Abstract
Pivotal response treatment (PRT) is an evidence-based behavioral intervention based on applied behavior analysis principles aimed to improve social communication skills in individuals with autism spectrum disorder (ASD). PRT adopts a more naturalistic approach and focuses on using a number of strategies to help increase children’s motivation during intervention. Since its conceptualization, PRT has received much empirical support for eliciting therapeutic gains in greater use of functional social communication skills in individuals with ASD. Building upon the empirical evidence supporting PRT, recent advancements have increasingly turned to using interdisciplinary research integrating neuroimaging techniques and behavioral measures to help identify objective biomarkers of treatment, which have two primary purposes. First, neuroimaging results can help characterize how PRT may elicit change, and facilitate partitioning of the heterogeneous profiles of neural mechanisms underlying similar profile of behavioral changes observed over PRT. Second, neuroimaging provides an objective means to both map and track how biomarkers may serve as reliable and sensitive predictors of responder profiles to PRT, assisting clinicians to identify who will most likely benefit from PRT. Together, a better understanding of both mechanisms of change and predictors of responder profile will help PRT to serve as a more precise and targeted intervention for individuals with ASD, thus moving towards the goal of precision medicine and improving quality of care. This review focuses on the recent emerging neuroimaging evidences supporting PRT, offering current perspectives on the importance of interdisciplinary research to help clinicians better understand how PRT works and predict who will respond to PRT.
History of autism spectrum disorder and behavioral treatments
Autism, from the Greek “autos” meaning self, was first poignantly captured by KannerCitation1 in his lucid account of Donald T, a child who experienced significant social impairments. Donald was described to be so “self-satisfied”Citation1 that “to get his attention almost requires one to break down a mental barrier between his inner consciousness and the outside world”.Citation1 Today, autism spectrum disorder (ASD) is still perceived as a pervasive developmental disorder, characterized by social communication deficit and narrow interest in objects and repetitive behavior.Citation2 Up to one-third of young children with ASD also experience clinically significant levels of maladaptive behaviors, such as withdrawal, inattention, and aggression.Citation3 Early behavioral interventions in the 1970s that aimed to increase social communication and reduce clinically significant maladaptive behaviors heavily relied on the use of operant conditioning principles,Citation4 the most influential model being applied behavioral analysis (ABA).Citation5
Traditional ABA aimed to elicit behavioral modifications through highly intensive and structured trials, where adult-chosen stimuli are repeatedly presented to induce target behaviors in individuals with ASD, with correct responses reinforced.Citation5,Citation6 More recently, advancements in ABA have led to more individualized and comprehensive treatments to target a wide range of adaptive behaviors that follow a more natural and normal developmental sequence, in children with ASD as young as three to four years of age.Citation7,Citation8 Although ABA has been demonstrated to be effective in both improving social functioning and reducing clinically significant maladaptive behaviors,Citation5,Citation6,Citation9,Citation10 clinical progress can be costly in both time and effort, which has received much criticism.Citation6,Citation10,Citation11 First, individuals often experience increased exposure to failed attempts on highly structured trials, which can further decrease motivation,Citation12 and induce a sense of learned helplessness.Citation13 Second, highly structured training bears limited ecological validity, further compromising the generalizability of any successfully acquired skills across other developmental domains, as well as outside of clinical settings.Citation6,Citation14,Citation15
A brief outline of pivotal response treatment
Pivotal response treatment (PRT) is a behavioral intervention aimed to improve social communication skills in individuals with ASD,Citation10,Citation11,Citation16,Citation17 which has accumulated a large evidence base with positive findings being replicated using a wide range of experimental designs across multiple settings.Citation17 Based on ABA principles, PRT adopts a more naturalistic approach that focuses on targeting skills that are pivotal to development across social, communication, and behavior.Citation11,Citation18 “Pivotal” refers to a set of targeted skills which, when successfully acquired, can elicit more widespread positive clinical gains in the child’s other domains of functioning.Citation18 Some pivotal areas identified include motivation, self-initiation, and self-management, which have been shown to be critical in eliciting broader improvements across multiple developmental domains, thus maximizing treatment gains.Citation11,Citation18,Citation19
PRT focuses on using a number of strategies to help increase children’s motivation during intervention, such as using a variety of child-chosen activities that are intrinsically motivating to each child,Citation12,Citation20 as well as interspersing maintenance and acquisition tasks to strengthen children’s exposure to well-established response-reinforcer contingency.Citation21,Citation22 Similar to ABA, the structure of PRT involves the presentation of repeated behavioral trials consisting of antecedent, behavior, and consequence,Citation15,Citation23 where the antecedent presents clear opportunities prompting the child for a desired behavior. In contrast to traditional ABA, PRT reinforces both correct behavioral responses and any valid attempts made by the child en route to skill acquisition, thus increasing frequency of exposure to response-reinforcement contingency, in order to help maintain and increase child’s motivation throughout the intervention.Citation20,Citation24
PRT – empirical evidence and questions to address
Since its original conception, PRT has received much empirical support for eliciting therapeutic gains for promoting greater use of functional social communication skills in individuals with ASD, ranging from increased self-initiated social responsesCitation25,Citation26 to advancing collateral language acquisition following increased question-asking behavior.Citation19,Citation27,Citation28 Increased adaptive use of language and social responses have also been linked to secondary clinical gains such as reduced disruptive behavior,Citation29 and restrictive and repetitive behaviors.Citation30 Such secondary clinical gains further support that targeting skills such as increasing social motivation and initiation of appropriate social responses may indeed be pivotal in securing changes in other behavioral domains that are less explicitly addressed during PRT intervention.Citation17,Citation30 For example, the use of child-preferred activities that carry high intrinsic motivational salience to children with ASD may be especially beneficial for providing opportunities to elicit joint attention,Citation31 as well as teaching children to engage in symbolic play.Citation32 Joint attention and pretend play are both crucial social skills underlying the emergence of higher-order social cognition, such as perspective taking and theory of mind development,Citation33–Citation37 and pivotal for securing children’s competence at navigating social situations.
