has also been shown to increase glutamate activity via antagonism of serotonin receptors 6 and 7 and agonism of serotonin receptor 1a.Citation66 Given the complexity of neurotransmitter systems regulating mood and emotional states, it is not surprising that mood disorders resulting from a disrupted balance of these circuits can take on various forms and manifest as highly variable symptoms ().Citation67
While the role of monoamine antidepressants in modulation of reward circuits has been the most extensively investigated, recent studies have demonstrated that substances that modulate other neurotransmitter systems, particularly N-nitrosodimethylamine receptors (glutamate), endogenous opioids, and acetylcholine, also affect reward pathways and hedonic tone. It has been demonstrated that symptoms of depression can be relieved with ketamine, an N-nitrosodimethylamine receptor antagonist,Citation68 scopolamine, a muscarinic cholinergic receptor antagonist,Citation69 and buprenorphine, a partial mu opioid receptor agonist,Citation70 and these pharmacological agents have also been shown to alter reward-processing circuits. A single infusion of ketamine was shown to reduce anhedonia levels in patients with TRD. The reduction in anhedonia was accompanied by an increase in glucose metabolism in the dorsal anterior cingulate cortex (ACC) and putamen,Citation71 suggesting that antidepressant properties of ketamine are mediated by its actions on reward processing. Clinical response to scopolamine is correlated with a greater neural activity in response to happy versus sad faces in the subgenual anterior cingulate cortex (sgACC).Citation72 Furthermore, opioid dependence is associated with a reduced hedonic response to positive stimuli and reduced neural activation in the rostral PFC and ventrolateral prefrontal cortex (VLPFC),Citation73 and buprenorphine and samidorphan exert their antidepressant effects by affecting the levels of hedonic tone and altering the circuitry of the PFC. Taken together, these data indicate that nonmonoamine antidepressants, such as ketamine, scopolamine, and buprenorphine, induce antidepressant effects by restoring hedonic tone to control levels. A better understanding of the effects of these agents on neural circuits that regulate mood and hedonic tone is an important strategy for identifying novel effective treatments of depression.
Dysfunction in LCSPT circuits and the associated neurotransmitter systems have been implicated as playing a key role in MDD.Citation19 Neuroimaging studies among depressed patients have demonstrated changes in the activity in LCSPT circuits in response to various treatments. For example, it has been shown that depression is associated with frontal hypometabolic activity accompanied by hypermetabolic activity in regions associated directly with the limbic system, including the VMPFC and sgACC.Citation19,Citation74–Citation76 Furthermore, it has been shown that in depressed patients, the activity in the sgACC is positively correlated with the severity of depression in response to sad stimuli and negatively correlated in response to happy stimuli. At the end of the study, following various antidepressant treatments, patients who showed the greatest reduction in the activity in the sgACC in response to sad stimuli experienced the greatest reductions in Hamilton Rating Scale for Depression scores.Citation77 For clarity in this review, it should be noted that the VMPFC overlaps with the region referred to as the ACC, particularly the pregenual and sgACC parts of the ACC. Therefore, the term “VMPFC” is used here for the region as a whole, and the term “sgACC” is used in describing studies that have focused on this particular area. The involvement of the PFC, the VMPFC, and the sgACC in depression is further highlighted by studies demonstrating normalization of functional activity to levels observed in nondepressed subjects following successful therapy, including treatment with antidepressants, deep brain stimulation (DBS), and vagus nerve stimulation (VNS) ().
Selective serotonin reuptake inhibitors (SSRIs) fluoxetine and paroxetine have been shown to increase glucose metabolism in the PFC, particularly the dorsolateral, ventrolateral, and medial aspects, parietal cortex, and the sgACC.Citation75,Citation78 In addition, fluoxetine reduced glucose metabolism in the sgACC, hippocampus, insula, and pallidum.Citation78 These findings suggest that dysfunction in serotonin reuptake in these regions accounts for symptoms of depression.
In patients with TRD, VNS has been shown to improve symptoms of depression.Citation79,Citation80 Imaging studies using either single photon emission computed tomography or blood oxygen level-dependent methods revealed that improvement in depression symptoms induced by VNS was associated with decreased activity in the sgACC, VMPFC, and ACC and increased activity of the superior temporal gyrus.Citation81,Citation82
DBS has also been shown to be useful in TRD with associated modulated activity of the sgACC and PFC in treatment-resistant patients. Mayberg et alCitation31 investigated whether chronic DBS could modulate the activity of the sgACC over 6 months among patients with TRD, defined as failure to respond to a minimum of four different antidepressant treatments during the current major depressive episode lasting at least 1 year. Positron emission tomography measures of regional blood flow at baseline identified increased blood flow in the sgACC and decreased blood flow in the PFC in depressed patients compared to healthy controls. After 6 months of treatment with DBS, blood flow in the DLPFC and VLPFC among responders increased to levels observed in healthy controls, while blood flow in the sgACC decreased to levels lower than those measured in healthy controls.
Taken together, the results of these studies show that hypoactivity in the VLPFC and DLPFC and increased activity in the VMPFC and sgACC are common findings among patients with depression and that reversal of activity in these regions to levels observed among healthy individuals is associated with improvement of depressive symptoms. Furthermore, the activity of these regions can be modulated not only by antidepressants in the responsive population but also by VNS and DBS in those who do not respond to pharmaceutical treatment.
Neurobiology of hedonic tone
It can be hypothesized that hedonic tone is closely related to mood, reward, and motivation and is modulated by LCSPT. Imaging studies have identified components of the LCSPT pathway that are involved in the regulation of hedonic tone. These studies have shown that the activity of various components of the LCSPT pathway is correlated with hedonic tone in healthy individuals and is altered in MDD.
In healthy subjects, presentation of positive stimuli increases the activation of regions involved in reward processing, including the caudate, putamen, NA, basal forebrain, medial frontal region, ACC, inferior parietal area, right fusiform, and lingual gyrus.Citation83–Citation85 When asked to suppress a positive emotion, healthy subjects show activation of the right VLPFC.Citation86 These studies show that these regions are involved in the perception of positive and rewarding stimuli.
