Abstract
Introduction
Sex-related differences in clinical manifestations and consequences of Parkinson’s disease (PD) have been poorly explored. Better understanding of sexual dimorphism in neurologic diseases such as PD has been announced as a research priority. The aim of our study was to determine independent sex differences in clinical manifestations and subtypes, psychosocial functioning, quality of life (QoL) and its domains between male and female individuals with PD.
Patients and methods
A comprehensive list of demographics, motor symptoms and subtypes, nonmotor features, health-related quality of life (HRQoL), psychosocial functioning and general aspects of daily life was assessed in 157 individuals (108 males and 49 females) with idiopathic PD. In order to control for potential confounding variables, we applied Orthogonal Partial Least Squares – Discriminant Analysis (OPLS-DA) to explore the strength of each feature to discriminate male and female patients with PD.
Results
While no sex difference was found in the total Unified Parkinson’s Disease Rating Scale (UPDRS) score and cumulative daily dose of levodopa, females had significantly more severe anxiety (mean difference =2.2 [95% confidence interval, CI: 0.5–4.0], P=0.011), worse nutritional status (23.8 [standard deviation, SD =4.2] vs 25.8 [SD =2.6], P=0.003) and poorer QoL (28.3 [SD =15.7] vs 17.9 [SD =14.2], P<0.001). Based on multivariate discriminant analysis, emotional well-being, bodily discomfort, social support, mobility and communication domains of HRQoL, together with anxiety, depression and psychosocial functioning, were the strongest features with more severe/worse status in females after adjustment for potential statistical confounders.
Conclusion
Our study provides a comprehensive understanding of sexual dimorphism in PD. Anxiety, depression, specific domains of HRQoL (mobility, emotional well-being, social support and bodily discomfort) and psychosocial functioning were significantly worse in female individuals with PD. Sexual dimorphism in PD highlights the features that are more likely to be affected in each sex and should be specifically targeted when managing male and female individuals with PD.
Introduction
Parkinson disease (PD) is a progressive neurodegenerative disorder with widely heterogeneous manifestations, including motor, nonmotor and neuropsychiatric symptoms. It affect~1% of the people older than 60 years.Citation1–Citation3 The major pathologic feature of PD is the loss of dopaminergic neurons in substantia nigra pars compacta and, consequently, basal ganglia.Citation4,Citation5
Sex differences in prevalence, clinical presentations and severity of PD have been reported in some previous studies.Citation6–Citation9 Although the exact sources of these differences in PD are unknown, sex hormones, genetic factors and variations in dopaminergic pathways have been proposed as possible underlying reasons.Citation10–Citation14 Identifying sexual variations influences prevention, therapeutic strategies and understanding of sex-related neurobiological differences.Citation15,Citation16 Exploration of sexual dimorphism in neurologic diseases such as PD has been recently announced as a research need.Citation3 While studies are unanimous about the higher prevalence of PD among men, there are conflicting data regarding sex differences in clinical manifestations, progression and treatment outcome.Citation17–Citation23 Motor presentations (ie, tremor and dyskinesia), psychological manifestations (especially depression), sleep behavior disorders and cognitive impairment have been reported as potential aspects of sexual dimorphism in PD.Citation17–Citation23 However, data are still controversial, inconclusive and even conflicting in some aspects. As an example, a recent randomized clinical trial did not find any sex differences in daily activities and main motor features.Citation24 In addition to lack of comprehensive data, these controversies can be partly due to interrelationship and confounding effects of clinical and demographic variables.Citation3 Some clinical features that were previously reported to be sex related failed to show any difference between males and females with PD when controlled for other demographic and clinical variables. For instance, cognitive impairment was reported to be more common in men;Citation9 however, the difference was much less pronounced when the effect of age, level of education and disease severity were taken into account.Citation23 In another large study, univariate analysis showed that daily activity was more impaired in females, but multivariate analysis revealed no significant sex difference.Citation24
Having collected comprehensive data on different PD- related and general features, we aimed to further investigate sexual dimorphism in PD. Our objective was to determine independent differences in clinical manifestations and subtypes, psychosocial functioning, quality of life (QoL) and its domains between males and females with idiopathic Parkinson’s disease (IPD) using a powerful statistical approach. Our a priori research hypothesis was that females with PD experienced more severe nonmotor manifestations, which would also influence their QoL and psychosocial functioning more prominently than males.