Building upon the empirical evidence supporting PRT, recent advancements have increasingly turned to using interdisciplinary research integrating neuroimaging techniques and behavioral measures to help identify biomarkers of treatment.Citation38–Citation40 The identification of sensitive and objective biomarkers serves two primary goals. First, neuroimaging facilitates better characterization of the heterogeneous profiles of neural mechanisms underlying behavioral changes observed over PRT, further increasing the evidence base surrounding how PRT may be eliciting changes across individuals with ASD.Citation38,Citation39 Second, mapping and tracking biomarkers across the course of development and intervention can instigate the generation of predictive models of responder profiles to PRT, thus assisting clinicians to identify who will most likely benefit from PRT in order to maximize therapeutic gains.Citation40 Together, a better understanding of both mechanisms of change and predictors of responder profile will help PRT to serve as a more precise and targeted intervention for individuals with ASD, thus moving towards the goal of precision medicine and improving quality of care.Citation41
The purpose of the current review is to review the recent emerging evidence from interdisciplinary perspective integrating behavioral and neuroscience research. Specifically, the current review examines (1) identification of neural biomarkers that objectively elucidate the underlying mechanisms of change following PRT and (2) behavioral and neural predictors of responder profile to PRT among individuals with ASD. Summaries of relevant studies are outlined in . Broader issues including limitations on the research quality supporting PRT, such as determining effective intervention study designs to help monitor change as well as conducting long-term follow-up, are discussed, with directions for future research outlined.
Table 1 Summary of behavioral and neural findings examining (a) neural mechanisms of change and (b) predictors of treatment response following pivotal response treatment
From behavior to neuroscience: identifying mechanisms of change
Although PRT has received much empirical support as an intervention targeting core social communication deficits in individuals with ASD from behavioral studies, few studies have investigated the possible underlying mechanisms and pathways that have elicited the changes observed.Citation38 From a clinical perspective, reliance on observable behavioral changes as the sole outcome measure often suffers from the problem of equifinality.Citation42 More precisely, similar profiles in behavioral changes could mask heterogeneous underlying mechanisms of change that are pivotal in eliciting therapeutic gains observed. Overcoming the problem of equifinality has important underlying rationales. Characterizing differential mechanisms of change underlying similar behavioral profiles can help clinicians understand how a child may be specifically benefitting from the intervention. Distilling down the key treatment components that can successfully tackle a specific behavioral profile can be especially informative for formulating treatment plans. Multiple strategies can be devised based around these crucial therapeutic components tailored to each child, generating a more focused and direct intervention plan that may be more cost-effective and time-efficient. Generating greater evidence highlighting differential mechanisms of treatment response can therefore guide clinicians to make evidence-based choices when reformulating treatment strategies. This is in line with moving towards the goal of precision medicine, where it is no longer sufficient to gather empirical evidence supporting if an intervention is working. The aim is to better partition how the intervention is working for each patient given his or her complex clinical profile.Citation41
Developing objective and sensitive biomarkers is crucial for helping clinicians to understand the range of clinical profiles that are likely to benefit from the same intervention via differential mechanisms of change. For PRT, recent advancements have focused on investigating whether neural markers for social cognition identified from neuroimaging studies can serve as reliable biomarkers to assist quantification, and characterization, of differential mechanisms of change associated with social gains following PRT.Citation38,Citation39,Citation43,Citation44 One well-validated paradigm in search for neural correlates of social cognition and perception is the biological motion paradigm, which uses point-light displays that contain sufficient information to depict social movements such as a person walking, dancing, or playing games familiar to children such as pat-a-cake ().Citation43,Citation45,Citation46 Biological motion capitalizes on the intrinsic preferential visual sensitivity found in typically developing (TD) children and adults to movements that are biologically coherent rather than scrambled motion, which is an early emerging mechanism that serves to bias one’s attention towards perceiving stimuli that carry greater social salience.Citation46,Citation47 Preferential bias towards biological motion perception is an important precursor to the emergence of higher-level social cognitionCitation34,Citation48 and affect regulation,Citation49 and thus serves as an important behavioral marker for social development.
To identify brain correlates relating to biological motion perception, Kaiser et alCitation43 compared and contrasted children with ASD (n=25) with their unaffected siblings (US; n=20) and TD children (n=17) to determine patterns of brain response that can serve as neural signatures underpinning social cognition differences in individuals with ASD when watching biological motion versus scrambled motion using functional magnetic resonance imaging (fMRI). The authors conducted group comparisons examining the effects of biological motion versus scrambled motion as follows: TD versus ASD, TD versus US, US versus ASD, and US versus TD; whole-brain analyses were conducted with activations noted at a voxel-wise uncorrected threshold level of P<0.05. To correct for multiple comparisons, clusters that exceeded the cluster threshold of k>20 contiguous voxels at α<0.05 from each group comparison were selected. Setting the cluster threshold at k>20 contiguous active voxels within each group contrast resulted in a <5% false-positive discovery rate of active voxels in the key group contrasts. The relative frequency of occurrence for each cluster size during 5,000 iterations using Monte Carlo simulation helped to inform assignment of α value for each cluster. Higher-level conjunction analyses to identify state, trait, and compensatory regions were conducted at the voxel-wise uncorrected threshold level of P<0.0025, and a cluster threshold of k>20, to further limit the rate of false positives in the final result output. The authors distinguished distinct patterns of (a) “state”, (b) “trait”, and (c) “compensatory” brain response to biological motion ().
Figure 2 Brain regions demonstrating differences in activation during biological motion relative to scrambled motion.
