Abstract
Specific serotonin norepinephrine reuptake inhibitors (SNRIs) have been described as “better tolerated tricyclic antidepressants” or as “boosted” selective serotonin reuptake inhibitors (SSRIs). Venlafaxine has become a therapeutic reference treatment for major depression. Although less widely studied, indirect comparisons with another SNRI, milnacipran, suggest an equivalent efficacy. This paper discusses these indirect comparisons and the recently published first double-blind, head-to-head comparison. Venlafaxine has potency at serotonin transporters which is about 30-fold greater than that at norepinephrine transporters while milnacipran has a similar potency at each transporter. Thus, at low doses, venlafaxine acts essentially as a SSRI, with significant noradrenergic activity only occurring at higher doses. To overcome the problem of the differing profile of venlafaxine at increasing doses, the first head-to-head study compared the therapeutic effects and tolerability of the two antidepressants when flexibly titrated to the high dose of 200 mg/day. The study showed that the two SNRIs have similar efficacy and safety profiles. Both drugs produced about 42% remissions at the end of the 20-week study. The most frequent adverse events in both groups were nausea, dizziness, headache, and sweating. Certain specific differences in tolerability are discussed.
Introduction
Major depression is a common psychiatric condition with a prevalence of 5%–13% in the general population.Citation1 Despite its prevalence and its consequences for both individuals and society in general, depression is only correctly diagnosed in 50% of cases, and less than 10% of patients with depression receive appropriate antidepressant treatment of adequate dose and duration.Citation2 It is now generally accepted that the minimum duration of antidepressant treatment in order to prevent relapse of the current episode is 6 months. Few randomized, double-blind, clinical studies of antidepressants, however, extend for this length of time.
Most antidepressants act to increase the synaptic concentration of serotonin (5-hydroxytryptamine, 5-HT) and/or norepinephrine (NE), generally by inhibiting the reuptake of one or both of these neurotransmitters. Serotonin and norepinephrine reuptake inhibitors (SNRIs) combine the efficacy of the tricyclic antidepressants (TCAs) with the tolerability of the selective serotonin reuptake inhibitors (SSRIs).Citation3 Milnacipran (Ixel®) and venlafaxine (Effexor®) are both SNRIs, although they differ in their selectivity inhibition of NE and 5-HT reuptake. Milnacipran has the most balanced ratio of potency for reuptake inhibition of the two neurotransmitters (5HT:NE 1:1.16).Citation4 One study has shown milnacipran to inhibit NE reuptake with greater potency than 5-HT (2.22:1).Citation5 Venlafaxine has a 30-fold greater potency for 5-HT.Citation4
TCAs remain the “gold standard” for antidepressant efficacy, although their poor tolerability complicates their use at fully effective doses.Citation6 Milnacipran has an efficacy comparable with that of the TCAsCitation7 including clomipramine.Citation8 A post hoc analysis has shown that milnacipran has greater efficacy than SSRIs in more severely depressed patients.Citation9 A global analysis of all dual-acting antidepressants concluded that three antidepressants, ie, milnacipran, duloxetine, and mirtazapine, had “probable superior efficacy” compared with the SSRIs.Citation10
Venlafaxine is a widely used antidepressant and has recently been described as a reference treatment for major depression.Citation11 In a global analysis of all antidepressants mentioned above, venlafaxine was classified, along with clomipramine, as having “definite superior efficacy”.Citation10 Juxtaposition of studies or meta-analyses comparing milnacipran with SSRIs and venlafaxine with SSRIs,Citation3 suggests that the two SNRIs had similar overall efficacy. A recent meta-analysis of studies comparing newer dual-action antidepressants with SSRIsCitation12 concluded a superior efficacy for all the dual-action antidepressants, including milnacipran and venlafaxine (as well as duloxetine, mirtazapine, mianserin, and moclobemide). Again the two antidepressants showed comparable efficacy across all measures.
With the exception of the study described below, there have been no direct comparisons between milnacipran and venlafaxine. One difficulty of directly comparing these two SNRIs is the choice of dose. In the FDA labeling for Effexor the recommended starting dose for venlafaxine is 75 mg/day, which may be increased to 150 mg/day. If needed, the dose may be further increased up to 225 mg/day. In outpatient settings there is no evidence of usefulness of doses greater than 225 mg/day for moderately depressed patients, but more severely depressed inpatients responded to a mean dose of 350 mg/day. Certain patients, including more severely depressed patients, may respond better to higher doses, up to a maximum of 375 mg/day. Because venlafaxine has a 30-fold selectivity for the reuptake of serotonin, at 75 mg/day it does not significantly modify noradrenergic neurotransmission and acts essentially as a SSRI.Citation13 A clinically meaningful effect on noradrenergic neurotransmission is estimated to occur from about 150–200 mg/day.Citation13 The adverse effects of venlafaxine have been shown to be dose-dependent.Citation14 Thus, depending on the dose chosen for the comparison, venlafaxine can be either a relatively well tolerated SSRI or a less well tolerated SNRI.