Patients and methods
Study setting
This study was conducted on 157 consecutive patients with IPD from an outpatient referral movement disorders clinic in Tehran, Iran, between October 2011 and December 2012. This was a collaborative project between Iran University of Medical Sciences (Tehran, Iran) and Karolinska Institute (Stockholm, Sweden).
Ethics
The study protocol was approved by the ethics committee of the Neurology Department at Firoozgar Clinical Research Development Center (FCRDC; affiliated to Iran University of Medical Sciences). Data were stored and treated according to the ethical guidelines of biomedical research. Prior to the launch of the study, all patients were informed about the aims and procedures. All participants provided their verbal informed consent to participate in this study. Since the project was designed as an observational research, verbal form of consent was approved by the aforementioned ethics committee. Participation in this study was voluntary, and the patients were free to withdraw from the project whenever they decided. Furthermore, the identity of research participants was protected, since the data files were anonymous and all names were omitted.
Patients’ requirement
Recruited patients fulfilled the following inclusion criteria: diagnosis of IPD based on the UK brain bank criteria,Citation25 which was assessed by the same neurologist who was specialized in movement disorders for all participants; current age of 30 years or older and motor disability in the mild-to-severe range but not in the advanced stages that needs wheelchair or hospitalization according to the Hoehn and Yahr (H&Y) criteria (stage <5).Citation26 Patients with moderate to severe dementia were excluded from the study, as were those with atypical Parkinsonism, including multiple system atrophy (MSA), progressive supranuclear palsy (PSP) and vascular or drug-induced Parkinsonism.
Assessments
Data collection was performed through face-to-face interviews with eligible patients by a trained group of medical interns by means of validated questionnaires and checklists. Patients were also examined for clinical assessments and diagnosis by one neurologist specialized in movement disorders. The demographic checklist consisted of baseline variables (age and sex), educational status, history of smoking, comorbidities, duration of PD (time passed from diagnosis) and history of levodopa administration. Total comorbidity burden was calculated by summing up the number of chronic comorbid conditions in each participant, including depression, hypertension, interstitial heart disease (IHD), diabetes, stroke/transient ischemic attack (TIA) and osteoporosis. Data were collected based on participants’ self-reports and their medical records at the referral center. Clinical characteristics of PD were assessed using the following scales and/or definitions:
Motor severity: Unified Parkinson’s Disease Rating Scale (UPDRS) subscales I–IV, dyskinesia score (sum of UPDRS – Part IV items 32–34), fluctuation score (sum of UPDRS – Part IV items 36–39), H&Y staging and Schwab and England activities of daily living (ADL);
Motor subtypes: postural instability–gait difficulty (PIGD) score (sum of UPDRS – Part III items concerning rise, gait and postural instability) and freezing–speech–swallowing (FOSS) score (sum of UPDRS – Part II items on freezing, speech and swallowing);
Predominance of core manifestations: proportion of UPDRS – Part III on motor scores accounted for tremor (items 20–21), rigidity (item 22), bradykinesia (items 23–26 and 31) and gait (items 27–30) in percentage;
Asymmetry Index: absolute differences in UPDRS between sides divided by the total UPDRS – Part III items 20–26;
Axial/limb ratio: sum of UPDRS – Part III items 18, 19, 22 and 27–30 divided by the sum of UPDRS – Part III items 20–26;
Other motor symptoms: presence of falls and freezing and
Nonmotor manifestations: the Hospital Anxiety and Depression Scale (HADS) questionnaire to measure anxiety and depression,Citation27 Fatigue Severity Scale (FSS) to investigate fatigue,Citation28 the Mini-Nutritional Assessment (MNA) questionnaire together with anthropometric measurements for nutritional status,Citation29 Persian-translated and validated version of the Scales for Outcomes in Parkinson’s Disease – Psychosocial (SCOPA-PS) questionnaireCitation30 to assess psychosocial functioning and validated Persian version of the 39-item PD Questionnaire (PDQ-39)Citation31 to evaluate health-related quality of life (HRQoL). All clinical assessments were done when the patients were in the “on” state.