Several studies have shown that anhedonia alters the activation of these regions, as it is negatively correlated with the activation of the NA, basal forebrain, and the hypothalamusCitation51,Citation84,Citation87 and positively correlated with the activity in the VMPFC.Citation51 It has also been shown that subjects with higher anhedonia scores have a decreased NA volume and a decreased resting activity in the rostral sgACC.Citation87 These findings suggest that anhedonia may at least partly be due to the insufficient activation of neural circuits that regulate feelings of pleasure. We propose that continued low activity of these circuits, particularly in depressed individuals, would suggest chronic low hedonic tone, an increased likelihood of developing anhedonia, and an increased probability of TRD. Furthermore, when presented with positive stimuli, patients with MDD show attenuated activation of the ventral striatum, medial frontal cortex, and the NACitation83,Citation85,Citation88,Citation89 and increased activation in the inferior frontal cortex, sgACC, thalamus, putamen, and the insula.Citation85 As such, it has also been reported that depression is correlated with a difficulty in sustaining the connectivity between the NA and the DLPFC, as well as between the NA and the middle temporal gyrus,Citation88,Citation90 showing that depressive state is associated with an inability to activate regions responsible for feelings of reward.Citation90,Citation91
Further in support are data examining the CNS differences in activity of adult females with both ADHD and MDD in comparison with those with pure MDD.Citation92 Gardner et al reported increased severity of depressive symptoms in patients with MDD + ADHD (vs MDD) in association with significantly enhanced activity in bilateral frontal regions (Brodmann areas 8, 9, 10, 32) in the “Depression + ADHD” subgroup compared to those in “the Depression group”. They also reported significantly decreased activity within the bilateral cerebellum in the “Depression + ADHD” subgroup, perhaps suggesting that chronic depression can be subtyped by the presence of ADHD and perhaps an associated low hedonic tone.Citation92
Furthermore, studies have also shown that the dysfunction in the activity and connections of these circuits observed in depression can be regulated by antidepressant treatment. After treatment with fluoxetine or venlafaxine, depressed patients reported increases in positive affect; interestingly, those patients demonstrating the largest increases in positive affect also demonstrated the largest increases in frontal activity.Citation88 Furthermore, depressed patients who did not exhibit positive emotions also showed decreased PFC activity, which changed with improvement in anhedonia in response to treatment with fluoxetine and venlafaxine.Citation86 Thus, given the similarities between activation of these regions in response to positive experiences in healthy populations with anhedonia and in patients with MDD, it has been postulated that anhedonia, or hedonic tone, may be predictive of development of MDD. The results of these studies demonstrate that depressed patients show decreased activation in the VMPFC and NA and increased activation in the sgACC, compared to healthy controls. Normalization of activity in these regions to levels observed among healthy individuals is associated with improvement of anhedonia. Thus, it has been postulated that patients with low hedonic tone look for ways to raise their hedonic tone by manipulating the neurobiology of the mood system.Citation12 Studies published in the literature showing changes in the activation of different regions are summarized in .
Table 1 Summary of studies published in the literature showing changes in the activation of different regions
Symptoms of low hedonic tone
Hedonic tone, also referred to as hedonic capacity or hedonic responsiveness, is the trait or genetic predisposition underlying one’s baseline range and lifelong ability to feel pleasure. High hedonic tone is associated with an increased likelihood of experiencing happiness, whereas low hedonic tone manifests as an increased risk and therefore a general feeling of sadness.Citation12 Low hedonic tone is suggestive of a reduced lifetime capacity to experience euthymia and a lower likelihood of experiencing pleasure at any given time. Thus, this results in the increased likelihood of experiencing anhedonia, depression, and/or dysthymia.Citation6,Citation7 Individuals with low hedonic tone may be unable to anticipate expected reward, to ascertain the amount of effort required to attain the anticipated reward, and to engage in goal-directed behaviors required to attain a reward.Citation5 In addition, it has been proposed that those with a low hedonic tone require higher volumes of positive experiences to raise their natural hedonic tone to a more euthymic tone, which is often accomplished through substance abuse or risky behaviors.Citation12 This phenomenon is commonly seen in conditions of low hedonic tone and in particular in MDD and ADHD ().Citation9,Citation12 Thus, this likely explains the findings reported by Gardner et alCitation92 of differences in severity between those with MDD + ADHD versus pure MDD as well as differences in activity in the frontal regions and the cerebellum.
The assessment of hedonic tone is complicated by the lack of reliable methods for its evaluation. Currently used methods include the Snaith-Hamilton Pleasure Scale, the Dimensional Anhedonia Rating Scale, and the Scale for the Assessment of Negative Symptoms. Of these, the Dimensional Anhedonia Rating Scale has the best reliability and validity.Citation93 However, currently available assessments evaluate the current state of hedonic tone and do not take into account its long-term aspects. Therefore, development of novel assessment methods is required in order to properly assess the life-long trait of hedonic tone.
Antidepressant side effects and low hedonic tone
Dysfunction of the neural circuits that regulate reward processing and motivation has also been implicated in the poor effectiveness of SSRIs used to treat anxiety and depression in patients with low hedonic tone.Citation94 Patients undergoing SSRI treatment frequently report having low energy levels and emotional blunting,Citation95 and symptoms of low hedonic tone often persist during SSRI treatment even if other clinical symptoms have been alleviated.Citation96 For example, it has also been demonstrated that citalopram, a commonly used SSRI, diminishes the neural activation of the striatum to both aversive and rewarding stimuli, suggesting that efficacy of SSRIs may be blunted due to their effects on reward processing.Citation94 This blunting may be related to the inhibitory effects of serotonin on dopamine and noradrenaline through the activity of serotonin on the serotonin 2C and 2A receptors, respectively.Citation97 This may well explain the commonly understood side effect of SSRI-induced emotional numbingCitation98 or indifferenceCitation99 as well as what has been described as an amotivational frontal lobe syndrome, which is accompanied by apathy and lack of motivation, as well as in some cases behavioral disinhibition (all of which were symptoms that were dose related and reversible).Citation100
Thus, this would suggest a specific differential beneficial role for norephinephrine reuptake inhibitors, serotonin–norephinephrine reuptake inhibitors, and stimulants in patients who suffer with SSRI-induced flat affect to directly elevate the extracellular DA and NA in areas such as the VMPFC and DLPFC.Citation101–Citation104 Furthermore, this would suggest a unique sensitivity to this side effect in those already having pretreatment lower catecholaminergic modulation of the PFC and potential higher risk of low hedonic tone.Citation95
This relationship between lowered noradrenergic and dopaminergic activity and depression has been supported most recently by Harmer et alCitation105 who reported that acute administration of a catecholaminergic acting antidepressant could increase positive affective processing. The authors reported that depressed patients receiving placebo showed reduced, 1) recognition of positive facial expressions, 2) decreased speed in responding to positive self-relevant personality adjectives, and 3) reduced memory for this positive information in comparison with healthy volunteers receiving placebo.Citation105 They then reported that this effect was reversed in depressed patients who received a single dose of reboxetine, which as a noradrenergic reuptake inhibitor raises synaptic catecholaminergic levels.Citation105 Interestingly, these effects were noted prior to changes in subjective ratings of mood or anxiety. Thus, the authors concluded that catecholaminergic enhancing antidepressant drug administration modulates emotional processing in depressed patients very early in treatment.Citation105 In fact, this effect happens even before changes occur in mood symptoms, resulting in the amelioration of the negative biases in information processing that characterize the mood disorders.Citation105
Hedonic tone and mood disorders
Low hedonic tone is a characteristic feature of several psychopathologies, including MDD, substance use, and schizophrenia,Citation8,Citation9 although variations in hedonic tone can also be observed in healthy individuals.Citation10 All these disorders are characterized by changes in reward processing, and altered monoamine signaling has been implicated as their underlying mechanism. These observations raise the possibility that low hedonic tone can be used to predict subtypes of these disorders as well as their comorbidities. While the possibility that low hedonic tone may predict other subtypes of MDD cannot be eliminated, this review focuses on TRD, as well as depression comorbid with ADHD and substance abuse as representative MDD subtypes that share low hedonic tone as their feature.