Abbreviations: pSTS, posterior superior temporal sulcus; AMG, amygdala; FFG, facial fusiform gyrus; vmPFC, ventromedial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; ITG, inferior temporal gyrus; vlPFC, ventrolateral prefrontal cortex.
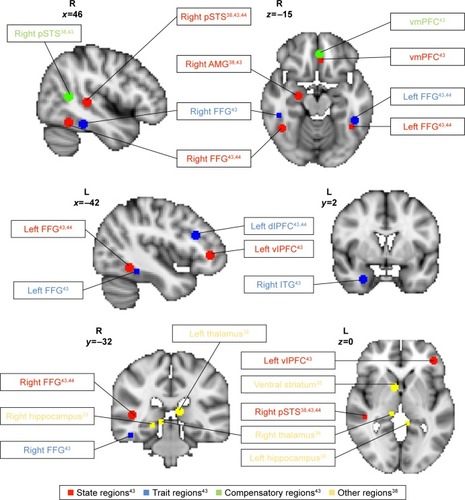
“State” regions demonstrated diminished activation in response to biological versus scrambled motion unique to ASD. “State” profile reflects disrupted brain processing of social stimuli specifically associated with the psychopathological state of ASD. “State” regions included left ventrolateral prefrontal cortex (vlPFC), ventromedial prefrontal cortex (vmPFC), right amygdala, right posterior superior temporal sulcus (rpSTS), and bilateral fusiform gyrus (FG). “Trait” regions demonstrated diminished activation in response to biological motion for both individuals with ASD and US relative to TD children. “Trait” profile may indicate possible disruption to social stimuli processing associated with shared genetic vulnerability between individuals with ASD and US, and a marker for endophenotype of ASD. “Trait” regions included left dorsolateral prefrontal cortex, right inferior temporal gyrus, and anterior bilateral FG. “Compensatory” regions demonstrated unique profile of activation to biological motion found in US group only, which may serve to overcome increased vulnerability to developing social perception deficits in US. “Compensatory” regions included caudal rpSTS and anterior rostral vmPFC. Taken together, the neural profiles identified included many brain areas that are associated with social responsiveness, mentalizing, and theory of mind.Citation50–Citation52
The “state”, “trait”, and “compensatory” neural profiles therefore serve as a guide for identifying biomarkers that are sensitive to differences in perception of social stimuli across children with ASD, US, and TD children. Using sensitive biomarkers to monitor changes in patterns of neural activity in response to biological motion provides an objective means to quantify whether behavioral changes in social cognition over the course of PRT are accompanied by underlying functional neural changes. Specifically, based on positive social gains from behavioral measures following PRT, one may hypothesize that individuals with ASD may also show increased brain activation when perceiving biological motion, thus normalizing attention bias towards socially salient stimuli.Citation44 Characterizing whether changes in brain response to biological motion perception may occur in “state” or “compensatory” regions may be especially informative for understanding how PRT may help improve social functioning in individuals with ASD. For example, improved social functioning may be driven by either increased normalization of developmental trajectory of social cognition in children with ASD compared to TD children (increased activation of “state” and “trait” regions to biological motion) or an increased recruitment of compensatory regions to overcome social perception difficulties similar to US, or both.
Voos et alCitation44 conducted the first fMRI study to evaluate changes in brain activation in response to biological motion following an open-label trial of 16 weeks of PRT in two high-functioning preschool-age children with ASD. The authors found that both children showed increased activation in “state” and “trait” regions in response to biological motion posttreatment, though distinct yet overlapping neural profiles emerged, which the authors concluded to be somewhat reflective of the level of social skills targeted for each child. For participant 1, goals were set to target lower-level adaptive social skills such as body positioning and voice modulation. For participant 2, goals were set to target higher-level social skills such as perspective taking, social reciprocity, and adaptive use of descriptive language. Both children made significant gains in adaptive social functioning as measured by both the Autism Diagnostic Observation ScheduleCitation53 and the Clinical Evaluation of Language Fundamentals Fourth Edition,Citation54 and demonstrated increased activation in bilateral FG (“state”) in response to biological motion, suggesting increased sensitivity to socially salient stimuli following PRT. However, only participant 2 demonstrated more widespread increased activation in state-defined rpSTS and vlPFC, which have been associated with higher-level social processingCitation55 such as perspective taking and understanding intention of others,Citation56 which may directly reflect the more advanced nature of social skills directly targeted during his intervention (). In contrast, no changes in “compensatory” regions were observed.
Given the small sample size of two, findings generated from Voos et al’sCitation44 study were largely preliminary, and could only offer a limited scope for interpretation. Nonetheless, the findings were promising and are important for future studies to consider due to three main reasons. First, behavioral clinical gains corresponded to brain activation changes in response to biological motion, suggesting that neural changes might serve as a sensitive and objective biomarker for measuring changes in social functioning over PRT. Second, the increased activation to biological motion was localized in “state” and “trait” regions only, suggesting that PRT may serve to normalize the developmental trajectory of neural mechanisms of social stimuli processing in children with ASD, by increasing recruitment of brain regions associated with social perception in TD children. Finally, the overlapping, yet distinct patterns of changes in brain activation following PRT across both participants further highlight the value of neuroimaging techniques to partition differential patterns of neural change underlying behavioral changes, and mark a step towards resolving the problem of equifinality. However, the small sample size of two children severely limits the ability to draw firm conclusions that can be generalized to a larger clinical population, especially to those of lower-functioning children with ASD. Furthermore, the lack of control group makes it unclear whether the neural changes observed were directly related to the therapeutic effects of PRT, some more general effects of development over the 16-week time window, or a combination.
