Abstract
Background
Radix Puerariae and hawthorn fruit have been demonstrated to treat diabetes. They offer potential benefits for preventing depression in diabetes.
Objective
The aim of this study was to investigate whether the combination of Radix Puerariae and hawthorn fruit (CRPHF) could prevent depression in a diabetic rat model generated by feeding the rats with a high-fat diet and a low-dose streptozotocin (STZ).
Methods
The CRPHF was provided by the Shanghai Chinese Traditional Medical University. Twenty-four rats were randomly divided into four groups: normal control, normal-given-CRPHF (NC), diabetic control, and diabetic-given-CRPHF (DC) groups. The type 2 diabetic model was created by feeding the rats with a high-fat diet for 4 weeks followed by injection of 25 mg/kg STZ. CRPHF was given at 2 g/kg/d to the rats of NC and DC groups by intragastric gavage daily for 4 weeks after the type 2 diabetic model was successfully created. Body weight, random blood glucose (RBG), oral glucose tolerance test, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured during the study. Depressive-like behavior was evaluated at the end of the treatment by using the open field test (OFT), the elevated plus-maze test (EPMT), locomotor activity test (LAT), and forced swimming test (FST). Levels of extracellular signal-regulated protein kinase (ERK) and brain-derived neurotrophic factor (BDNF) in the prefrontal cortex were evaluated by using Western blot.
Results
1) CRPHF reduced RBG and improved glucose tolerance in diabetic rats; 2) CRPHF reduced TC and TG but did not significantly change HDL-C or LDL-C in diabetic rats; 3) CRPHF reversed the loss in body weights observed in diabetic rats; 4) CRPHF reduced depressive-like behavior as measured by OFT, EPMT, LAT, and FST; 5) BDNF was upregulated, and ERK was activated in the prefrontal cortex of diabetic rats treated with CRPHF.
Conclusion
CRPHF has the potential of preventing depression in patients with diabetes.
Introduction
Diabetes mellitus (DM) and depression are both chronic and potentially life-threatening illnesses with a high prevalence in the modern era. The International Diabetes Federation reported that 382 million people suffered from DM worldwide in 2013, and this number would exceed 592 million in <25 years (Federation 2013). Similarly, in 2014, the World Health Organization estimated that depression affects >350 million people globally. Previous studies demonstrated that there is a bidirectional association between depression and type 2 diabetes. Depression is a major risk factor in the development of type 2 diabetes,Citation1,Citation2 and patients with diabetes show about twice the prevalence of depression compared with the general population.Citation3 It is reported that the presence of diabetic complications, low monthly family income, negative life event, and poor social support were the statistically significant risk factors associated with depression in diabetic patients.Citation4,Citation5 However, the pathophysiological mechanism remains unclear. Further research is required to identify the physiological and pathological relations between diabetes and depression.
As depression is a common comorbidity characterized by suicidal tendency and recurrence of morbidity among patients with type 2 diabetes, there is a requirement to find effective drugs to prevent the onset of depression among people with type 2 diabetes. Previous studies have shown the therapeutic effects of some Traditional Chinese Medicine in the treatment of depression.Citation6,Citation7 The antidepressant effect of the ethanol extract of Radix Puerariae has been reported in mice exposed to cerebral ischemia reperfusionCitation6 and in C57BL/6 N mice with spared nerve injury,Citation8 and the hawthorn fruit is a promising remedy in the treatment of anxietyCitation9 and has also been reported to reduce depressive-like behaviors in mice with chronic mild stress.Citation7 Furthermore, a possible mechanism of reducing depression is increasing brain-derived neurotrophic factor (BDNF) levels.Citation10 However, the effect of Radix Puerariae and hawthorn fruit on depression associated with type 2 diabetes remains unknown. In the present study, the antidepressant effect of ethanol extract of Radix Puerariae and hawthorn fruit on a rat model with diabetes was assessed, and by using open field test (OFT), elevated plus-maze test (EPMT), locomotor activity test (LAT), and the forced swimming test (FST), it was observed that the combination markedly restored the body weight and improved the performances of rats with type 2 diabetes, indicating a possible antidepressant activity. The levels of extracellular signal-regulated protein kinases (ERKs) and BDNF in the prefrontal cortex of rats were also assessed in order to obtain further insight into the mechanism of the antidepressant action of the combination of Radix Puerariae and hawthorn fruit (CRPHF).