Statistical analysis
Statistical descriptions and univariate and multivariate regression analyses were performed using the IBM SPSS Statistics for Windows, version 23 (IBM Corporation, Armonk, NY, USA). Mean (standard deviation [SD]) and frequency percentages were used to describe numerical and categorical variables, respectively. Kolmogorov–Smirnov test was applied to check the normality assumption for continuous variables. Since the normality of distribution was met, we used parametric tests for between-group comparisons. Univariate comparisons between males and females were performed using either independent samples t-test, chi-square test or Fisher’s exact test where appropriate. We used multivariate linear regression model to adjust the between-group differences in some numeric variables of interest for the baseline differences in disease duration and level of education. A two-tailed P-value of <0.05 was considered as the threshold to show statistical significant differences.
In order to evaluate the strength of each variable to discriminate male and female patients with PD, we applied Orthogonal Partial Least Squares – Discriminant Analysis (OPLS-DA) method using SIMCA software, version 14.1 (MKS Umetrics AB, Umeå, Sweden). Unit variance (UV) scaling was used to transform crude data prior to the OPLS-DA modeling. The OPLS-DA method divides the systematic variation in the X-block consisting of a comprehensive list of demographic and PD-related features to separate males and females with PD into two model parts. One part models the co-variation between X and Y, and another part expresses the X-variation that is not related to Y and is shown by the orthogonal component(s). This method results in a better class resolution for a discriminant problem such as the case in our study. Furthermore, the OPLS-DA method made it possible to compare the strength of different variables with various measurement units and scales to discriminate males and females with PD by means of values of standardized loading, their standard error (SE) and 95% confidence interval (CI). We visualized findings from the OPLS-DA method by three different plots with the following parameters:
Score scatter plot: to show the performance of the entire OPLS-DA model to separate males and females where t1 refers to the score of each participant from the main discriminant component in the X-block (horizontal axis), while t2 shows the score of each participant from the Y-orthogonal component (vertical axis);
Loadings bars: this is a one-dimensional plot representing loading value (p1) of each feature to discriminate males and females based on the main discriminant component of the OPLS-DA model;
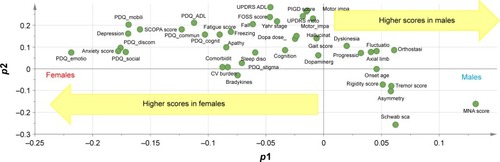
Loadings scatter plot: in this plot, p1 refers to the loading values of each feature from the main discriminant component to discriminate males and females (horizontal axis) and p2 represents the loadings of each variable from the Y-orthogonal component for the differences between features that are not related to sex (vertical axis).
Results
Overall, 157 individuals consisting of 108 males and 49 females with PD were recruited in our study. summarizes the results for univariate comparison of the demography, motor severity and medications, motor subtypes, nonmotor features and QoL indicators between the two subgroups.