Another way to directly address the issue of equifinality is to capitalize on the idea that heterogeneous behavioral and neural response profiles at baseline may undergo differential trajectories of change following PRT. Heterogeneity at baseline can thus serve as a marker for dividing participants into meaningful groups, where subsequent changes over the course of PRT can be directly compared and contrasted to evaluate differences in mechanisms of change. Utilizing fMRI and the biological motion paradigm, Ventola et alCitation38 compared and contrasted the baseline neural response to biological versus scrambled motion in rpSTS of each of 10 high-functioning school-aged children with ASD to the group average response of five TD children. Two distinct patterns of neural responses were identified: a “hyperactive” group (hyperactive rpSTS activation) and a “hypoactive” group (hypoactive rpSTS activation), relative to TD group average. The authors hypothesized that the hyperactive group may experience increased sensitivity to external stimuli, as a result of deficits in neural systems responsible for sensory gating (eg, increased activation in thalamus, amygdala, temporal cortical regions), and effective attention control.Citation57 In contrast, the hypoactive group may experience diminished social motivation, as a result of deficits in neural systems responsible for processing reward in response to social stimuliCitation58 (eg, reduced activation in nucleus accumbens, ventral striatum [VS], and amygdala). Despite distinct neural profiles in response to biological motion at baseline, both groups presented similar levels of reduced attention to faces and poor eye contact at baseline, thus highlighting the importance of using neuroimaging tools as an objective way of capturing underlying heterogeneity in the absence of distinct behavioral markers.
Following PRT, distinct neural patterns of mechanisms of change across the two groups emerged.Citation38 Given that PRT primarily targets social communication skills and appropriate response to social stimuli, the authors examined changes in response to biological versus scrambled motion that were driven primarily by differential response to biological motion pre- and post-PRT. The hyperactive group demonstrated reduced activation in rpSTS, amygdala, thalamus, and hippocampus in response to biological motion following PRT, as well as reduced functional connectivity between left amygdala and left prefrontal regions. For the hyperactive group, PRT may have elicited positive gains in neural processing of sensory gating and attention control. In contrast, the hypoactive group demonstrated increased activation in rpSTS and VS in response to biological motion following PRT, and significant increases in functional connectivity between bilateral VS and pSTS as well as nodes of the mirror neuron system (eg, right inferior frontal gyrus, precentral cingulate) (). For the hypoactive group, PRT may have elicited positive gains in neural processing of reward in response to socially meaningful stimuli. It is important to highlight that these distinct mechanisms of change were accompanied by considerable clinical gains as well as “normalized” patterns of response to perceiving socially meaningful stimuli, further suggesting that individuals may benefit from PRT in different ways in terms of increasing both self-regulation and social cognition. Translating back to clinical work, distinct neural responses may serve as objective biomarkers that help clinicians to identify and better monitor different therapeutic elements of PRT at work, an especially powerful tool when used in combination with behavioral data and subjective questionnaire ratings to better monitor how PRT may elicit positive therapeutic gains across a wider range of responder profiles.
Building upon positive gains in normalizing trajectories of functional connectivity in neural circuits underlying reduced social motivation and inefficient sensory gating and attention control,Citation38 it is important to investigate how PRT may influence neural circuitries involved in processing socially meaningful stimuli at a global level.Citation39 The nature of social cognition deficits in individuals with ASD may arise from multiple levels of social information processing, ranging from the coordination of social motivation and the ability to attune one’s attention to socially meaningful stimuli,Citation58,Citation59 to the ability to recognize and interpret perceived social stimuli such as biological motion and faces for higher-level processing.Citation46,Citation47,Citation60 In a recent study, Venkataraman et alCitation39 analyzed brain response to biological motion using fMRI and found that PRT might prompt changes in functional connectivity in social processing circuitry. The authors adopted a data-driven approach and estimated that posterior cingulate cortex (PCC), an area involved in both self-regulation and social cognition,Citation61–Citation63 is a locus representing aggregated functional change in response to biological motion among 19 high-functioning school-aged children with ASD following PRT. Changes in PCC activity were accompanied by both decreased functional synchrony to orbitofrontal cortex, implicated in evaluating reward value associated with external stimuli,Citation64 and increased functional synchrony to occipital–temporal cortex, implicated in perception of biological motion and facial recognition.Citation65 The authors interpreted this shift in functional synchrony as a result of PRT prompting increased recognition of socially meaningful stimuli, and engagement in higher-order social perception areas.
However, a major limitation of Venkataraman et al’sCitation39 study is the lack of control group, since neither a TD group nor a waitlist control group was included for comparison. It thus remains unclear whether changes in functional synchrony observed may be elicited by PRT per se rather than reflecting naturally occurring changes over the course of development among individuals with ASD. Furthermore, one cannot assess whether the quality of changes in functional synchrony may represent a closer alignment to that seen during social information processing found in typical development. Nonetheless, this study represents a promising first step towards the use of data-driven approach to investigate changes in functional synchrony at a brain circuitry level in response to social information following PRT.
From behavior to neuroscience: identifying responder profiles and predictors of change
Another important area critical to improving quality of care for children and young people with ASD is to characterize responder profiles to the treatment in question. The benefit of developing objective biomarkers that may help clinicians predict treatment outcome at baseline is twofold. First, such biomarkers can help clinicians select the most appropriate form of intervention to maximize treatment gain for the individual in question. Given that there are a wide variety of behavioral-based interventions for children with ASD, identifying predictors that are specific to the active ingredients of PRT is important, in order to aid clinicians assess whether the fit of PRT is appropriate for each child. Second, identifying behavioral and brain response profiles of nonresponders at baseline can indicate problem areas that are more treatment resistant, and guide clinicians to be more mindful of these issues when formulating treatment plans and identifying specific goals. Being more mindful, clinicians may consider implementing strategies to provide additional support for these more challenging and persistent issues, as well as taking into account how such adaptations may influence fidelity of treatment. To our knowledge, only three studies up to date have evaluated predictors of treatment outcome in children with ASD in response to PRT, identifying a mixture of behavioral and brain response biomarkers to characterize responder profile. Here, we summarize each study, highlighting the limitations arising from the paucity of literature in this area.