Materials and methods
Drugs and reagents
The extracts of Radix Puerariae and hawthorn fruit were provided by the Shanghai Chinese Traditional Medical University. These extracts were separated by using the following process: 1 kg of each extract was reflux-extracted twice with 60% alcohol; the amount of solvent used was 5 L, and the extraction time was 90 min for each extraction.Citation11 The extract was evaporated at reduced pressure until the volume became 590 mL. The Radix Puerariae and hawthorn fruit solutions were mixed at a volume ratio of 1:1 and used for treating the rats in this study.
Animals and experimental design
A total of 24 male Sprague Dawley rats, aged 6–8 weeks (200–250 g), were obtained from the Experimental Animal Center of Zhejiang Province (Zhejiang, People’s Republic of China). Rats were given free access to food and water throughout the experiment. All the animal-handling procedures were in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals. The study was approved by the Ethics Committee of Ningbo University. All the surgical procedures were performed under anesthesia, and all efforts were made to minimize suffering.
The rats were randomly assigned to four groups (n=6 per group) as follows: 1) normal control (N) group, consisting of rats fed with the regular diet; 2) normal-given-CRPHF (NC) group, consisting of rats fed with the regular diet and treated with CRPHF; 3) diabetic control (D) group, consisting of rats fed with the high-fat diet; and 4) diabetic-given-CRPHF (DC) group, consisting of rats fed with the high-fat diet and treated with CRPHF. The entire experimental period was 9 weeks, and the rats were fed a regular or high-fat diet throughout this time period according to their group assignment. Streptozotocin (STZ) was injected at the end of the 4th week. Rats in the D and DC groups were given an intraperitoneal injection of 25 mg/kg STZ (Sigma, St Louis, MO, USA) dissolved in a citrate buffer with a pH of 4.5. Rats in the N and NC groups were injected with the same volume of the citrate buffer. After STZ injection, random blood glucose (RBG) levels were measured daily, and an additional equivalent dose of STZ was given to the rats whose RBG was <11.1 mmol/L on the 3rd day. On the 7th day after the initial STZ injection, RBG of all the rats in D and DC groups was found to be >11.1 mmol/L, indicating the successful induction of diabetes. In the last 4 weeks, the rats were treated with CRPHF or vehicle. For the treatment, the rats were administered CRPHF (5 mL/kg/d, NC and DC groups), or an equivalent volume of normal saline (N and D groups), daily for 4 weeks through an intragastric feeding tube.
Tissue preparation
After the treatment was completed, all the rats were euthanized by intraperitoneal injection of a lethal dose of pentobarbital. The abdominal cavity was opened, and blood samples were collected by direct puncture of the descending aorta into ethylenediaminetetraacetic acid anticoagulant tubes and then centrifuged at 3,000 rpm/min for 15 min to collect the serum. Sera were stored frozen until use. The skull was opened, and the brain was immediately frozen in liquid nitrogen for further analysis or fixed in 4% paraformaldehyde at 4°C for 24 h.
Measurement of body weight and RBG
Body weights were recorded at 7 days after the injection of STZ and again at the end of the experiment. Blood was collected from the tail vein, and RBG was measured with a blood glucose meter once every 4 days in the last 4 weeks of the experiment (Johnson & Johnson, New Brunswick, NJ, USA). Fasting blood glucose measurement and oral glucose tolerance test (OGTT) were performed on overnight-fasted rats in order to evaluate glucose tolerance at the end of the study. Rats were fasted for 12 h and then given a glucose solution (2 g/kg body weight), and blood samples were collected before and at 30, 60, and 120 min after the glucose solution was given.
Determination of blood lipid parameters
Blood lipid parameters were measured at the end of the study. Sera prepared as described earlier were used for the detection of high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, total cholesterol (TC), and triglyceride (TG) by using a MODULAR P800 Automated Biochemist Analyzer (Roche, Basel, Switzerland).
OFT
The OFT was conducted in order to measure spontaneous activity in rodents in a novel environment.Citation12 Briefly, the rats were placed individually in a white Plexiglas box (100×100×40 cm) with the bottom divided into four identical squares on the floor of the arena. The test room was dimly illuminated. A single rat was placed in the center of the cage. Line crossing (four paws placed into a new square) and rearing (with both front paws raised from the floor) were recorded over 5 min by using the EthoVision video tracking system (Noldus, Leesburg, VA, USA). All behaviors were recorded by using a video camera located 150 cm above the arena. After each trial, the arena was cleaned with 75% alcohol.