Dysfunctional reward processing in MDD has been well characterized,Citation106 with depression having been shown to affect various aspects of reward processing, such as evaluating the value of rewards and motivation to obtain a reward. Pizzagalli et alCitation107 have shown that depressed patients have a reduced capacity to integrate reinforcement over time and to respond to more frequently rewarded cues. Similar observations were reported by studies in those with history of depression.Citation108,Citation109 Depressed patients are also less able to assess the value of the reward and exhibit a dissociation between perceived effort required to obtain a reward and reward value, suggesting that low hedonic tone in depression manifests as an impaired ability to modify behavior in response to a reward.Citation106 Taken together, these findings suggest that in depressed patients, low hedonic tone and anhedonia are associated with an altered ability to feel pleasure and a reduced motivation for goal-directed behaviors ().
Anhedonia has also been shown to be a predictor of TRD.Citation110,Citation111 In addition, DBS of the regions hypoactive in individuals with low hedonic tone, such as the NA,Citation112 the PFC,Citation113 and the sgACC,Citation78 has been shown to improve symptoms in TRD. These observations raise the possibility that hedonic tone can be used as a tool to predict treatment outcomes and select best treatments for MDD.
In ADHD, low hedonic tone is manifested as an altered sensitivity to reinforcement.Citation12 Children with ADHD have been reported to respond only to immediate rewards but not when the rewards are delayed and therefore only exhibit conditioning to immediate rewards.Citation114 It has also been reported that children with ADHD have an increased sensitivity to rewards and therefore suffer from a heightened frustration when the reward is not presented.Citation115 Yet another theory, proposed by Quay,Citation116,Citation117 is that children with ADHD are less responsive to punishment or nonreward. Although these studies report different manifestations of reduced hedonic tone in ADHD, the common finding is that ADHD is associated with altered feelings of pleasure. These behavioral features of ADHD are paralleled by changes in the neural pathways that regulate reward and motivation. A meta-analysis published by Dickstein et alCitation118 found significant hypoactivity in several frontal regions in individuals with ADHD compared with control subjects, specifically the orbitofrontal cortices, inferior prefrontal, dorsolateral prefrontal, and the anterior cingulate. In addition, hypoactivity was detected in related regions, such as areas of the thalamus, basal ganglia, and parietal cortices.Citation109 The meta-analysis also determined that some areas (eg, hypoactivated areas of the frontal and parietal regions) showed hyperactivation in individuals with ADHD. While this may suggest a compensatory mechanism, it has been postulated that this pattern may reflect “invasion” in areas where activation would be expected to diminish during a cognitive task.Citation119 Furthermore, highly correlated brain activity can be seen during rest and a reduction in activation throughout tasks that require attention. Others have suggested that the performance deficit in individuals with ADHD may be related to the persistence of spontaneous and low-frequency activation of the default-mode network, which compete with task-specific processing.Citation120
Alterations in the reward circuitry are also observed in substance abuse, and addictive substances are known to alter hedonic tone.Citation121 As dependence on a given substance develops, an increasing dose of that substance is needed to elicit the same pleasurable effect. For example, heroin addicts often report that after being addicted for some time, they no longer administer the drug to get high, but simply to “get straight”, meaning that the goal of the drug is to return their baseline hedonic tone back toward normal levels.Citation121 In addition, low hedonic tone is considered to be one of the risk factors for substance abuse, since many addictive substances, including prescription painkillers, work by raising the hedonic tone.Citation121 In adolescents, increased attentional processing of pleasant stimuli has been shown to increase the likelihood of alcohol use later in life.Citation122 Anhedonia is also positively correlated with drug cravings in detoxified alcohol- and opioid-dependent usersCitation123 and smokers.Citation124,Citation125 Positron emission tomography studies have shown that alcohol intake induces dopamine release in the ventral striatum of human subjects,Citation126,Citation127 suggesting that alcohol use alters the function on dopaminergic reward pathways. It has also been shown that reduced VLPFC activation, during shifting in the probabilistic reversal learning task, may be a marker of cocaine addiction and pathological gambling.Citation128 This finding further supports the notion that drug abuse is associated with alterations in reward processing, which underlie low hedonic tone.
In addition to sharing low hedonic tone as a key feature, MDD, ADHD, and substance abuse also share a dysfunction in monoamine signaling, particularly in the ventral striatum. Neurotransmitter studies have revealed abnormalities in DA and NE signaling in both MDD and ADHD, suggesting a potentially shared underlying pathophysiology, at least in some individuals.Citation129–Citation132 Imaging studies have reported decreased striatal activation in depressed patients.Citation106 More specifically, depressed patients display a stronger striatal–ACC connectivity in response to negative stimuli, but a weaker connectivity of the same pathway in response to positive stimuli.Citation133 Support for the dopaminergic theory of ADHD, which states that ADHD results from DA deficits and therefore lowered activity in the frontal cortex and striatum,Citation134 comes from a variety of pharmacological and imaging studies that have revealed hypoperfusion and lowered activations in those areas.Citation135–Citation138 Interestingly, with treatment with methylphenidate, normalization of the hypoperfusion of prefrontal areas is associated with the corresponding improvement in ADHD symptoms.Citation135,Citation139–Citation142
Furthermore, the ability of methylphenidate to elevate DA levels in the ventral striatum is attenuated in alcoholics, suggesting that DA plays a role in the alterations in reward pathways in alcoholism.Citation143 It has also been shown that the severity of anhedonia is negatively correlated with the ventral striatal activity and volume.Citation11,Citation51,Citation95,Citation144 Observations that dysfunction of this circuitry is also shared by MDD, ADHD, and substance abuse further support the idea that hedonic tone is the common link between MDD, ADHD, and depression.