Milnacipran has a similar effect on both serotonin and norepinephrine reuptake and thus acts as an SNRI at all doses.Citation3 The recommended dose of milnacipran in major depression is 100 mg/day, although various studies carried out with doses 150–300 mg/day have suggested that some patients may benefit from higher doses.Citation15–Citation17 These studies have shown that these higher doses of milnacipran are well tolerated by most patients. No new adverse effects are seen at higher doses.
Milnacipran and venlafaxine IR flexibly titrated to 200 mg/day
The first direct comparison of milnacipran and venlafaxine IR (immediate-release)Citation18 was carried out at doses flexibly titrated up to a maximum of 200 mg/day for each compound. In addition, because in clinical practice a major objective is to achieve a long-lasting remission from depression, the study was carried out over a duration of 24 weeks. The choice of the IR formulation of venlafaxine rather than the XR (extended-release) formulation was probably chosen to enable identical twice-daily dosing, although this is not mentioned in the publication.Citation18
The recommended dose of milnacipran in major depression is 100 mg/day, but studies carried out at doses of 150–300 mg/day suggest that some patients may benefit from higher dosesCitation18 and milnacipran is frequently used at these doses. The recommended dose range for venlafaxine in outpatients is 75–225 mg/day. Thus, the objective of this study was to explore the efficacy and long-term safety/tolerability of milnacipran and venlafaxine administered in an outpatient setting at doses of up to 200 mg/day in patients suffering from a major depressive episode.
This multicenter, randomized, double-blind study was carried out in 195 adult outpatients presenting with an episode of recurrent, unipolar, moderate-to-severe (Montgomery- Asberg Depression Rating Scale [MADRS]Citation19 score ≥ 23) major depressive disorder. During the first 4 weeks of the study, the dose of each antidepressant was uptitrated (days 1–3, 25 mg/day; days 4–6, 50 mg/day; days 7–13, 100 mg/day; days 14–28, 150 mg/day). At day 28, at the clinician’s discretion, the dose was maintained at 150 mg/day, reduced to 100 mg/day, or increased to 200 mg/day. At the end of the 24-week study, the dose was downtitrated by 50 mg/day every 5 days. The daily doses were administered in two identical doses morning and evening, except the initial dose of 25 mg/day (one morning dose only) and the dose of 150 mg/day (50 mg morning and 100 mg evening). Depressive symptoms were assessed weekly using the MADRS.
Of the 195 patients randomized (milnacipran 97, venlafaxine 98), 134 completed 24 weeks of treatment (milnacipran 60, venlafaxine 74). In both groups, the main reason for premature withdrawal was adverse events (AEs, 51% on milnacipran, 42% on venlafaxine). The other reasons for premature withdrawal, such as lack of efficacy or patient decision after recovery, were not stated, so it is not possible to analyze the dropout data in detail.
The baseline characteristics of the patients () were similar in both groups, except that there were significantly more patients with a severe depressive episode in the milnacipran group (63.3% versus 54% in the venlafaxine group). Most patients (87%) had had previous depressive episodes which had been treated with antidepressants. The mean baseline MADRS score of 31.0 in the overall population indicated moderate to severe intensity of the depression.
Table 1 Demographic characteristics at baseline
At the end of the titration period, approximately 90% of patients were taking an antidepressant dose of either 150 or 200 mg/day (). This percentage remained constant for both antidepressants through to the end of the study.
Table 2 Doses of antidepressants received by patients at the end of the titration period
The MADRS score decreased progressively throughout the study and was comparable in the two treatment groups (). The rate of response (≥ 50% reduction in initial MADRS score) and remission (a MADRS score ≤ 10, ) was not clinically or statistically different between the two treatment groups at week 8 or week 24. Rates of response and remission were also similar with both antidepressants in a subgroup of patients with a severe depressive episode (n = 104, ) and in patients with a measurable suicide risk (“low” or “moderate” according to the Mini International Neuropsychiatric Interview) at baseline (n = 75, ).