EPMT
EPMT is a widely used behavioral assay for anxiety responses to evaluate risk-taking behavior.Citation12 The elevated plus-maze apparatus has a plus sign-like shape and consists of two “open” (no walls, 50×10 cm) and two “closed” (50×10×40 cm) arms, arranged perpendicularly and elevated 70 cm above the floor. Each animal was placed at the center of the device, facing an open arm, and then were allowed to freely explore the maze for 5 min, and all the testing sessions were conducted between 5:00 pm and 10:00 pm in a sound-attenuated room. After each trial, the test maze was wiped with 75% ethanol. The number of entries into the open and closed arms, and the time spent in the open and closed arms were recorded. The percentage of time spent in the open arms and the percentage of open arm entries were calculated on the basis of the total time and the total number of entries (number of entries in open + closed arms) and were used as anxiety and locomotor parameters.
LAT
The LAT was used to evaluate depressive-like and anxiety-like behaviorsCitation13 as a complement to OFT. Each rat was placed in a black box (100×100×40 cm) without cover, which was enclosed in a soundproof box. The test room was kept relatively quiet. Horizontal distance was measured by the sequential breaking of infrared beams. Movement time was incremented when a rat was active for 0.1 s. The average speed of movement was obtained by dividing the total horizontal distance moved by the total time in motion for each rat in the 60 min testing period, and locomotor activity (distance moved in cm) was used to evaluate depressive-like and anxiety-like behaviors. After each trial, the apparatus was cleaned with 75% ethanol.
FST
The FST was conducted to assess behavioral despair in a sound-attenuated room, which was dimly illuminated.Citation14 Rats were placed individually in a clear plastic cylinder (height =40 cm; diameter =30 cm) filled with water to 21.5±1.5 cm high at 24°C±0.5°C for 5 min. The rats were allowed to swim in the cylinder under the condition that escape was not possible. The duration of immobility, which is defined as the lack of motion of the whole body except for small movement necessary to keep the head above the water, was recorded, and the extent of despair symptoms was evaluated in the rats. After each trial, the cylinder was cleaned and filled with freshwater.
Protein extraction and Western blot analysis
Proteins were extracted from rat brain tissues that were frozen in liquid nitrogen; 10 mg of brain tissues was homogenized in a lysis buffer containing 200 µL RIPA (Solarbio Science & Technology Co., Ltd, Beijing, People’s Republic of China) and 1 mM aprotinin, and the lysate was centrifuged at 12,000× rpm for 5 min. The protein concentration of the supernatant was quantified by using a Bicinchoninic Acid Protein Assay kit (Beyotime Institute of Biotechnology, Shanghai, People’s Republic of China). For Western blot analysis,Citation15 the protein samples were mixed in a ratio of 4:1 with a sample buffer (Beyotime Institute of Biotechnology) and boiled for 5 min. Samples (containing 80 µg of proteins in total) were resolved by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Separated proteins were electrophoretically transferred to polyvinylidene fluoride membranes (Immobilon-P; Millipore Corp., Billerica, MA, USA) in a buffer containing 38.67 mM glycine, 47.9 mM Tris, and 20% methanol. Nonspecific binding was blocked by incubation of the membranes with 10% nonfat milk in phosphate-buffered solution (PBS) containing 0.1% Tween-20 (PBST) for 1 h at room temperature. Then, the membranes were incubated with the following primary antibodies diluted in 1% milk in PBST overnight at 4°C: primary antibodies for BDNF (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:200), p-ERK (Cell Signaling Technology, Danvers, MA, USA; 1:500), ERK (Cell Signaling Technology, 1:1,000), and β-actin (Cell Signaling Technology, 1:5,000). Anti-rabbit and anti-mouse secondary antibodies were used at a ratio of 1:5,000. The membranes were washed with PBST, and then, they were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h and visualized by chemiluminescence (Tanon-5,200 gel imager, Shanghai, People’s Republic of China). The relative expression of proteins was quantified by densitometry scanning of the blots and calculated by using the ImageJ 2X software (National Institutes of Health, Bethesda, MD, USA).
Statistics
Statistical analysis was conducted by using GraphPad Prism® version 5.02 (GraphPad Software, La Jolla, CA, USA), and the accepted significance level for all the tests was P<0.05. All the data were expressed as mean ± standard deviation; t-test of independent samples was used to compare the differences between the two groups, whereas one-way analysis of variance was used to compare the differences among the three or more groups.