Given that MDD, ADHD, and substance abuse share the characteristic of low hedonic tone and underlying neurobiology, it is not surprising that these disorders also have high levels of comorbidities. For example, most individuals with depression experience psychiatric comorbidity, according to the National Comorbidity Survey Replication.Citation145,Citation146 It has been reported that depression occurs in 9%–50% of patients with ADHD,Citation147 and STAR*D study has reported that approximately a third of patients with MDD also have a substance use disorder. When MDD is comorbid with ADHD, it is associated with a much more severe course, an earlier age of illness onset, more complex psychiatric comorbidities, decreased quality of life, greater burden of illness, greater illness complexity, lower social functioning, lower work productivity, and lower employment rates than patients with MDD alone.Citation148,Citation149 As well, individuals with MDD and ADHD experience higher levels of substance use and psychosocial impairment than each disorder in isolation,Citation150–Citation153 suggesting a potential variant of MDD, and therefore explaining the treatment resistance in this population when treated with traditional SSRIs. Among respondents with ADHD, there is significant comorbidity with a wide range of Diagnostic and Statistical Manual of Mental Disorders, fourth edition disorders, with odds ratios of 2.7–7.5 for mood disorders, 1.5–5.5 for anxiety disorders, 1.5–7.9 for substance use disorders, and 3.7 for intermittent explosive disorder.Citation145 Sternat et al reported that in patients referred to a tertiary care center, 32.5% of TRD met criteria for ADHD.Citation154 Furthermore, the best predictors of comorbid ADHD with TRD were the number of SSRIs and less specifically the number of antidepressants that the patient had failed.
Thus, it has been proposed that low hedonic tone is a predictor of comorbidities of various mood disorders.Citation12 Neuroimaging studies have identified morphological and functional features common to both MDD and ADHD, including decreased brain volumes and altered activity of frontal lobe structures (and specifically the DLPFC and ACC), areas that are associated with attention regulation, behavior selection, and emotionCitation155,Citation156 and significantly worsened outcome. In ADHD, other than hyperactivity and inattention, impulsivity is one of the most prevalent diagnostic symptoms. Impulse control has been associated with prefrontal functioning, specifically in the DLPFC, VMPFC, VLPFC, inferior frontal gyrus, the rostral and dorsal ACC, and the insula.Citation157–Citation159 Dysregulation in these regions is associated with the impulsivity observed in patients with ADHD and overlaps with the dysregulation observed among depressed individuals, as well as individuals with substance abuse disorders. Interestingly, altered activity of these regions is also observed in people with low hedonic tone, suggesting that low hedonic tone may be a behavioral manifestation of dysregulation of these circuits and consequent mood disorders. Thus, these patient populations share low hedonic tone as a common endophenotype, which may lead to dysthymia and double depression. Furthermore, studies investigating the role of monoamines in these disorders point to a key role for DA dysfunction in the pathophysiology of ADHD and its comorbidities, including MDD. With evidence pointing to dysfunctional neuromodulation of the DLPFC in relation to executive dysfunction in ADHD,Citation160–Citation164 it is not too much of a stretch to consider low hedonic tone as an affective correlate in ADHD explaining the lack of responsiveness to SSRIs in depression. Low dopaminergic activity has been reported in patients with MDD and ADHD, as well as those with low hedonic tone.Citation134,Citation165 Furthermore, SSRI treatment is commonly associated with symptoms of low hedonic tone, even in the absence of other MDD symptoms.Citation67,Citation96 Taken together, these observations suggest that low hedonic tone results from reduced baseline catecholaminergic activity in the regions involved in reward processing and executive functions, which may also be a predictive factor of resistance to SSRI treatment as well as a predictor of comorbidity of various disorders. In support of this suggestion, it has been reported in both the STAR*D and GENDEP studies that the percent reduction on the primary outcome scale over 12 weeks of treatment was best predicted by loss of interest and diminished activity (perhaps proxy measures for low hedonic tone) even after adjustment for overall depression severity and other clinical covariates such as age, sex, and center differences.Citation166
Conclusion
Low hedonic tone is characterized by a dysfunction of neural circuits that regulate motivation and reward processing and is a common feature of MDD, ADHD, and substance abuse. Anhedonic, depressed patients consistently show decreased prefrontal activity and increased activity in the VMPFC and sgACC. These patients with low hedonic tone attempt to cope with their depression and enter into a euthymic state by raising their hedonic tone, thus achieving a shift toward a more neutral hedonic tone, and therefore a rise in their mood. In the absence of antidepressant therapy, or in TRD where altered activity in these regions cannot be normalized with pharmacologic therapy, patients will often choose ways to self-medicate, often through substance abuse, in order to modify the neurobiology of the mood system to correct their chronic low hedonic tone. Similarly, patients with ADHD share a similar functional dysregulation in the frontal cortex and similar abnormalities in DA and NE signaling. Perhaps, this explains the high comorbidity between MDD and ADHD. If one imagines then that the presence of anomalous activity of the PFC and the cingulate gyrus is a function of a chronic disturbance in catecholaminergic innervation (and specifically lower dopaminergic and noradrenergic tone),Citation167,Citation168 then activities that raise dopaminergic and noradrenergic tone, such as substance abuse, would be favored by patients suffering with this neurobiology.
Since low hedonic tone is a shared feature of these disorders, evaluation of hedonic tone may become a diagnostic feature used to predict subtypes of MDD, such as TRD, as well as comorbidities of MDD with ADHD and substance abuse.
Acknowledgments
The authors would like to thank Tanja Babic for her assistance in the preparation of this paper, specifically contributions to fact finding, writing, and editing in association with the development of this manuscript.