Using a retrospective design, Sherer and SchreibmanCitation66 compared the baseline profiles of six children with ASD (average age 3–4 years) who received 90-minute sessions of PRT, four to five times per week, for 5–6 months. Three children showed significant improvements and were characterized as responders to PRT, and three children did not show any significant change and were characterized as nonresponders to PRT. The children were matched for IQ, use of functional language, and autism symptom severity at baseline. The study aimed to examine whether differences in social behavior and play skills at baseline served as behavioral markers predicting treatment outcome. Both social and play skills were assessed using a child–mother interaction social probe, where mothers were given specific instructions to initiate language, reciprocal play, and responding to requests from their child over 5-minute intervals. Social behaviors included both initiation of interaction and the ability to sustain interaction. Play behaviors included functional use of toys, symbolic and pretend play, and the ability to engage in varied play. The authors found that compared to nonresponders, all three responders showed greater engagement in functional, symbolic, and varied play at baseline, and experienced improvements in all three areas over the course of PRT. In addition, responders also showed significant gains in ability to sustain social engagement following PRT. In contrast, nonresponders demonstrated lower levels of baseline play and social skills compared to responders, and saw little change in these functional behaviors over the course of PRT.
Given the well-matched cognitive, language, and autism symptom severity profiles at baseline, differences in therapeutic gains over the course of PRT could not be attributed to differences in cognitive capacity or language and social competency. It may be that those who displayed poorer social and play skills at baseline experienced a floor effect. PRT primarily serves to increase social communication skills through utilizing varied play activities that are naturally motivating for children,Citation20,Citation21 and creating opportunities naturally embedded in social interactions for teaching appropriate social responses. Therefore, such a floor effect in both play skills and social reciprocity at baseline might compromise individuals’ ability to learn through play during PRT, affecting long-term prognosis. However, given the small sample size and lack of alternative treatment for comparison, it is unclear whether such nonresponder profiles and the possible floor effects of low baseline social engagement may be a predictor of poor outcome following PRT only, or represent a generalized floor effect impacting treatment response to behavioral interventions in general. Finally, the identified responder and nonresponder profiles included a complex range of social and play-based behaviors, therefore making it difficult to identify whether there may be a specific active predictor that may account for a majority of variance associated with treatment outcome.
To address the question of treatment specificity, Schreibman et alCitation67 employed a prospective design by recruiting six children with ASD (age 2–4 years) who matched the nonresponder behavior profile identified from the above study at baseline, and compared intervention outcome following PRT and an alternative behavioral training known as discrete trial training (DTT). Compared to PRT, DTT is a highly structured behavioral intervention where target behavior is broken down into smaller components, each of which is taught using repeated discrete trials.Citation14,Citation16 All children received 18 hours of PRT first, followed by 18–36 hours of DTT. To identify whether poor treatment response may be related to either social avoidance or play-based skills measured at baseline, the authors identified that the six participants were matched to the nonresponder profile with three children demonstrating high toy contact and three children demonstrating low social avoidance at baseline.
The authors found that the nonresponder profile only predicted treatment outcome of PRT, and not that of DTT, suggesting PRT specificity. Furthermore, children who demonstrated high toy contact at baseline showed some minimal, yet relative greater, response to PRT compared to the group with low social avoidance. Degree of social avoidance and contact at baseline did not predict treatment response differences, suggesting that having low social avoidance at baseline may be neither necessary nor sufficient to help children respond to PRT. There are several limitations to this study. First, all children received PRT first, and therefore, it is unclear whether any therapeutic potentials following subsequent DTT may in fact reflect either delayed response to PRT or the result of cumulative benefits from prior PRT in combination with DTT.Citation67 Second, with a small sample size of three children in each group, it cannot be concluded whether interest in objects and toy contact measured at baseline may be a necessary factor for eliciting positive treatment response following PRT.
One way to partition how each behavioral predictor identified at baseline may account for variances in changes elicited over the course of PRTCitation66,Citation67 is to identify objective biomarkers such as neural clusters that can serve as correlates of baseline behaviors such as motivation, social responsiveness, and attention.Citation40 Differential baseline neurobiological responsiveness to social stimuli in each distinct neural cluster can serve as an objective proxy measure of how much its behavioral correlate may affect an individual’s overall social cognition capacity. So far, only one study has identified neurobiological markers that can predict treatment response to PRT. Using biological motion paradigm and fMRI, Yang et alCitation40 identified four neural clusters whose activation pattern during biological motion at baseline predicted treatment gains in social competency following 16 weeks of PRT in 20 children with ASD (mean age 5.9 years). Structurally, the clusters included areas such as the STS, superior parietal lobule, FG, vmPFC, VS, and putamen. Functionally, the clusters corresponded to a range of lower- and higher-level cognitive processes, ranging from motion and object perception, visuospatial attention, emotion regulation, and response inhibition, to reward and motivation.
The authors employed regression-based multivariate pattern analyses (MVPAs) to examine how unique activation patterns of voxels within neural networks may contribute to the function and efficiency of complex cognitive processes.Citation40 Using cross-validation design, MVPA modeling of pretreatment response to biological motion trained using one cohort of patients’ data significantly predicted changes in social impairment severity following PRT in an independent second cohort of patients’ data, thus illustrating that the biomarkers identified were both robust and generalizable across patient samples. However, the lack of comparison treatment group makes it difficult to conclude whether the neurobiological biomarkers identified were predictors specific to PRT, rather than a general neural profile indicative of brain’s readiness to respond to behavioral-based social skills interventions. Nonetheless, this study demonstrated an important first step toward using novel neuroimaging analyses to identify sensitive and robust biomarkers to predict treatment outcomes.