Results
RBG and OGTT
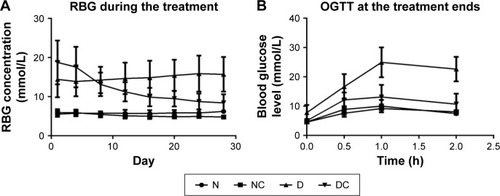
A total of 24 rats were used in the in vivo experiment, and they all survived until the end of the experiment. As shown in , between- and within-group analyses were performed to identify the differences in RBG across the various groups and time points, respectively. The rats in N group exhibited stable RBG levels (5.53–6.23 mmol/L) throughout the treatment period, whereas the RBG of rats in D group showed an increasing trend and the RBG of NC and DC groups given the CRPHF showed decreasing trend. The percent reduction in RBG was 18.7 and 54.9 on Day 28 (the day after the last CRPHF treatment, 4.82±0.50 mmol/L, 8.45±2.17 mmol/L) compared with Day 1 (the first day of CRPHF treatment, 5.93±0.86 mmol/L, 18.75±5.59 mmol/L) in NC and DC groups, respectively. Between-group analysis showed that the RBG level of rats in the DC group was significantly lower than that of rats in the D group on Day 28 (P<0.01). OGTT was conducted to determine whether CRPHF improved glucose homeostasis in these animals. As shown in , all the groups showed significant increases (P<0.001) in RBG 1 h following oral glucose challenge caused by the oral glucose. It was found that RBG showed a greater increase in the D group than in the DC group from 0.5 h to 1 h, indicating a postponed RBG peak. Moreover, rats in the DC group had lower RBG compared with the D group at 2 h after the glucose challenge. Interestingly, the glucose levels in the DC group rats remained 52.8% lower than that of the D group at 2 h after the glucose challenge, indicating that rats treated with CRPHF had improved glucose tolerance.
Figure 1 CRPHF reduced random blood glucose and improved glucose tolerance in diabetic rats.
Abbreviations: CRPHF, combination of Radix Puerariae and hawthorn fruit; D, diabetic control; DC, diabetic-given-CRPHF; N, normal control; NC, normal-given-CRPHF; OGTT, oral glucose tolerance test; RBG, random blood glucose.
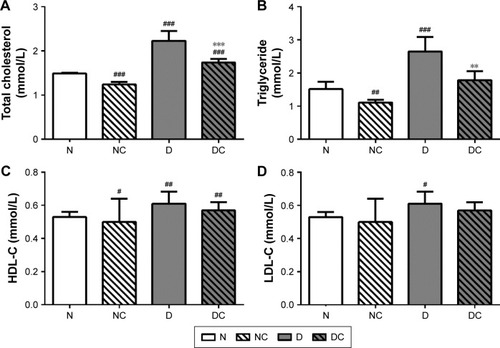
CRPHF improved lipid metabolism in diabetic rats
CRPHF reduced TG (1.79±0.27 mmol/L, 2.65±0.44 mmol/L, P<0.01) and TC (1.74±0.08 mmol/L, 2.23±0.22 mmol/L, P<0.001) in diabetic rats (). Similarly, the NC group showed significantly decreased TG (1.11±0.08 mmol/L, 1.52±0.22 mmol/L, P<0.05) and TC (1.24±0.06 mmol/L, 1.49±0.02 mmol/L, P<0.001) and significantly increased HDL-C (1.20±0.18 mmol/L, 0.92±0.17 mmol/L, P<0.05) compared with the N group. These data indicate that CRPHF effectively improved lipid metabolism in type 2 diabetic rats and lowered blood lipid in normal rats to a certain extent.
Figure 2 CRPHF reduced blood lipid in diabetic rats.
Abbreviations: CRPHF, combination of Radix Puerariae and hawthorn fruit; D, diabetic control; DC, diabetic-given-CRPHF; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; N, normal control; NC, normal-given-CRPHF; TC, total cholesterol; TG, triglyceride.