Disclosure
Tia Sternat reports no conflicts of interest in this work. Martin A Katzman has received grant support, participated in advisory boards, and/or received honorarium for giving lectures from the following: Canadian Foundation for Innovation, Lotte & John Hecht Memorial Foundation, Allergan, Astra Zeneca, Bedrocan, Biotics, Bristol-Myers Squibb, Eli Lilly, Genuine Health, Janssen, Lundbeck, Merck, Pfizer, Purdue, Shire, and Tweed.
References
- EkkekakisPThe Measurement of Affect, Mood, and Emotion: A Guide for Health-Behavioral ResearchCambridge, UK, New YorkCambridge University Press2013
- RusselJEmotion, core affect, and psychological constructionCogn Emotion200923712591283
- GorwoodPNeurobiological mechanisms of anhedoniaDialogues Clin Neurosci200810329129918979942
- SteinDJDepression, anhedonia, and psychomotor symptoms: the role of dopaminergic neurocircuitryCNS Spectr200813756156518622360
- Der-AvakianAMarkouAThe neurobiology of anhedonia and other reward-related deficitsTrends Neurosci2012351687722177980
- Di NicolaMDe RisioLBattagliaCReduced hedonic capacity in euthymic bipolar subjects: a trait-like feature?J Affect Disord20121471–344645023122985
- LoasGVulnerability to depression: a model centered on anhedoniaJ Affect Disord199641139538938204
- SnaithRPIdentifying depression: the significance of anhedoniaHosp Pract (Off Ed)1993289A55608408359
- SnaithRPHamiltonMMorleySHumayanAHargreavesDTrigwellPA scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure ScaleBr J Psychiatry19951671991037551619
- JankowskiKSCiarkowskaWDiurnal variation in energetic arousal, tense arousal, and hedonic tone in extreme morning and evening typesChronobiol Int200825457759518622817
- KeedwellPAAndrewCWilliamsSCBrammerMJPhillipsMLThe neural correlates of anhedonia in major depressive disorderBiol Psychiatry2005581184385316043128
- SternatTLodzinskiAKatzmanMAHedonic tone: a bridge between the psychobiology of depression and its comorbiditiesJ Depress Anxiety201431
- BertonOHahnCGThaseMEAre we getting closer to valid translational models for major depression?Science20123386103757923042886
- NestlerEJCarlezonWAJrThe mesolimbic dopamine reward circuit in depressionBiol Psychiatry200659121151115916566899
- BevinsRABesheerJNovelty reward as a measure of anhedoniaNeurosci Biobehav Rev2005294–570771415876456
- KnutsonBFongGWAdamsCMVarnerJLHommerDDissociation of reward anticipation and outcome with event-related fMRINeuroreport200112173683368711726774
- LammelSTyeKMWardenMRProgress in understanding mood disorders: optogenetic dissection of neural circuitsGenes Brain Behav2013131385123682971
- OngurDFerryATPriceJLArchitectonic subdivision of the human orbital and medial prefrontal cortexJ Comp Neurol2003460342544912692859
- DrevetsWCPriceJLFureyMLBrain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depressionBrain Struct Funct20082131–29311818704495
- GrimmSBeckJSchuepbachDImbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorderBiol Psychiatry200863436937617888408
- TekinSCummingsJLFrontal-subcortical neuronal circuits and clinical neuropsychiatry: an updateJ Psychosom Res200253264765412169339
- KoenigsMGrafmanJThe functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortexBehav Brain Res2009201223924319428640
- CarmichaelSTPriceJLConnectional networks within the orbital and medial prefrontal cortex of macaque monkeysJ Comp Neurol199637121792078835726
- OngurDPriceJLThe organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humansCereb Cortex200010320621910731217
- AnXBandlerROngurDPriceJLPrefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeysJ Comp Neurol199840144554799826273
- OngurDAnXPriceJLPrefrontal cortical projections to the hypothalamus in macaque monkeysJ Comp Neurol199840144805059826274
- SchultzWTremblayLHollermanJRReward prediction in primate basal ganglia and frontal cortexNeuropharmacology1998374–54214299704983
- SebastianAJungPKrause-UtzALiebKSchmahlCTuscherOFrontal dysfunctions of impulse control – a systematic review in borderline personality disorder and attention-deficit/hyperactivity disorderFront Hum Neurosci2014869825232313
- KayserASAllenDCNavarro-CebrianAMitchellJMFieldsHLDopamine, corticostriatal connectivity, and intertemporal choiceJ Neurosci201232279402940922764248
- PattersonJC2ndUngerleiderLGBandettiniPATask-independent functional brain activity correlation with skin conductance changes: an fMRI studyNeuroimage20021741797180612498753
- MaybergHSLozanoAMVoonVDeep brain stimulation for treatment-resistant depressionNeuron200545565166015748841
- CritchleyHDElliottRMathiasCJDolanRJNeural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging studyJ Neurosci20002083033304010751455
- KimJJJungMWNeural circuits and mechanisms involved in Pavlovian fear conditioning: a critical reviewNeurosci Biobehav Rev200630218820216120461
- PhelpsEALeDouxJEContributions of the amygdala to emotion processing: from animal models to human behaviorNeuron200548217518716242399
- PhelpsEADelgadoMRNearingKILeDouxJEExtinction learning in humans: role of the amygdala and vmPFCNeuron200443689790515363399
- JanakPHTyeKMFrom circuits to behaviour in the amygdalaNature2015517753428429225592533
- SwartzJRWilliamsonDEHaririARDevelopmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life eventsAm J Psychiatry2015172327628325526599
- DavisMWhalenPJThe amygdala: vigilance and emotionMol Psychiatry200161133411244481
- MarenSPhanKLLiberzonIThe contextual brain: implications for fear conditioning, extinction and psychopathologyNat Rev Neurosci201314641742823635870
- MiladMRQuirkGJNeurons in medial prefrontal cortex signal memory for fear extinctionNature20024206911707412422216
- GormanJMKentJMSullivanGMCoplanJDNeuroanatomical hypothesis of panic disorder, revisedAm J Psychiatry2000157449350510739407
- CarlsonJMGreenbergTRubinDMujica-ParodiLRFeeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipationSoc Cogn Affect Neurosci201161748120207692
- SimmonsAMatthewsSCSteinMBPaulusMPAnticipation of emotionally aversive visual stimuli activates right insulaNeuroreport200415142261226515371746
- StarrCJSawakiLWittenbergGFRoles of the insular cortex in the modulation of pain: insights from brain lesionsJ Neurosci20092992684269419261863