It is important to consider the value of such biomarkers in combination with individuals’ behavioral profiles, to gain a better understanding of how certain neurobiological profiles may be associated with behavioral predictors such as social avoidance and toy contact.Citation66,Citation67 Techniques such as MVPAs provide a more sensitive means to detect inter-participant variations in neurobiological response to social stimuli at baseline, and provide a useful means to help partition heterogeneity underlying similar behavioral manifestations at baseline to further increase precision when predicting response to treatment. Therefore, it is important to consider the complementary value of employing interdisciplinary research techniques, such as qualitative behavioral measures and neuroimaging analyses, when moving towards the goal of precision medicine.Citation41 One limitation of the current preliminary findings is the lack of information on broader systemic influences such as the type of educational settings the participants were in, socioeconomic status, and other environmental factors that may have played a role in shaping individual’s response to PRT over the course of treatment. Future studies should seek to characterize how external environmental factors outside the clinical setting may interact with individual’s biology, and better understand the gene–environment interaction underlying differential profiles of treatment response.
Limitations and future directions
Current neuroimaging findings of PRT are limited to studies with small sample sizes consisting of high-functioning school-aged children with ASD. Intervention studies with small sizes may be more influenced by inter-participant variance, and making it questionable whether heterogeneity in mechanisms of change captured is clinically meaningful and can be generalized to a wider clinical population.Citation68 Comparing effect sizes of therapeutic gains from both published and unpublished PRT studies, Sham and SmithCitation69 found that reported therapeutic gains were much greater in published studies, suggesting possible publication bias in empirical evidence supporting PRT.Citation70 However, despite potential publication bias, the average reported effect size still demonstrated PRT to be an effective intervention, and did not significantly impact PRT’s status as an evidence-based treatment for individuals with ASD.Citation69 Nonetheless, future studies should evaluate whether existing neuroimaging findings may be replicated using larger sample sizes, as well as assess generalizability to other age groups, particularly younger children with ASD, to further expand the evidence surrounding PRT and investigate its effectiveness as an early intervention for children with ASD. Future studies should also include lower-functioning individuals with ASD, in order to further increase reliability of findings discussed in the present review.
In addition, no neuroimaging studies up to date have used an effective control group, nor conducted follow-up assessments to examine long-term treatment outcomes following termination of PRT treatment.Citation71 From a developmental perspective, it is important to include either an age-matched waitlist control or a treatment-as-usual group in order to examine if functional neural changes cannot be fully explained by development alone, but are rather treatment-specific effects in response to PRT. Future studies should also aim to adopt a longitudinal design with a follow-up period to examine whether changes in functional response and connectivity when processing social information may last beyond the course of PRT. In addition, there is a need to evaluate whether clinical gains and predictors of treatment are specific to PRT, as opposed to other modes of behavioral- and communication-based interventions for children with ASD. Few randomized controlled trials (RCTs) have been conducted up to date.Citation72–Citation74 Although two RCTs have shown that PRT was able to elicit greater gains in social communication skillsCitation72 and reductions in disruptive behaviorCitation73 when compared to highly structured ABA, another RCT found no differences in overall therapeutic gains in spoken language skills when comparing PRT to pictorial naturalistic communication strategies.Citation74 Future studies should seek to include comparative intervention as well as treatment-as-usual groups, to further evaluate whether predictors and underlying mechanisms of response may be specific to PRT, to continue address the question of treatment specificity.
Future studies can also examine broader changes in other areas of brain, both within and beyond that of the social brain network. For example, using a broader range of tasks that require subjects to utilize other skills acquired during PRT, such as effective downregulation during affectively salient situations (top-down versus bottom-up affect regulation within the vmPFC–amygdala circuit), and the change in motivation when engaging in social versus nonsocial interchanges (ventral versus dorsal striatal response) might help better characterize other neural correlates outside the social brain, further expanding the range of objective neural biomarkers to help monitor and track progress over the course of PRT.
Finally, future studies can also consider using other modes of neuroimaging techniques such as electrophysiology (EEG) and event-related potentials (ERPs), as well as eye tracking, to assess changes in social competency following PRT. Both EEG/ERP and eye tracking can be used to investigate social information processing at a better temporal resolution compared to fMRI, and may cross-validate and extend existing findings from fMRI studies. Furthermore, cost per experiment is considerably lower for EEG/ERP and eye-tracking studies, and may be considered in a more favorable light for larger-scale studies with more frequent time points of assessment to monitor changes over the course of PRT, as well as at longer-term follow-ups.
Taken together, current findings presented based on fMRI neuroimaging provide a foundation for feasibility of finding objective biomarkers to help better understand mechanisms of change and predictors of therapeutic response in young children with ASD. At present, the neural findings presented have been solely generated based on fMRI, which is leading the scientific field in relation to identification of biological markers of treatment response and prediction. However, due to the expense of fMRI neuroimaging technique, there is a greater need to continue interdisciplinary research to examine the degree of convergence on finding biomarkers that can help predict treatment response by using other more cost-effective neuroimaging tools such as EEG/ERP, and eye tracking to facilitate more widespread use of technology as a clinical tool. Adapting existing and developing novel paradigms compatible with alternative modes of neuroimaging tools can generate results that can enable researchers to cross-validate against current fMRI results, to not only increase the robustness of biomarkers identified but also increase the scalability of such research tools to better serve a larger clinical population.
Conclusion
Recent studies using neuroimaging techniques mark a first step towards partitioning the underlying heterogeneous mechanisms of change driving improvements in social competency following PRT. The tight coupling between neural and behavioral changes observed over the course of PRT provides promising preliminary data to support the use of these neural regions involved in social processing as potential objective biomarkers to help predict and capture both the quality and magnitude of changes in social information processing throughout intervention. Such findings highlight that interdisciplinary research implementing neuroimaging techniques and qualitative behavioral measures can better aid the characterization of heterogeneous trajectories of change across multiple levels, ranging from behaviors to brain regional and circuitry level, which can be especially meaningful when used to address the issue of equifinality. By helping clinicians better understand how PRT may be eliciting positive therapeutic gains for each individual, as well as predicting who may be most likely to benefit from PRT, neuroimaging research serves as an important milestone along the journey towards the development of precision medicine for individuals with ASD.Citation41
Acknowledgments
Funding for PRT in the authors’ laboratory came from the Autism Science Foundation, Simons Foundation (#383661), Women’s Health Research at Yale University (#1087045), Deitz Family, Esme Usdan and Family, and Dwek Family to PV.