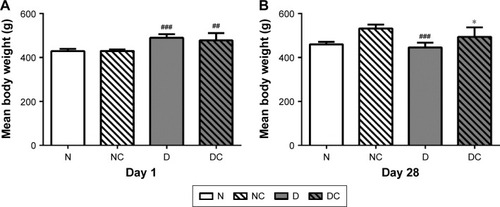
CRPHF restored the body weight of diabetic rats
There was a gradual increase in the body weights of the rats during the first 4 weeks of feeding period. As shown in , on the first day of treatment, the mean body weights of the high-fat diet groups (430±6.50 g) were significantly higher than those in the corresponding normal diet groups (485±20.56 g, P<0.05). At the completion of the study (), the mean body weights in the DC group (494±44.44 g) were significantly higher than those in the D group (446±22.10 g; P<0.05), and similarly, the mean body weights in the NC group were significantly higher than those in the N group (P<0.001). The body weights of rats in the D group were less than that of the N group, resembling the weight loss seen in diabetic patients. These findings suggested that the CRPHF could significantly restore the reduction in body weight caused by STZ.
Figure 3 CRPHF restored the body weights in diabetic rats.
Abbreviations: CRPHF, combination of Radix Puerariae and hawthorn fruit; D, diabetic control; DC, diabetic-given-CRPHF; N, normal control; NC, normal-given-CRPHF; STZ, streptozotocin.
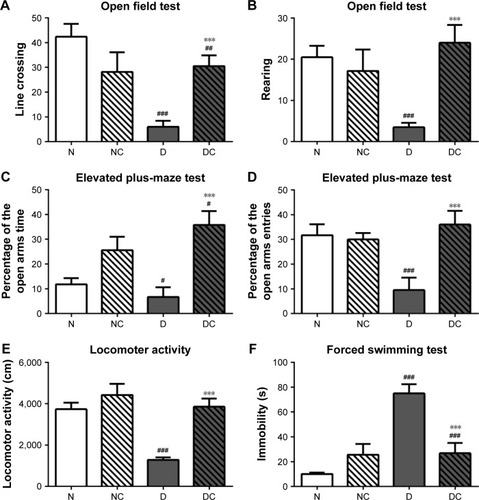
CRPHF reduced depressive-like behaviors in diabetic rats
Depressive-like behaviors were assessed by using four different tests (). High-fat diet and STZ injection induced a marked decrease in the frequency of line crossing and rearing during OFT (; P<0.001 for both), a significant decrease in the total time and entries in the open arms in the EPMT (; P<0.05, P<0.001, respectively) and a pronounced decrease in LAT (; P<0.001) compared with the N group. CRPHF significantly reversed the decreases in the measured parameters in OFT, EPMT, and LAT (P<0.001 for all) induced by DM. For FST (), immobility time was prolonged in the D group versus the N group (P<0.001). CRPHF treatment significantly alleviated the STZ-induced increase in immobility time (P<0.001).
Figure 4 CRPHF reduced depressive-like behaviors in diabetic rats.
Abbreviations: CRPHF, combination of Radix Puerariae and hawthorn fruit; D, diabetic control; DC, diabetic-given-CRPHF; N, normal control; NC, normal-given-CRPHF.
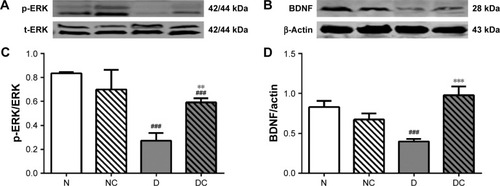
CRPHF increased the protein level of phosphorylated extracellular signal-regulated kinase (p-ERK) and BDNF in the brain of diabetic rats
BDNF is a neurotrophin that is critical for the control of mood, emotion, learning, and cognition, and a number of animal and human studies have suggested the role of BDNF in the pathogenesis of recurrent mood disorders.Citation16 In addition, serum BDNF levels are abnormally low in patients with depressive disorder.Citation17 Moreover, a meta-analysis suggests a potential role of BDNF as a marker of treatment response after treatment in patients with mood disorders,Citation18 and another study showed that p-ERK levels decreased in depression rats.Citation19 Therefore, the BDNF and p-ERK expressions were measured to ensure the success of depression rat models and to evaluate the therapeutic effect of CRPHF at the molecular level. As shown in , the protein levels of p-ERK and BDNF were significantly higher (P<0.05 and P<0.001, respectively) in the brain of the rats in DC group than in the rats in the D group. Moreover, the protein levels of p-ERK and BDNF were lower in rats of the D group than in rats of the N group (P<0.05 and P<0.01, respectively).
Figure 5 CRPHF increased the protein levels of p-ERK and BDNF in the brain of diabetic rats.