- GuXHofPRFristonKJFanJAnterior insular cortex and emotional awarenessJ Comp Neurol2013521153371338823749500
- FanselowMSDongHWAre the dorsal and ventral hippocampus functionally distinct structures?Neuron201065171920152109
- HenkePGHippocampal pathway to the amygdala and stress ulcer developmentBrain Res Bull19902556916952289157
- KheirbekMAHenRDorsal vs ventral hippocampal neurogenesis: implications for cognition and moodNeuropsychopharmacology201136137337421116266
- RingHASerra-MestresJNeuropsychiatry of the basal gangliaJ Neurol Neurosurg Psychiatry2002721122111784818
- WangYDengYFungGDistinct structural neural patterns of trait physical and social anhedonia: evidence from cortical thickness, subcortical volumes and inter-regional correlationsPsychiatry Res2014224318419125288478
- HarveyPOPruessnerJCzechowskaYLepageMIndividual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjectsMol Psychiatry200712870376777517505465
- DelgadoMRLockeHMStengerVAFiezJADorsal striatum responses to reward and punishment: effects of valence and magnitude manipulationsCogn Affect Behav Neurosci200331273812822596
- GrahnJAParkinsonJAOwenAMThe cognitive functions of the caudate nucleusProg Neurobiol200886314115518824075
- CarlsonPJSinghJBZarateCAJrDrevetsWCManjiHKNeural circuitry and neuroplasticity in mood disorders: insights for novel therapeutic targetsNeuroRx200631224116490411
- Roberts-WolfeDJKalivasPWGlutamate transporter GLT-1 as a therapeutic target for substance use disordersCNS Neurol Disord Drug Targets201514674575626022265
- BrambillaPPerezJBaraleFSchettiniGSoaresJCGABAergic dysfunction in mood disordersMol Psychiatry20038872173771512888801
- HayesDJJuppBSawiakSJMerloECaprioliDDalleyJWBrain gamma-aminobutyric acid: a neglected role in impulsivityEur J Neurosci201439111921193224460847
- LupicaCRRiegelACHoffmanAFMarijuana and cannabinoid regulation of brain reward circuitsBr J Pharmacol2004143222723415313883
- ManzoniOJWilliamsJTPresynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawalJ Neurosci199919156629663610414991
- TermanGWDrakeCTSimmonsMLMilnerTAChavkinCOpioid modulation of recurrent excitation in the hippocampal dentate gyrusJ Neurosci200020124379438810844006
- PehrsonALSanchezCSerotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunctionCNS Spectr201419212113323903233
- KrishnanVNestlerEJThe molecular neurobiology of depressionNature2008455721589490218923511
- GlennonRADukatMWestkaemperRBSerotonin Receptor Subtypes and LigandsAmerican College of Neuropsychopharmacology2000 Available from: http://www.acnp.org/g4/GN401000039/Ch039.htmlAccessed August 2, 2016
- LuckiISerotonin receptor specificity in anxiety disordersJ Clin Psychiatry199657suppl 65108647798
- NestlerEJHymanSEMalenkaRCMolecular Neuropharmacology: A Foundation for Clinical Neuroscience2nd edNew YorkMcGraw-Hill Medical2009
- StahlSMModes and nodes explain the mechanism of action of vortioxetine, a multimodal agent (MMA): modifying serotonin’s downstream effects on glutamate and GABA (gamma amino butyric acid) releaseCNS Spectr201520433133626062900
- PriceJLDrevetsWCNeural circuits underlying the pathophysiology of mood disordersTrends Cogn Sci2012161617122197477
- IonescuDFRosenbaumJFAlpertJEPharmacological approaches to the challenge of treatment-resistant depressionDialogues Clin Neurosci201517211112626246787
- DrevetsWCFureyMLReplication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trialBiol Psychiatry201067543243820074703
- EhrichETurncliffRDuYEvaluation of opioid modulation in major depressive disorderNeuropsychopharmacology20154061448145525518754
- LallyNNugentACLuckenbaughDAAmeliRRoiserJPZarateCAAnti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depressionTransl Psychiatry20144e46925313512
- FureyMLDrevetsWCSzczepanikJKhannaANugentAZarateCAJrPretreatment differences in BOLD response to emotional faces correlate with antidepressant response to scopolamineInt J Neuropsychopharmacol2015188110
- HuhnASMeyerREHarrisJDEvidence of anhedonia and differential reward processing in prefrontal cortex among post-withdrawal patients with prescription opiate dependenceBrain Res Bull201612310210926711857
- GreiciusMDFloresBHMenonVResting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamusBiol Psychiatry200762542943717210143
- KennedySHEvansKRKrügerSChanges in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depressionAm J Psychiatry2001158689990511384897
- LiottiMMaybergHSBrannanSKMcGinnisSJerabekPFoxPTDifferential limbic – cortical correlates of sadness and anxiety in healthy subjects: implications for affective disordersBiol Psychiatry2000481304210913505
- KeedwellPADrapierDSurguladzeSGiampietroVBrammerMPhillipsMSubgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depressionJ Affect Disord20101201–312012519539998
- MaybergHSLiottiMBrannanSKReciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadnessAm J Psychiatry1999156567568210327898
- OldaniLDell’OssoBAltamuraACLong-term effects of vagus nerve stimulation in treatment-resistant depression: a 5-year follow up case seriesBrain Stimulat20158612291230
- YuanTFLiASunXArias-CarriónOMachadoSVagus nerve stimulation in treating depression: a tale of two storiesCurr Mol Med2016161333926695696
- NahasZTenebackCChaeJHSerial vagus nerve stimulation functional MRI in treatment-resistant depressionNeuropsychopharmacology20073281649166017203016
- ZobelAJoeAFreymannNChanges in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: an exploratory approachPsychiatry Res2005139316517916043331
- EpsteinJPanHKocsisJHLack of ventral striatal response to positive stimuli in depressed versus normal subjectsAm J Psychiatry2006163101784179017012690
- KellerJYoungCBKelleyEPraterKLevitinDJMenonVTrait anhedonia is associated with reduced reactivity and connectivity of mesolimbic and paralimbic reward pathwaysJ Psychiatr Res201347101319132823791396
- MitterschiffthalerMTKumariVMalhiGSNeural response to pleasant stimuli in anhedonia: an fMRI studyNeuroreport200314217718212598724
- LightSNHellerASJohnstoneTReduced right ventrolateral prefrontal cortex activity while inhibiting positive affect is associated with improvement in hedonic capacity after 8 weeks of antidepressant treatment in major depressive disorderBiol Psychiatry2011701096296821867991