Figure 1 MADRS total score (mean changes from baseline) over the first 8 weeks of treatment
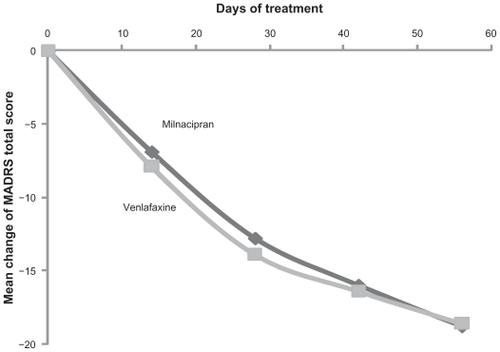
Figure 2 Responders and remissions (MADRS) after 8 weeks
Key: filled columns = responders (≥ 50% reduction in initial MADRS score); hatched columns = remissions (MADRS score ≤ 10).
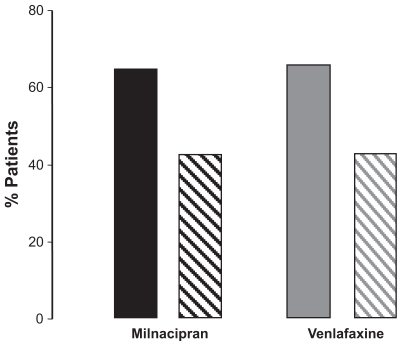
Table 3 Rates of response and remission
In the vast majority of cases, the therapeutic benefit achieved at week 8 was maintained over the long term. Overall, 93% of responders at week 8 were also responders at week 24 (milnacipran, 95%, venlafaxine 87%), while 89.3% of the patients in remission at week 8 were also in remission at week 24 (milnacipran 87%, venlafaxine 92%).
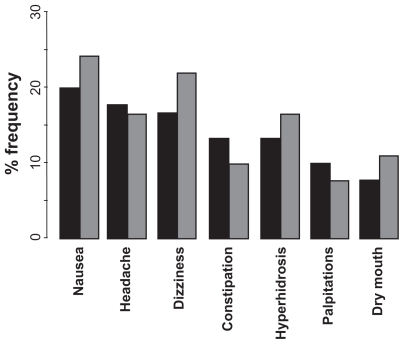
During treatment, AEs were reported by 72% patients in the milnacipran group and 74% patients in the venlafaxine group. In both groups, the most common treatment-emergent AEs (occurring in ≥ 10% of patients) were nausea, headache, dizziness, constipation, hyperhidrosis, palpitations, and dry mouth (). After the end of treatment during the down-titration, the incidence of AEs was greater in the venlafaxine group (14% versus 7% in the milnacipran group). In males, dysuria was experienced only in the milnacipran group (20%), while orgasmic disorders were only reported in the venlafaxine group (17%). Most of the AEs occurred during the first 3 weeks of treatment, and 85% of them were of mild or moderate severity. Five patients in the milnacipran group and seven patients in the venlafaxine group experienced at least one serious AE. Only three serious AEs (all in the venlafaxine group) were considered to be possibly related to the study treatment (one worsening depression, one anxiety related to an overdose of venlafaxine, and one biliary colic).
Figure 3 Principal adverse events experienced with milnacipran and venlafaxine during the study.
Key: black columns = milnacipran; grey columns = venlafaxine.
Dysuria during treatment with milnacipran has been reported previously.Citation7,Citation20 It is probably a consequence of increased noradrenergic tone of the urethral sphincter.Citation21 This is consistent with the fact that the dysuria induced by milnacipran can be reversed by administration of a urinary tract-specific alphaCitation1 antagonist, such as tamsulosin and alfuzosin. Citation22 Dysuria is also experienced with other antidepressants having a major impact on the noradrenergic system, such as reboxetine.Citation23
Orgasmic disorder was spontaneously reported in 17% of patients treated with venlafaxine but in no patients treated with milnacipran. The absence of sexual dysfunction with milnacipran is consistent with other studiesCitation24,Citation25 which have shown that milnacipran does not cause treatment-emergent sexual dysfunction. Antidepressant-induced sexual dysfunction is generally thought to result from stimulation of 5-HT2 receptors,Citation26 although this is probably not the only mechanism. The absence of these effects with milnacipran, which also stimulates serotonergic transmission but does not act as an antagonist at 5-HT2 receptors, suggests that sexual dysfunction induced via stimulation of postsynaptic 5-HT2 receptors may be attenuated or completely blocked by simultaneous noradrenergic stimulation, although the mechanism of this effect remains to be elucidated.