Abbreviations: BDNF, brain-derived neurotrophic factor; CRPHF, combination of Radix Puerariae and hawthorn fruit; D, diabetic control; DC, diabetic-given-CRPHF; N, normal control; NC, normal-given-CRPHF; p-ERK, phosphorylated extracellular signal-regulated protein kinase.
Discussion
Chronic depression is frequent among diabetic patients, aggravates DM-induced complications, and increases mortality.Citation20,Citation21 The pathophysiological connections between DM and depression have not yet been widely studied. The present study showed that DM induced behavioral changes that can be measured by OFT, EPMT, LAT, and FST, whereas CRPHF reversed these behavioral changes. It further showed that the p-ERK–BDNF pathway might be associated with the comorbidity of these diseases.
Appropriate diabetic animal models play an important role in illustrating the underlying mechanisms of DMCitation22 and the associated depression. The high-fat diet- and STZ-induced type 2 diabetic rat model is well accepted as this model is closely analogous to the clinical situation in humans,Citation23 and the hyperglycemia state () and hyperlipidemia state () also conform to the transitions of depression in the progression of type 2 diabetes. To the best of the authors’ knowledge, the present study is the first to demonstrate that the high-fat diet and STZ-induced type 2 diabetic rat model exhibits depressive-like behaviors.
Previous studies have shown the therapeutic effects of Traditional Chinese Medicine in treating patients with depression or DM. The present study showed the dual function of a novel herbal treatment to reduce RBG and depressive-like behaviors. Treatment with a dose of 2 g/kg/d CRPHF for 4 weeks reversed DM-induced depressive-like moving pattern in type 2 diabetic rats, suggesting that long-term administration of CRPHF reduces depressive-like behaviors.
Further research into the mechanism of the observed effects showed that treatments with CRPHF have increased p-ERK and BDNF levels in the prefrontal cortex. p-ERK is implicated in rapid antidepressant effects;Citation24 thus, it is possible that p-ERK contributes to the antidepressant effects of CRPHF. BDNF is a critical regulator of the formation, regulation, and integrity of neurons in brain circuits that regulate emotion.Citation25 The neurotrophic hypothesis suggests that reduced activity of BDNF causes a depressive state.Citation26 Decreased centralCitation27 and peripheral levels of BDNFCitation28 have been reported in patients with major depression disorder. Associations between BDNF gene and major depression disorder have been reported in several human genetic studies.Citation29,Citation30 Some other studies have found reduced mRNA levels of BDNF in the prefrontal cortex and hippocampus of suicide subjects, compared with the control subjects.Citation31 Consistent with these findings, the present study showed that BDNF is decreased when rats are exposed to a high-fat diet for 4 weeks followed by an STZ injection, and CRPHF treatment increases BDNF levels in the prefrontal cortex in these diabetic rats.
Conclusion
In conclusion, a depression-like rat model was developed based on a high-fat diet and STZ injection. The results of the present study suggest that CRPHF is a putative antidepressant for patients with diabetes, and it possibly acts through the p-ERK–BDNF pathway. Further research is required to investigate the optimum dose of CRPHF and exact mechanisms underlying these effects.
Acknowledgments
This work was supported by the Ningbo Science and Technology Innovation Team Program (2014B82002); the Natural Science Foundation of Ningbo (2015A610176); the Natural Science Foundation of Zhejiang province (Q16H100001); the National Natural Science Foundation of China (281370165, 81501421); the Fang Runhua Fund of Hong Kong; and KC Wong Magna Fund in Ningbo University.
Disclosure
The authors report no conflicts of interest in this work.