Table 1 Comparison of demographic characteristics, disease severity scales, psychiatric features, nutritional status and QoL scores between males and females with PD
Univariate and multivariate comparisons
The age at PD onset did not significantly differ between males and females (55.2 [SD =11.6] year vs 53.4 [SD =12.4] year, P=0.384), however, disease duration was longer in females at the time of recruitment (6.1 [SD =4.9] year vs 8.2 [SD =5.7] year, P=0.023). PD-related medication profile did not differ between males and females. Univariate differences in motor features such as total UPDRS and freezing score disappeared after adjustment for baseline difference. Among nonmotor features, anxiety, depression, fatigue and psychosocial functioning were all significantly more severe in female PD patients (). After statistical adjustment for the baseline differences in disease duration and level of education, female individuals still showed significantly higher score in anxiety (mean difference =2.2 [95% CI: 0.5–4.0], P=0.011) and borderline higher score in depression (mean difference =1.3 [95% CI: −0.2–2.7], P=0.079). Furthermore, female PD patients had significantly worse nutritional status and higher Parkinson’s Disease Summary Index (PDSI), both of which remained statistically significant after multivariate adjustment (mean difference for MNA score =−1.5 [95% CI: −2.5 to −0.4], P=0.009; mean difference for PDSI score =6.9 [95% CI: 1.9–11.8], P=0.006).
Among different domains of QoL, females were significantly worse in mobility (40.8 [SD =27.4] vs 22.5 [SD =24.0], P<0.001), emotional well-being (39.4 [SD =24.4] vs 23.5 [SD =21.7], P<0.001), social support (20.1 [SD =25.9] vs 7.5 [SD =15.9], P<0.001) and bodily discomfort (33.0 [SD =25.3] vs 17.2 [SD =20.1], P<0.001). All of these differences were still significant even after multivariate adjustment for the baseline differences in disease duration and level of education between males and females, resulting in the mean difference of 11.9 (95% CI: 3.5–20.3, P=0.006) in mobility, 12.5 (95% CI: 4.7–20.3, P=0.002) in emotional well-being and 11.0 (95% CI: 0.9–21.5, P=0.001) and 14.2 (95% CI: 6.4–21.9, P<0.001) in bodily discomfort domain of QoL.
Discriminant analysis
The OPLS-DA model that fitted the best to our dataset had the overall cross-validated predictive R2 value of 0.33. The score scatter plot in shows the performance of this model to discriminate males and females with PD. The corresponding loading value of each variable in the OPLS-DA model and its 95% CI are shown in , and the loadings scatter plot is illustrated in . Based on the absolute loading value for each variable in the OPLS-DA model, emotional well-being (mean =−0.22, SE =0.14), bodily discomfort (mean =−0.18, SE =0.13), social support (mean =−0.17, SE =0.19), mobility (mean =−0.17, SE =0.04) and communication (mean =−0.12, SE =0.15) domains of QoL, together with anxiety (mean =−0.18, SE =0.10), depression (mean =−0.17, SE =0.08) and psychosocial functioning (mean =−0.16, SE =0.11), were the strongest features with higher values in favor of females to discriminate the two sexes in PD population ( and ). Nutritional status (mean =0.13, SE =0.07), Schwab and England ADL score (mean =0.06, SE =0.13), and orthostasis (mean =0.06, SE =0.05) were also found to be strong discriminators in the model, nevertheless, with higher values in favor of the male PD patients.
Figure 1 Score scatter plot to show the performance of the OPLS model to separate male and female PD patients.
Abbreviations: OPLS, Orthogonal Partial Least Squares; PD, Parkinson’s disease; t1, score of each participant from the main discriminant component in the X-block (horizontal axis); t2, score of each participant from the Y-orthogonal component (vertical axis).
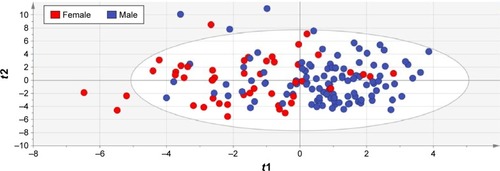
Figure 2 Loading plots of the OPLS model to separate male and female PD patients showing the importance of each feature for this discrimination.
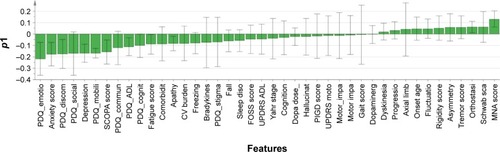
Figure 3 Loadings scatter plot p1 vs p2 to show the performance each feature/characteristic of the OPLS model to separate male and female PD patients.