- WackerJDillonDGPizzagalliDAThe role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniquesNeuroimage200946132733719457367
- HellerASJohnstoneTLightSNRelationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatmentAm J Psychiatry2013170219720623223803
- PizzagalliDAHolmesAJDillonDGReduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorderAm J Psychiatry2009166670271019411368
- GabbayVElyBALiQStriatum-based circuitry of adolescent depression and anhedoniaJ Am Acad Child Adolesc Psychiatry2013526628641.e1323702452
- HellerASJohnstoneTShackmanAJReduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activationProc Natl Acad Sci U S A200910652224452245020080793
- GardnerASalmasoDVarroneADifferences at brain SPECT between depressed females with and without adult ADHD and healthy controls: etiological considerationsBehav Brain Funct200953719723308
- RizviSJQuiltyLCSprouleBACyriacAMichael BagbyRKennedySHDevelopment and validation of the Dimensional Anhedonia Rating Scale (DARS) in a community sample and individuals with major depressionPsychiatry Res20152291–210911926250147
- McCabeCMishorZCowenPJHarmerCJDiminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatmentBiol Psychiatry201067543944520034615
- PriceJColeVGoodwinGMEmotional side-effects of selective serotonin reuptake inhibitors: qualitative studyBr J Psychiatry2009195321121719721109
- HaslerGDrevetsWCManjiHKCharneyDSDiscovering endophenotypes for major depressionNeuropsychopharmacology200429101765178115213704
- TrivediMHHollanderENuttDBlierPClinical evidence and potential neurobiological underpinnings of unresolved symptoms of depressionJ Clin Psychiatry200869224625818363453
- ArnstenAFRubiaKNeurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disordersJ Am Acad Child Adolesc Psychiatry201251435636722449642
- SansoneRASansoneLASSRI-induced IndifferencePsychiatry (Edgmont)20107101418
- GarlandEJBaergEAAmotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescentsJ Child Adolesc Psychopharmacol200111218118611436958
- CarboniETandaGLFrauRDi ChiaraGBlockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminalsJ Neurochem1990553106710702117046
- TandaGCarboniEFrauRDi ChiaraGIncrease of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential?Psychopharmacology (Berl)19941151–22852887862908
- MillanMJNewman-TancrediAAudinotVAgonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive statesSynapse2000352799510611634
- LinnerLEnderszHOhmanDBengtssonFSchallingMSvenssonTHReboxetine modulates the firing pattern of dopamine cells in the ventral tegmental area and selectively increases dopamine availability in the prefrontal cortexJ Pharmacol Exp Ther2001297254054611303041
- HarmerCJO’SullivanUFavaronEEffect of acute antidepressant administration on negative affective bias in depressed patientsAm J Psychiatry2009166101178118419755572
- AdmonRPizzagalliDADysfunctional reward processing in depressionCurr Opin Psychol2015411411826258159
- PizzagalliDAIosifescuDHallettLARatnerKGFavaMReduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward taskJ Psychiatr Res2008431768718433774
- PechtelPDutraSJGoetzELPizzagalliDABlunted reward responsiveness in remitted depressionJ Psychiatr Res201347121864186924064208
- PizzagalliDAJahnALO’SheaJPToward an objective characterization of an anhedonic phenotype: a signal-detection approachBiol Psychiatry200557431932715705346
- McMakinDLOlinoTMPortaGAnhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depressionJ Am Acad Child Adolesc Psychiatry201251440441122449646
- RubinDHJoy returns last: anhedonia and treatment resistance in depressed adolescentsJ Am Acad Child Adolesc Psychiatry201251435335522449641
- EggersAETreatment of depression with deep brain stimulation works by altering in specific ways the conscious perception of the core symptoms of sadness or anhedonia, not by modulating network circuitryMed Hypotheses2014831626424767178
- ReaERummelJSchmidtTTAnti-anhedonic effect of deep brain stimulation of the prefrontal cortex and the dopaminergic reward system in a genetic rat model of depression: an intracranial self-stimulation paradigm studyBrain Stimulat2014712128
- SagvoldenTAaseHZeinerPBergerDAltered reinforcement mechanisms in attention-deficit/hyperactivity disorderBehav Brain Res199894161719708840
- LumanMOosterlaanJSergeantJAThe impact of reinforcement contingencies on AD/HD: a review and theoretical appraisalClin Psychol Rev200525218321315642646
- QuayHCTheories of ADDHJ Am Acad Child Adolesc Psychiatry19882722622633360739
- QuayHCInhibition and attention deficit hyperactivity disorderJ Abnorm Child Psychol19972517139093895
- DicksteinSGBannonKCastellanosFXMilhamMPThe neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysisJ Child Psychol Psychiatry200647101051106217073984
- RaichleMEMacLeodAMSnyderAZPowersWJGusnardDAShulmanGLA default mode of brain functionProc Natl Acad Sci U S A200198267668211209064
- Sonuga-BarkeEJCastellanosFXSpontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesisNeurosci Biobehav Rev200731797798617445893
- GardnerELAddiction and brain reward and antireward pathwaysAdv Psychosom Med201130226021508625
- GarfieldJBAllenNBCheethamASimmonsJGLubmanDIAttention to pleasant stimuli in early adolescence predicts alcohol-related problems in mid-adolescenceBiol Psychol2015108435025818044
- MartinottiGCloningerCRJaniriLTemperament and character inventory dimensions and anhedonia in detoxified substance-dependent subjectsAm J Drug Alcohol Abuse200834217718318293234
- CookJWSpringBMcChargueDHedekerDHedonic capacity, cigarette craving, and diminished positive moodNicotine Tob Res200461394714982686
- LeventhalAMWatersAJKahlerCWRayLASussmanSRelations between anhedonia and smoking motivationNicotine Tob Res20091191047105419571250
- AaltoSIngmanKAlakurttiKIntravenous ethanol increases dopamine release in the ventral striatum in humans: PET study using bolus-plus-infusion administration of [(11)C]racloprideJ Cereb Blood Flow Metab201535342443125492110
- BoileauIAssaadJMPihlROAlcohol promotes dopamine release in the human nucleus accumbensSynapse200349422623112827641
- Verdejo-GarciaAClarkLVerdejo-RomanJNeural substrates of cognitive flexibility in cocaine and gambling addictionsBr J Psychiatry2015207215816426045346