Disclosure
The authors report no conflicts of interest in this work.
References
- KannerLAutistic disturbances of affective contactNerv Child19432217250
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders5th edWashington, DCAmerican Psychiatric Association2013
- HartleySLSikoraDMMcCoyRPrevalence and risk factors of maladaptive behaviour in young children with autistic disorderJ Intellect Disabil Res2008521081982918444989
- SkinnerBThe Behavior of Organisms: An Experimental AnalysisOxfordAppleton-Century1938
- LovaasOIBehavioral treatment and normal educational and intellectual functioning in young autistic childrenJ Consult Clin Psychol1987551393571656
- VismaraLARogersSJBehavioral treatments in autism spectrum disorder: what do we know?Annu Rev Clin Psychol2010644746820192785
- Virués-OrtegaJApplied behavior analytic intervention for autism in early childhood: meta-analysis, meta-regression and dose–response meta-analysis of multiple outcomesClin Psychol Rev201030438739920223569
- MauriceCGreenGFoxxRMMaking a Difference: Behavioral Intervention for AutismAustin, TXPro-Ed, Inc2001
- Peters-SchefferNDiddenRKorziliusHSturmeyPA meta-analytic study on the effectiveness of comprehensive ABA-based early intervention programs for children with autism spectrum disordersPubMed Health2011 [cited January 13, 2017]. Available from: https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0032150/Accessed February 1, 2017
- SmithTWhat is evidence-based behavior analysis?Behav Anal201336173325729130
- KoegelLKAshbaughKKoegelRLPivotal response treatmentLangRHancockTBSinghNNEarly Intervention for Young Children with Autism Spectrum Disorder (Evidence-Based Practices in Behavioral Health)BaselSpringer International Publishing2016 [cited September 15, 2016]85112 Available from: http://link.springer.com/chapter/10.1007/978-3-319-30925-5_4
- KoegelRLEgelALMotivating autistic childrenJ Abnorm Psychol1979884418426479464
- SeligmanMEMaierSFFailure to escape traumatic shockJ Exp Psychol1967741196032570
- SteegeMWMaceFCPerryLLongeneckerHApplied behavior analysis: beyond discrete trial teachingPsychol Sch20074419199.0
- LovaasOIKoegelRSimmonsJQLongJSSome generalization and follow-up measures on autistic children in behavior therapyJ Appl Behav Anal19736113116516795385
- DelpratoDJComparisons of discrete-trial and normalized behavioral language intervention for young children with autismJ Autism Dev Disord200131331532511518484
- VerschuurRDiddenRLangRSigafoosJHuskensBPivotal response treatment for children with autism spectrum disorders: a systematic reviewRev J Autism Dev Disord2013113461
- KoegelRLKoegelLKTreatment of pivotal areasThe PRT Pocket Guide: Pivotal Response Treatment for Autism Spectrum DisordersBaltimore, MDPaul H. Brookes Publishing Co20121338
- VentolaPEOostingDRKeiferCMFriedmanHEToward optimal outcome following pivotal response treatment: a case seriesYale J Biol Med2015881374425745373
- KoegelRLDyerKBellLKThe influence of child-preferred activities on autistic children’s social behaviorJ Appl Behav Anal19872032432523667475
- DunlapGKoegelRLMotivating autistic children through stimulus variationJ Appl Behav Anal19801346196277204282
- DunlapGThe influence of task variation and maintenance tasks on the learning and affect of autistic childrenJ Exp Child Psychol198437141646707578
- VentolaPFriedmanHOostingDPivotal response treatment: case reportsPsychoanal Study Child20156924226027337818
- KoegelRLO’DellMDunlapGProducing speech use in nonverbal autistic children by reinforcing attemptsJ Autism Dev Disord19881845255383215880
- KoegelLKKoegelRLShoshanYMcNerneyEPivotal response intervention II: preliminary long-term outcome dataRes Pract Pers Sev Disabil1999243186198
- KoegelLKCarterCMKoegelRLTeaching children with autism self-initiations as a pivotal responseTop Lang Disord2003232134145
- KoegelLKKoegelRLGreen-HopkinsIBarnesCCBrief report: question-asking and collateral language acquisition in children with autismJ Autism Dev Disord2009404509515
- KoegelLSinghAKoegelRHollingsworthJBradshawJAssessing and improving early social engagement in infantsJ Posit Behav Interv2014162698025313271
- KoegelLKKoegelRLHurleyCFreaWDImproving social skills and disruptive behavior in children with autism through self-managementJ Appl Behav Anal19922523413531634427
- VentolaPEYangDAbdullahiSMPaisleyCABraconnierMLSukhodolskyDGBrief report: reduced restricted and repetitive behaviors after pivotal response treatmentJ Autism Dev Disord20164682813282027230762
- VismaraLALyonsGLUsing perseverative interests to elicit joint attention behaviors in young children with autism theoretical and clinical implications for understanding motivationJ Posit Behav Interv200794214228
- StahmerACTeaching symbolic play skills to children with autism using pivotal response trainingJ Autism Dev Disord19952521231417559281
- Baron-CohenSLeslieAMFrithUDoes the autistic child have a “theory of mind”?Cognition198521137462934210
- Baron-CohenSThe autistic child’s theory of mind: a case of specific developmental delayJ Child Psychol Psychiatry19893022852972523408
- Baron-CohenSAutism and symbolic playBr J Dev Psychol198752139148
- Baron-CohenSTheory of mind and autism: a reviewInt Rev Res Ment Retard200023169184
- JarroldCBoucherJSmithPSymbolic play in autism: a reviewJ Autism Dev Disord19932322813077687245
- VentolaPYangDYFriedmanHEHeterogeneity of neural mechanisms of response to pivotal response treatmentBrain Imaging Behav201591748825370452