Abbreviations: OPLS, Orthogonal Partial Least Squares; PD, Parkinson’s disease; PDQ, PD questionnaire; SCOPA, Scales for Outcomes in Parkinson’s Disease; ADL, activities of daily living; CV, cardiovascular; FOSS, freezing–speech–swallowing; UPDRS, Unified Parkinson’s Disease Rating Scale; PIGD, postural instability–gait difficulty; MNA, Mini-Nutritional Assessment.
Discussion
Our study is one of the few attempts to comprehensively investigate sexual dimorphism in PD. We applied powerful multivariate statistical methods to explore independent sex differences in clinical manifestations, HRQoL and psychosocial functioning of people with PD. In general, females with PD were significantly worse in psychological features such as anxiety and depression, nutritional status and specific domains of QoL, namely, mobility, emotional well-being, social support and bodily discomfort. Neuropsychiatric symptoms are among the main manifestations of sexual dimorphism in PD. Studies have suggested that emotional symptoms of PD, especially depression, are more common and more severe in females than in males.Citation32–Citation34 However, these findings were not approved by a large prospective study, which found no difference in the prevalence or severity of depression evaluated by Beck Depression Inventory (BDI) between females and males.Citation24 In our study, females with PD tended to be more affected from psychological symptoms. We found that the scores for anxiety, psychosocial functioning and emotional well-being were all higher among females demonstrating a worse status. Conflicting results can be, in part, explained by ethnic, cultural and environmental differences as well as the use of different depression scales. In some cultures and environments, women are more prone to be stigmatized by disabling conditions,Citation35,Citation36 which make them more vulnerable to emotional and psychosocial adverse effects of PD.
Social and physical well-being, measured separately or as parts of QoL questionnaires, are important contributors of patient’s QoL.Citation37,Citation38 In our study, physical discomfort, including mobility, fatigue and bodily discomfort, was more severe among female participants. This finding is confirmed by previous studies of PD populations,Citation17,Citation34,Citation39 as well as those of general population showing more severe physical discomfort in healthy older women compared to males of the same age group.Citation40–Citation42 Our results showed that despite the lack of statistically significant difference in social stigma, females displayed worse communication and psychosocial functioning. These sex-related differences can be the consequences of more severe emotional disturbance, physical discomfort, and poorer social supports in females compared to males with PD.
In our study, self-reported cognitive impairment was higher in female participants based on the cognition dimension of PDQ-39. In contrast, some studies have suggested that cognitive decline in PD is more severe among males.Citation18,Citation23 Nonetheless, as different dimensions of cognitive performance have been shown to be sex related in healthy elderly adults (for instance, males have better visuospatial ability, while females perform better on face and verbal recognition and semantic fluency testsCitation43–Citation45), different dimensions of cognitive performance must be investigated in order to clarify sexual dimorphism of cognition in PD.
Studies have reported several sex differences in motor symptoms of PD. Tremor and dyskinesia are reported to occur more frequently in females,Citation8,Citation18,Citation33 whereas gait disturbance is more common in males.Citation19 However, in line to one prospective study that had also adjusted the differences for the confounding effects of demographics and clinical covariates,Citation24 we also could not find sex-related differences in motor symptoms.
Sleep quality is the other symptom of PD, which is believed to be sex related, but still remained a controversial issue. Rapid eye movement (REM) sleep behavior disorder (RBD) was reported as a male-related symptom in PD;Citation33,Citation46–Citation48 some other sleep disturbances were found to be more severe among females.Citation34,Citation49 We did not find any difference in sleep disturbance between males and females using the nonmotor items of the UPDRS. A more thorough assessment with a valid and specific tool such as polysomnography is required to investigate sexual dimorphism in sleep disorders in people with PD.