The study discussed in this review shows that milnacipran and venlafaxine, when administered at flexible doses of up to 200 mg/day, have similar efficacy and safety profiles. There are, however, certain differences between the two SNRIs that were not explored in this study. Whereas milnacipran has no effect on any cytochrome (CYP) P450 enzyme,Citation27 venlafaxine is a substrate for CYP2D6 which is responsible for its transformation into the active metabolite, desvenlafaxine.Citation28 There is considerable polymorphism of CYP2D6, with nearly 10% of “slow metabolizers” in the Caucasian population.Citation29 This property is likely to induce unexpected variation in therapeutic effect and tolerability.Citation28 In addition, as stated in the FDA labeling, venlafaxine’s tolerability can be influenced by coadministration of drugs that inhibit CYP2D6, such as cimetidine and certain antipsychotics.Citation30 A recent study has shown that venlafaxine intoxication frequently (in 46% of cases) occurs in the presence of drugs interacting at the CYP P450 2DC isoenzyme, resulting in higher venlafaxine concentrations.Citation31
Finally, the two antidepressants vary considerably in terms of safety in overdose. Milnacipran has been shown to be safe in overdose, with doses as high as 2.8 g causing no serious effects and no mortality.Citation32 No deaths have been reported following milnacipran overdose, with the exception of a case of multiple drug overdose where high blood levels of milnacipran, fluoxetine, norfluoxetine, sertraline, cyamemazine, nordiazepam, and oxazepam were found.Citation33 In contrast, lethal consequences of venlafaxine overdose have been reported, with a frequency of over 34 per 1000 person-years. This was significantly greater than other antidepressants, including the TCAs.Citation34 A study carried out in the UK showed that the fatal toxicity index (deaths/million prescriptions) of venlafaxine (13) was similar to that of TCAs such as amitriptyline (15).Citation35 Another more recent fatal toxicity survey, however, showed greater toxicity for TCAs (13.8) than for venlafaxine (2.5), although the latter was considerably more toxic than the SSRIs (0.5).Citation36 A prominent feature of cases of venlafaxine intoxication is the frequent presence (in 46% of cases) of drugs interacting at the CYP P450, resulting in higher venlafaxine concentrations.Citation36
Conclusion
Indirect comparisonsCitation3,Citation12 and everyday clinical experience has suggested that milnacipran and venlafaxine have similar efficacy. The first head-to-head comparison at a dose of 200 mg/dayCitation18 has confirmed this suggestion. The study also showed that concerns about tolerability at higher doses were unfounded. When the SNRIs were titrated flexibly over a period of 4 weeks, both milnacipran and venlafaxine were relatively well tolerated, with about 90% of patients achieving doses of 150 or 200 mg/day.
Venlafaxine has become a therapeutic reference for major depression.Citation11 Although less widely studied, indirect comparisons and the head-to-head study discussed here show that milnacipran can be considered to be clinically equivalent to venlafaxine in terms of efficacy, with certain advantages when sexual dysfunction, overdose, or polymedication are of concern.
Disclosure
The author is employed by Pierre Fabre Médicament manufacturers of milnacipran.
References
- O’ConnorEAWhitlockEPBeilTLGaynesBNScreening for depression in adult patients in primary care settings: A systematic evidence reviewAnn Intern Med200915179380319949145
- LépineJPGastparMMendlewiczJDepression in the community: The first pan-European study DEPRES (Depression Research in European Society)Int Clin Psychopharmacol199711929
- StahlSMGradyMMMoretCBrileyMSNRIs: Their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressantsCNS Spectr20051073274716142213
- KochSHemrick-LueckeSKThompsonLKComparison of effects of dual transporter inhibitors on monoamine transporters and extracellular levels in ratsNeuropharmacology200345793594414573386
- VaishnaviSNNemeroffCBPlottSJMilnacipran: A comparative analysis of human monoamine uptake and transporter binding affinityBiol Psychiatry200455332032214744476
- PerettiSJudgeRHindmarchISafety and tolerability considerations: Tricyclic antidepressants vs selective serotonin reuptake inhibitorsActa Psychiatr Scand2000403Suppl1725
- KasperSPletanYSollesATournouxAComparative studies with milnacipran and tricyclic antidepressants in the treatment of patients with major depression: A summary of clinical trial resultsInt Clin Psychopharmacol199611Suppl 435398923125