- DunlopBWNemeroffCBThe role of dopamine in the pathophysiology of depressionArch Gen Psychiatry200764332733717339521
- Garnock-JonesKPKeatingGMSpotlight on atomoxetine in attention-deficit hyperactivity disorder in children and adolescentsCNS Drugs2010241858820030421
- NiggJTCaseyBJAn integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciencesDev Psychopathol200517378580616262992
- SergeantJAGeurtsHHuijbregtsSScheresAOosterlaanJThe top and the bottom of ADHD: a neuropsychological perspectiveNeurosci Biobehav Rev200327758359214624803
- AdmonRNickersonLDDillonDGDissociable cortico-striatal connectivity abnormalities in major depression in response to monetary gains and penaltiesPsychol Med201545112113125055809
- LevyFThe dopamine theory of attention deficit hyperactivity disorder (ADHD)Aust N Z J Psychiatry19912522772831652243
- KimBNLeeJSChoSCLeeDSMethylphenidate increased regional cerebral blood flow in subjects with attention deficit/hyperactivity disorderYonsei Med J2001421192911293498
- KimBNLeeJSShinMSChoSCLeeDSRegional cerebral perfusion abnormalities in attention deficit/hyperactivity disorder. Statistical parametric mapping analysisEur Arch Psychiatry Clin Neurosci2002252521922512451463
- LouHCHenriksenLBruhnPFocal cerebral hypoperfusion in children with dysphasia and/or attention deficit disorderArch Neurol19844188258296331818
- LouHCHenriksenLBruhnPBørnerHNielsenJBStriatal dysfunction in attention deficit and hyperkinetic disorderArch Neurol198946148522783366
- LeeJSKimBNKangERegional cerebral blood flow in children with attention deficit hyperactivity disorder: comparison before and after methylphenidate treatmentHum Brain Mapp200524315716415486990
- MatochikJALiebenauerLLKingACSzymanskiHVCohenRMZametkinAJCerebral glucose metabolism in adults with attention deficit hyperactivity disorder after chronic stimulant treatmentAm J Psychiatry199415156586648166305
- MatochikJANordahlTEGrossMEffects of acute stimulant medication on cerebral metabolism in adults with hyperactivityNeuropsychopharmacology1993843773868512624
- VaidyaCJAustinGKirkorianGSelective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance studyProc Natl Acad Sci U S A1998952414494144999826728
- VolkowNDWangGJTelangFProfound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvementJ Neurosci20072746127001270618003850
- DowdECBarchDMAnhedonia and emotional experience in schizophrenia: neural and behavioral indicatorsBiol Psychiatry2010671090291120004364
- KesslerRCAdlerLBarkleyRThe prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey ReplicationAm J Psychiatry2006163471672316585449
- KesslerRCBerglundPDemlerOThe epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R)JAMA2003289233095310512813115
- McIntoshDKutcherSBinderCLevittAFalluARosenbluthMAdult ADHD and comorbid depression: a consensus-derived diagnostic algorithm for ADHDNeuropsychiatr Dis Treat2009513715019557108
- BondDJHadjipavlouGLamRWThe Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid attention-deficit/hyperactivity disorderAnn Clin Psychiatry2012241233722303520
- McIntyreRSKennedySHSoczynskaJKAttention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the international mood disorders collaborative projectPrim Care Companion J Clin Psychiatry2010123 PCC.09m00861
- BiedermanJBallSWMonuteauxMCNew insights into the comorbidity between ADHD and major depression in adolescent and young adult femalesJ Am Acad Child Adolesc Psychiatry200847442643418388760
- BiedermanJMickEFaraoneSVDepression in attention deficit hyperactivity disorder (ADHD) children: “true” depression or demoralization?J Affect Disord1998471–31131229476751
- Chronis-TuscanoAMolinaBSPelhamWEVery early predictors of adolescent depression and suicide attempts in children with attention-deficit/hyperactivity disorderArch Gen Psychiatry201067101044105120921120
- WardenDRiggsPDMinSJMajor depression and treatment response in adolescents with ADHD and substance use disorderDrug Alcohol Depend20121201–321421921885210
- SternatTMohamedMFurtadoMAttention deficit hyperactivity disorder and depression: Sequential and concurrent disordersPoster presented at: Annual Meeting of the American Society of Clinical PsychopharmacologyJune 1; 2016Phoenix, AZ
- KonarskiJZMcIntyreRSKennedySHRafi-TariSSoczynskaJKKetterTAVolumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorderBipolar Disord200810113718199239
- SeidmanLJValeraEMMakrisNDorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imagingBiol Psychiatry200660101071108016876137
- AlvarezJAEmoryEExecutive function and the frontal lobes: a meta-analytic reviewNeuropsychol Rev2006161174216794878
- AronARRobbinsTWPoldrackRAInhibition and the right inferior frontal cortex: one decade onTrends Cogn Sci201418417718524440116
- LairdARMcMillanKMLancasterJLA comparison of label-based review and ALE meta-analysis in the Stroop taskHum Brain Mapp200525162115846823
- CunhaPJGonçalvesPDOmettoMExecutive cognitive dysfunction and ADHD in cocaine dependence: searching for a common cognitive endophenotype for addictive disordersFront Psychiatry2013412624155725
- FuermaierABTuchaLKoertsJComplex prospective memory in adults with attention deficit hyperactivity disorderPLoS One201383e5833823484020
- MadhooMKeefeRSRothRMLisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorderNeuropsychopharmacology20133961388139824309905
- MissonnierPHaslerRPerroudNEEG anomalies in adult ADHD subjects performing a working memory taskNeuroscience201324113514623518223
- QianYShuaiLChanRCQianQJWangYThe developmental trajectories of executive function of children and adolescents with attention deficit hyperactivity disorderRes Dev Disabil20133451434144523474996
- BooijLVan der DoesAJHaffmansPMRiedelWJFekkesDBlomMJThe effects of high-dose and low-dose tryptophan depletion on mood and cognitive functions of remitted depressed patientsJ Psychopharmacol200519326727515888512
- UherRPerlisRHHenigsbergNDepression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptomsPsychol Med201242596798021929846
- RajkowskaGHistopathology of the prefrontal cortex in major depression: what does it tell us about dysfunctional monoaminergic circuits?Prog Brain Res200012639741211105659
- HarrisonPJThe neuropathology of primary mood disorderBrain2002125pt 71428144912076995