The comprehensive list of PD-related features and general aspects of daily life is the most important strength of our study. Previous studies have investigated sexual dimorphism in manifestations of PD with rather small sample size not being controlled for the potential confounders.Citation3,Citation50 In our study, we evaluated a large list of variables (some of which were considered for sexual dimorphism in PD for the first time) and applied a strong multivariate statistical approach, OPLS-DA, to take into account all potential confounding interactions. Nevertheless, we also acknowledge our limitations. This study was designed as a cross-sectional research that restricts any causal inferences and going beyond associations. There are other aspects of sexual dimorphism in PD that need to be addressed in future research, namely, course of progression and mortality. There is a potential risk for selection bias since all recruited patients were from an outpatient neurology clinic where the majority of patients had mild-to-moderate IPD. As a result, our findings may not be generalized to all people with PD, particularly those with end-stage disease. Considering time restriction for data collection spent in each participant, we ought to rely on the items from UPDRS for some variables, which might not be sensitive enough to show between-group differences on this summary scale. Therefore, more sensitive gold-standard tools might potentially show different results for such features. Our rather small sample size in comparison with few large previous studies should also be noted. Augustine et alCitation24 reported that studies with smaller sample sizes were more likely to report sex differences in different clinical features. We should also add the uneven number of males and females in our study as another limitation; however, it could be expected for PD that affects men more frequently.Citation51 Yet the comprehensive list of variables and appropriate multivariate statistical methods of the present study overcame these issues and strengthened our findings.
Conclusion
This study provides a comprehensive understanding of sexual dimorphism in different motor and nonmotor features of PD and various domains of daily life by using sophisticated statistical analysis to evaluate the independent differences between males and females. After controlling for potential confounders, anxiety, depression, mobility, emotional well-being, social support, bodily discomfort and psychosocial functioning were significantly worse in female individuals with PD. On the other hand, male PD patients had better nutritional status (though with rather small effect size for difference) and ADL but also more severe orthostasis. Conclusively, there are considerable differences in psychiatric nonmotor manifestations. Concordance of depression, anxiety and overall psychosocial burden in being more severe in women is in favor of the presence of a considerable sex dimorphism in neuropsychiatric symptoms, which in turn suggest its clinical importance. QoL and psychosocial consequences of PD between males and females should be addressed for developing a more personalized caring approach. These aspects of sexual dimorphism in PD also enlighten the features that are more likely to be affected in each sex and should be specifically targeted when managing male and female individuals with PD. As for clinical implication, clinicians should consider sex differences in the evaluation and management of patients with PD. For example, they should be aware of the higher risk of psychological manifestations in women and therefore should screen them accurately in a timely manner and, if necessary, refer them to a psychologist in the early stages of these manifestations. Sex-specific symptoms highlight the importance of sex-specific treatments; hence, future studies should investigate the effects of specific interventions for females. Moreover, educational interventions are also needed in order to improve social support for women with PD and reduce their extra psychosocial burden.
Acknowledgments
The authors are grateful to the colleagues from the Movement Disorder Clinic who contributed in data collection: Hasti Hadizadeh, Mahdiyeh Shafieesabet, Nader Naderi, Dena Khaefpanah, and Ms Mahmoudi. The authors also thank all patients and their caregivers for their collaboration to collect the data for this project.
Disclosure
The authors report no conflicts of interest in this work.