- SteenADen BoerJAA double-blind six months comparative study of milnacipran and clomipramine in major depressive disorderInt Clin Psychopharmacol19971252692819466161
- Lopez-IborJGuelfiJDPletanYMilnacipran and selective serotonin reuptake inhibitors in major depressionInt Clin Psychopharmacol199611Suppl 441468923126
- MontgomerySABaldwinDSBlierPWhich antidepressants have demonstrated superior efficacy? A review of the evidenceInt Clin Psychopharmacol200722632332917917550
- BauerMTharmanathanPVolzHPThe effect of venlafaxine compared with other antidepressants and placebo in the treatment of major depression: A meta-analysisEur Arch Psychiatry Clin Neurosci2009259317218519165525
- PapakostasGIThaseMEFavaMAre antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agentsBiol Psychiatry200762111217122717588546
- DebonnelGSaint-AndréEHébertCDifferential physiological effects of a low dose and high doses of venlafaxine in major depressionInt J Neuropsychopharmacol2007101516116690006
- GutierrezMAStimmelGLAisoJYVenlafaxine: A 2003 updateClin Ther20032582138215414512125
- AnsseauMVon FrenckellRGérardMAInterest of a loading dose of milnacipran in endogenous depressive inpatients. Comparison with the standard regimen and with fluvoxamineEur Neuropsychopharmacol199111131211821700
- HayashiMMimuraMOtsuboTEffect of high-dose milnacipran in patients with depressionNeuropsychiatric Dis Treat200735699702
- OkumuraKFurukawaTARemission rates with milnacipran 100 mg/day and 150 mg/day in the long term treatment of major depressionClin Drug Invest2006263135142
- OliéJ-PGourionDMontagneAMilnacipran and venlafaxine at flexible doses (up to 200 mg/day) in the outpatient treatment of adults with moderate-to-severe major depressive disorder: A 24-week randomized, double-blind exploratory studyNeuropsychiatr Dis Treat20106717920396639
- MontgomerySAAsbergMA new depression scale designed to be sensitive to changeBr J Psychiatry197913382389444788
- SpencerCMWildeMIMilnacipran. A review of its use in depressionDrugs19985634054279777315
- DemyttenaereKHuygensRVan BuggenhoutRTamsulosin as an effective treatment for reboxetine-associated urinary hesitancyInt Clin Psychopharmacol200116635335511712624
- KasperSWolfRSuccessful treatment of reboxetine-induced urinary hesitancy with tamsulosinEur Neuropsychopharmacol200212211912211872327
- ScatesACDoraiswamyPMReboxetine: A selective norepinephrine reuptake inhibitor for the treatment of depressionAnn Pharmacother200034111302131211098346
- BaldwinDMorenoRABrileyMResolution of sexual dysfunction during acute treatment of major depression with milnacipranHum Psychopharmacol200823652753218536065
- TakahashiHIshigookaJImprovement of selective serotonin reuptake inhibitor-induced sexual dysfunction without worsening of depressive symptom after switching to milnacipranClin Neuropharmacol200932317717819483494
- BaldwinDThomasSBirtwistleJEffects of antidepressant drugs on sexual functionInt J Psychiatry Clin Pract199714758
- ParisBLOgilvieBWScheinkoenigJAIn vitro inhibition and induction of human liver cytochrome p450 enzymes by milnacipranDrug Metab Dispos200937102045205419608694
- ShamsMEArnethBHiemkeCCYP2D6 polymorphism and clinical effect of the antidepressant venlafaxineJ Clin Pharm Ther200631549350216958828
- AlvánGBechtelPIseliusLGundert-RemyUHydroxylation polymorphisms of debrisoquine and mephenytoin in European populationsEur J Clin Pharmacol19903965335372151318
- CuppMJTracyTSCytochrome P450: New nomenclature and clinical implicationsAm Fam Physician19985711071169447218
- LauniainenTRasanenIVuoriEOjanperäIFatal venlafaxine poisonings are associated with a high prevalence of drug interactionsInt J Legal Med2010430 [Epub ahead of print]
- MontgomerySATolerability of serotonin norepinephrine reuptake inhibitor antidepressantsCNS Spectr2008137 Suppl 11273318622372
- FantonLBévalotFGraitHFatal intoxication with milnacipranJ Forensic Leg Med20081538839018586210
- TiihonenJLönnqvistJWahlbeckKAntidepressants and the risk of suicide, attempted suicide, and overall mortality in a nationwide cohortArch Gen Psychiatry200663121358136717146010
- CheetaSSchifanoFOyefesoAAntidepressant-related deaths antidepressant prescriptions in England and Wales, 1998–2000Br J Psychiatry2004184414714702226
- HawtonKBergenHSimkinSToxicity of antidepressants: Rates of suicide relative to prescribing and non-fatal overdoseBr J Psychiatry201019635435820435959