References
- MusselmanDLBetanELarsenHPhillipsLSRelationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatmentBiol Psychiatry200354331732912893107
- BognerHRMoralesKHde VriesHFCappolaARIntegrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trialAnn Fam Med2012101152222230826
- AndersonRJFreedlandKEClouseRELustmanPJThe prevalence of comorbid depression in adults with diabetes: a meta-analysisDiabetes Care20012461069107811375373
- HabtewoldTDAlemuSMHaileYGSociodemographic, clinical, and psychosocial factors associated with depression among type 2 diabetic outpatients in Black Lion General Specialized Hospital, Addis Ababa, Ethiopia: a cross-sectional studyBMC Psychiatry201616110327083154
- IshizawaKBabazonoTHoribaYThe relationship between depressive symptoms and diabetic complications in elderly patients with diabetes: analysis using the Diabetes Study from the Center of Tokyo Women’s Medical University (DIACET)J Diabetes Complications201630459760226987919
- YanBWangDYXingDMThe antidepressant effect of ethanol extract of radix puerariae in mice exposed to cerebral ischemia reperfusionPharmacol Biochem Behav200478231932515219773
- DoronRLotanDEinatNA novel herbal treatment reduces depressive-like behaviors and increases BDNF levels in the brain of stressed miceLife Sci201494215115724184295
- ZhaoJLuoDLiangZLaoLRongJPlant natural product puerarin ameliorates depressive behaviors and chronic pain in mice with spared nerve injury (SNI)Mol Neurobiol Epub2016324
- HaselbeckAEpoetins: differences and their relevance to immunogenicityCurr Med Res Opin200319543043213678480
- LiQZhaoHFZhangZFLong-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascadeNeuroscience200915941208121519409206
- FuXSongBTianGWLiJLThe effects of the water-extraction of Astragali Radix and Lycopi herba on the Pathway of TGF-smads-UPP in a rat model of diabetic nephropathyPharmacogn Mag2014104049149625422551
- MeirsmanACRobéAde Kerchove d’ExaerdeAKiefferBLGPR88 in A2AR neurons enhances anxiety-like behaviorseNeuro201634 pii
- BurkeNNCoppingerJDeaverDRRocheMFinnDPKellyJSex differences and similarities in depressive- and anxiety-like behaviour in the Wistar-Kyoto ratPhysiol Behav2016167283427591842
- NelsonLHLenzKMMicroglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female ratsBehav Brain Res201731627929327613230
- FujitaNShimotakeNOhkiIMechanism of transcriptional regulation by methyl-CpG binding protein MBD1Molecular and Cellular Biology200020145107511810866667
- PostRMRole of BDNF in bipolar and unipolar disorder: clinical and theoretical implicationsJ Psychiatr Res2007411297999017239400
- SenSDumanRSanacoraGSerum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implicationsBiol Psychiatry200864652753218571629
- RochaRBDondossolaERGrandeAJIncreased BDNF levels after electroconvulsive therapy in patients with major depressive disorder: a meta-analysis studyJ Psychiatr Res201683475327552533
- WangXXieYZhangTResveratrol reverses chronic restraint stress-induced depression-like behaviour: involvement of BDNF level, ERK phosphorylation and expression of Bcl-2 and Bax in ratsBrain Res Bull201612513414327346276
- KatonWFanMYUnützerJTaylorJPincusHSchoenbaumMDepression and diabetes: a potentially lethal combinationJ Gen Intern Med200823101571157518649108
- ZelenaDBarnaIMlynarikMGuptaOPJezovaDMakaraGBStress symptoms induced by repeated morphine withdrawal in comparison to other chronic stress models in miceNeuroendocrinology200581320521516020930
- SrinivasanKViswanadBAsratLKaulCLRamaraoPCombination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screeningPharmacol Res200552431332015979893
- SkovsoSModeling type 2 diabetes in rats using high fat diet and streptozotocinJ Diabetes Investig201454349358
- XueWZhouXYiNYueju pill rapidly induces antidepressant-like effects and acutely enhances BDNF expression in mouse brainEvid Based Complement Alternat Med2013201318436723710213
- DumanRSMonteggiaLMA neurotrophic model for stress-related mood disordersBiol Psychiatry200659121116112716631126
- ChourbajiSBrandweinCGassPAltering BDNF expression by genetics and/or environment: impact for emotional and depression-like behaviour in laboratory miceNeurosci Biobehav Rev201135359961120621121
- KaregeFBondolfiGGervasoniNSchwaldMAubryJMBertschyGLow brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivityBiol Psychiatry20055791068107215860348
- MolendijkMLBusBASpinhovenPSerum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatmentMol Psychiatry201116111088109520856249
- LicinioJDongCWongMLNovel sequence variations in the brain-derived neurotrophic factor gene and association with major depression and antidepressant treatment responseArch Gen Psychiatry200966548849719414708
- SchumacherJJamraRABeckerTEvidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BDNF) locus and major depressionBiol Psychiatry200558430731416005437
- DwivediYRizaviHSConleyRRRobertsRCTammingaCAPandeyGNAltered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjectsArch Gen Psychiatry200360880481512912764