References
- LangstonJWThe Parkinson’s complex: parkinsonism is just the tip of the icebergAnn Neurol200659459159616566021
- ElbazABowerJHMaraganoreDMRisk tables for parkinsonism and Parkinson’s diseaseJ Clin Epidemiol2002551253111781119
- PavonJMWhitsonHEOkunMSParkinson’s disease in women: a call for improved clinical studies and for comparative effectiveness researchMaturitas201065435235820117891
- StuendlAKunadtMKruseNInduction of alpha-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with lewy bodiesBrain2016139pt 248149426647156
- KaliaLVKaliaSKalpha-synuclein and lewy pathology in Parkinson’s diseaseCurr Opin Neurol201528437538126110807
- KowalSLDallTMChakrabartiRStormMVJainAThe current and projected economic burden of Parkinson’s disease in the United StatesMov Disord201328331131823436720
- AmanteaDRussoRBagettaGCorasanitiMTFrom clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogensPharmacol Res200552211913215967377
- HaaxmaCABloemBRBormGFGender differences in Parkinson’s diseaseJ Neurol Neurosurg Psychiatry200778881982417098842
- MillerINCronin-GolombAGender differences in Parkinson’s disease: clinical characteristics and cognitionMov Disord201025162695270320925068
- SmithKMDahodwalaNSex differences in Parkinson’s disease and other movement disordersExp Neurol2014259445624681088
- CzechDPLeeJSimHParishCLVilainEHarleyVRThe human testis-determining factor SRY localizes in midbrain dopamine neurons and regulates multiple components of catecholamine synthesis and metabolismJ Neurochem2012122226027122568433
- DewingPChiangCWSinchakKDirect regulation of adult brain function by the male-specific factor SRYCurr Biol200616441542016488877
- LavalayeJBooijJRenemanLHabrakenJBvan RoyenEAEffect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteersEur J Nucl Med200027786786910952500
- SimunovicFYiMWangYStephensRSonntagKCEvidence for gender-specific transcriptional profiles of nigral dopamine neurons in Parkinson diseasePLoS One201051e885620111594
- PancholySBSharmaPSPancholyDSPatelTMCallansDJMarchlinskiFEMeta-analysis of gender differences in residual stroke risk and major bleeding in patients with nonvalvular atrial fibrillation treated with oral anticoagulantsAm J Cardiol2014113348549024315113
- AndersonGDGender differences in pharmacological responseInt Rev Neurobiol20088311018929073
- ShulmanLMGender differences in Parkinson’s diseaseGend Med20074181817584622
- LyonsKEHubbleJPTrosterAIPahwaRKollerWCGender differences in Parkinson’s diseaseClin Neuropharmacol19982121181219579298
- ScottBBorgmanAEnglerHJohnelsBAquiloniusSMGender differences in Parkinson’s disease symptom profileActa Neurol Scand20001021374310893061
- KompolitiKComellaCLJaglinJALeurgansSRamanRGoetzCGMenstrual-related changes in motoric function in women with Parkinson’s diseaseNeurology200055101572157511094119
- MozleyLHGurRCMozleyPDGurREStriatal dopamine transporters and cognitive functioning in healthy men and womenAm J Psychiatry200115891492149911532737
- ElbersRvan WegenEERochesterLIs impact of fatigue an independent factor associated with physical activity in patients with idiopathic Parkinson’s disease?Mov Disord200924101512151819514069
- NazemSSiderowfADDudaJEMontreal cognitive assessment performance in patients with Parkinson’s disease with “normal” global cognition according to mini-mental state examination scoreJ Am Geriatr Soc200957230430819170786
- AugustineEFPerezADhallRSex differences in clinical features of early, treated Parkinson’s diseasePLoS One2015107e013300226171861
- HughesAJDanielSEKilfordLLeesAJAccuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 casesJ Neurol Neurosurg Psychiatry19925531811841564476
- HoehnMMYahrMDParkinsonism: onset, progression, and mortalityNeurology19671754274426067254
- MontazeriAVahdaniniaMEbrahimiMJarvandiSThe hospital anxiety and depression scale (HADS): translation and validation study of the Iranian versionHealth Qual Life Outcomes200311412816545