- VenkataramanAYangDYDvornekNPivotal response treatment prompts a functional rewiring of the brain among individuals with autism spectrum disorderNeuroreport201627141081108527532879
- YangDPelphreyKASukhodolskyDGBrain responses to biological motion predict treatment outcome in young children with autismTransl Psychiatry2016611e94827845779
- InselTRThe NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatryAm J Psychiatry2014171439539724687194
- CicchettiDRogoschFAEquifinality and multifinality in developmental psychopathologyDev Psychopathol199684597600
- KaiserMDHudacCMShultzSNeural signatures of autismProc Natl Acad Sci U S A201010749212232122821078973
- VoosACPelphreyKATirrellJNeural mechanisms of improvements in social motivation after pivotal response treatment: two case studiesJ Autism Dev Disord201343111023104615
- JohanssonGVisual perception of biological motion and a model for its analysisPercept Psychophys1973142201211
- KlinALinDJGorrindoPRamsayGJonesWTwo-year-olds with autism orient to non-social contingencies rather than biological motionNature2009459724425726119329996
- SimionFRegolinLBulfHA predisposition for biological motion in the newborn babyProc Natl Acad Sci U S A2008105280981318174333
- FrithCDFrithUInteracting minds – a biological basisScience199928654451692169510576727
- DittrichWHTrosciankoTLeaSEMorganDPerception of emotion from dynamic point-light displays represented in dancePerception19962567277388888304
- CastelliFHappéFFrithUFrithCMovement and mind: a functional imaging study of perception and interpretation of complex intentional movement patternsNeuroimage200012331432510944414
- CastelliFFrithCHappéFFrithUAutism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapesBrain2002125Pt 81839184912135974
- HillELFrithUUnderstanding autism: insights from mind and brainPhilos Trans R Soc Lond B Biol Sci2003358143028128912639326
- LordCRutterMDiLavorePCRisiSGothamKBishopSLAutism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part I): Modules 1–4Torrence, CAWestern Psychological Services2012
- PaslawskiTThe Clinical Evaluation of Language Fundamentals, Fourth Edition (CELF-4): a reviewCan J Sch Psychol2005201–2129134
- PinkhamAEHopfingerJBPelphreyKAPivenJPennDLNeural bases for impaired social cognition in schizophrenia and autism spectrum disordersSchizophr Res2008991–316417518053686
- WykBCHudacCMCarterEJSobelDMPelphreyKAAction understanding in the superior temporal sulcus regionPsychol Sci200920677177719422619
- MarkramKMarkramHThe intense world theory – a unifying theory of the neurobiology of autismFront Hum Neurosci2010422421191475
- ChevallierCKohlsGTroianiVBrodkinESSchultzRTThe social motivation theory of autismTrends Cogn Sci201216423123922425667
- ElsabbaghMMercureEHudryKBASIS TeamInfant neural sensitivity to dynamic eye gaze is associated with later emerging autismCurr Biol201222433834222285033
- DawsonGWebbSJMcPartlandJUnderstanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studiesDev Neuropsychol200527340342415843104
- JohnsonMKRayeCLMitchellKJTouryanSRGreeneEJNolen-HoeksemaSDissociating medial frontal and posterior cingulate activity during self-reflectionSoc Cogn Affect Neurosci200611566418574518
- Andrews-HannaJRSmallwoodJSprengRNThe default network and self-generated thought: component processes, dynamic control, and clinical relevanceAnn N Y Acad Sci20141316295224502540
- DayanESellaIMukovskiyAThe default mode network differentiates biological from non-biological motionCereb Cortex201626123424525217472
- Scott-Van ZeelandAAAbrahamsBSAlvarez-RetuertoAIAltered functional connectivity in frontal lobe circuits is associated with variation in the autism risk gene CNTNAP2Sci Transl Med201025656ra80
- SchultzRTGauthierIKlinAAbnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndromeArch Gen Psychiatry200057433134010768694
- ShererMRSchreibmanLIndividual behavioral profiles and predictors of treatment effectiveness for children with autismJ Consult Clin Psychol200573352553815982150
- SchreibmanLStahmerACBarlettVCDufekSBrief report: toward refinement of a predictive behavioral profile for treatment outcome in children with autismRes Autism Spectr Disord20093116317220046210
- IoannidisJPWhy most published research findings are falsePLoS Med200528e12416060722
- ShamESmithTPublication bias in studies of an applied behavior-analytic intervention: an initial analysisJ Appl Behav Anal201447366367824990802
- EasterbrookPJBerlinJAGopalanRMatthewsDRPublication bias in clinical researchLancet199133787468678721672966
- CadoganSMcCrimmonAWPivotal response treatment for children with autism spectrum disorder: a systematic review of research qualityDev Neurorehabil201518213714424180635
- MohammadzaheriFKoegelLKRezaeeMRafieeSMA randomized clinical trial comparison between pivotal response treatment (PRT) and structured applied behavior analysis (ABA) intervention for children with autismJ Autism Dev Disord201444112769277724840596
- MohammadzaheriFKoegelLKRezaeiMBakhshiEA randomized clinical trial comparison between pivotal response treatment (PRT) and adult-driven applied behavior analysis (ABA) intervention on disruptive behaviors in public school children with autismJ Autism Dev Disord20154592899290725953148
- SchreibmanLStahmerACA randomized trial comparison of the effects of verbal and pictorial naturalistic communication strategies on spoken language for young children with autismJ Autism Dev Disord20144451244125124272416