- FereshtehnejadSMHadizadehHFarhadiFShahidiGADelbariALokkJReliability and validity of the Persian version of the fatigue severity scale in idiopathic Parkinson’s disease patientsParkinsons Dis2013201393542924089644
- GhaziLFereshtehnejadSMAbbasi FardSSadeghiMShahidiGALokkJMini nutritional assessment (MNA) is rather a reliable and valid instrument to assess nutritional status in Iranian Healthy Adults and Elderly with a chronic diseaseEcol Food Nutr201554434235725714475
- FereshtehnejadSMFarhadiFHadizadehHShahidiGADelbariALökkJCross-cultural validity, reliability, and psychometric properties of the Persian version of the scales for outcomes in Parkinson’s disease-psychosocial questionnaireNeurol Res Int2014201426068424804096
- NojomiMMostafavianZShahidiGAJenkinsonCQuality of life in patients with Parkinson’s disease: translation and psychometric evaluation of the Iranian version of PDQ-39J Res Med Sci2010152636921526061
- FernandezHHLapaneKLOttBRFriedmanJHGender differences in the frequency and treatment of behavior problems in Parkinson’s disease. SAGE Study Group. Systematic assessment and geriatric drug use via epidemiologyMov Disord200015349049610830414
- Martinez-MartinPFalup PecurariuCOdinPGender-related differences in the burden of non-motor symptoms in Parkinson’s diseaseJ Neurol201225981639164722237822
- KovacsMMakkosAAschermannZImpact of sex on the non-motor symptoms and the health-related quality of life in Parkinson’s diseaseParkinsons Dis20162016795184027293959
- KhanNKausarRKhalidAFarooqAGender differences among discrimination & stigma experienced by depressive patients in PakistanPak J Med Sci20153161432143626870110
- AsieduGBMyers-BowmanKSGender differences in the experiences of HIV/AIDS-related stigma: a qualitative study in GhanaHealth Care Women Int2014357–970372724564483
- KalfossMHalvorsrudLImportant issues to quality of life among Norwegian older adults: an exploratory studyOpen Nurs J20093455519738913
- MolzahnAEKalfossMSchick MakaroffKSkevingtonSMComparing the importance of different aspects of quality of life to older adults across diverse culturesAge Ageing201140219219921186234
- BabaYPutzkeJDWhaleyNRWszolekZKUittiRJGender and the Parkinson’s disease phenotypeJ Neurol2005252101201120516151602
- MantonKGA longitudinal study of functional change and mortality in the United StatesJ Gerontol1988435S153S1612971088
- Kelly-HayesMJetteAMWolfPAD’AgostinoRBOdellPMFunctional limitations and disability among elders in the Framingham StudyAm J Public Health19928268418451533995
- GuerraROAlvaradoBEZunzuneguiMVLife course, gender and ethnic inequalities in functional disability in a Brazilian urban elderly populationAging Clin Exp Res2008201536118283229
- RuitenbergAOttAvan SwietenJCHofmanABretelerMMIncidence of dementia: does gender make a difference?Neurobiol Aging200122457558011445258
- LetenneurLGilleronVCommengesDHelmerCOrgogozoJMDartiguesJFAre sex and educational level independent predictors of dementia and Alzheimer’s disease? Incidence data from the PAQUID projectJ Neurol Neurosurg Psychiatry199966217718310071096
- ShinJYPohligRTHabermannBSelf-reported symptoms of Parkinson’s disease by sex and disease durationWest J Nurs Res Epub2016923
- ScaglioneCVignatelliLPlazziGREM sleep behaviour disorder in Parkinson’s disease: a questionnaire-based studyNeurol Sci200525631632115729494
- OzekmekciSApaydinHKilicEClinical features of 35 patients with Parkinson’s disease displaying REM behavior disorderClin Neurol Neurosurg2005107430630915885389
- YoritakaAOhizumiHTanakaSHattoriNParkinson’s disease with and without REM sleep behaviour disorder: are there any clinical differences?Eur Neurol200961316417019129703
- PandeySBajajBKWadhwaAAnandKSImpact of sleep quality on the quality of life of patients with Parkinson’s disease: a questionnaire based studyClin Neurol Neurosurg2016148293427372436
- Van Den EedenSKTannerCMBernsteinALIncidence of Parkinson’s disease: variation by age, gender, and race/ethnicityAm J Epidemiol2003157111015102212777365
- KaliaLVLangAEParkinson’s diseaseLancet2015386999689691225904081
