Abstract
This study aimed to perform a comprehensive meta-analysis of topiramate-augmentation therapy in patients with schizophrenia receiving antipsychotic agents. Data published up to June 20, 2016 were obtained from the PubMed, PsycINFO, and Cochrane Library databases. Twelve randomized controlled trials comparing topiramate to placebo or antipsychotic only were included (n=676 patients). The primary outcome was change in overall symptoms. Relative risk (RR) and standardized mean difference (SMD), along with 95% confidence intervals, were calculated using random effects model for each outcome. Topiramate-augmentation therapy was superior to the control for decreasing overall symptoms (SMD −0.55, 95% confidence interval −0.86 to −0.24; P=0.001; I2=55%, eight comparisons, n=380), positive symptoms (SMD −0.4), negative symptoms (SMD −0.47), and Positive and Negative Syndrome Scale general subscale scores (SMD −0.67). Furthermore, topiramate-augmentation therapy decreased weight (SMD −0.69) and body mass index (SMD −0.95) compared with the control. Topiramate was similar to the control with respect to discontinuation due to all causes (RR 1.19), inefficacy (RR 1.71), and adverse events (RR 1.09). Topiramate was associated with higher incidence of paresthesia (RR 2.67) and attention difficulty (RR 8.97) compared with the control. Our results seemed to suggest that topiramate-augmentation therapy improves the psychopathology of schizophrenia with good tolerability and has the additional advantage of weight maintenance. However, because there were some limitations (numbers of studies and patients included in the meta-analysis were small, some studies used completer analysis, Chinese studies were included in the meta-analysis, and studies that had a risk of bias were included in the meta-analysis) in this study, we cannot apply the results of this study in daily clinical practice.
Introduction
Schizophrenia is characterized by positive symptoms, such as hallucinations and delusions, negative symptoms, such as abulia and autism, and cognitive impairments.Citation1 At its 15-year follow-up, a Dutch cohort study of psychotic disorder reported that two-thirds of patients suffered at least one relapse. Of those patients, one in six failed to recover fully and one in ten committed suicide.Citation2 A previous meta-analysis that compared relapse rates between antipsychotic and placebo groups at 7–12 months found that antipsychotics significantly reduced relapse rates at 1 year over placebo, measured by the number needed to treat to benefit, which was three.Citation3 Nonetheless, it appears difficult to maintain drug adherence by outpatients, which leads to relapse and readmission to hospital.Citation4 In an anonymous online-survey study (n=113), patients with schizophrenia reported discontinuing medication for the following reasons: side effects (80%), stigma (31%), mistrusting the physician/therapist (31%), and rejection of medication in general (28%).Citation5 Therefore, side effects are considered the cardinal reason for poor compliance or noncompliance with antipsychotic medication.Citation6
A recent network meta-analysis comparing 15 antipsychotics to placebo found that antipsychotics were superior for reducing overall symptoms of schizophrenia.Citation7 However, all individual effect sizes were moderate, with the exception of clozapine, which had a large effect size compared with placebo.Citation7 On the other hand, olanzapine and clozapine have been demonstrated to have a higher risk of metabolic abnormalities compared with other second-generation antipsychotics.Citation8 For example, olanzapine revealed a higher risk of weight gain compared with amisulpride, aripiprazole, asenapine, lurasidone, paliperidone, and risperidone.Citation8 Risperidone was also associated with the need for more use of antiparkinsonian medication compared with clozapine, olanzapine, quetiapine, and ziprasidone.Citation9 When comparing antipsychotics (regarding overall symptoms as efficacy, and weight gain as safety), because the effect size of efficacy is smaller than that of safety, such as weight gain, several guidelines for the management of schizophrenia have recommended that the safer antipsychotic should be used for patients with schizophrenia.Citation10 However, not all patients with schizophrenia respond to the safer antipsychotics.
Topiramate is approved for the treatment of epilepsy and prophylaxis of migraine by the US Food and Drug Administration.Citation11 A previous review suggested that topiramate effects on psychopathology may be mediated through glutamatergic neurons, especially those expressing kainic acid and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors, and by inhibition of nitric oxide production.Citation12 Although the mechanisms for improving metabolic disturbances are unclear, a previous report demonstrated that topiramate enhances insulin action and glucose transport in adipose cells from obese and insulin-resistant rodents.Citation13 Further, appetite suppression through hypothalamic AMPA-receptor antagonism is thought to contribute to weight loss.Citation14
To our knowledge, 12 randomized controlled trials (RCTs) of topiramate have been conducted for the treatment of schizophrenia and related psychoses.Citation15–Citation26 There were inconsistent results among these RCTs, however, regarding psychopathology and metabolic outcomes. Although four studiesCitation15,Citation19,Citation21,Citation25 reported that topiramate was superior to control in improving psychopathological outcomes, six studiesCitation16–Citation18,Citation20,Citation23,Citation26 reported that topiramate was similar to the control in this aspect, and the remaining studiesCitation22,Citation24 did not report any psychopathological outcomes. To date, two meta-analyses have evaluated topiramate for the treatment of schizophrenia.Citation27,Citation28 Sommer et alCitation27 reviewed three RCTs,Citation15,Citation20,Citation23 and reported that topiramate augmentation for clozapine showed trend-level improvement regarding overall symptoms compared with placebo. Mizuno et alCitation28 conducted a meta-analysis of multiple pharmacological strategies to combat weight gain in antipsychotic-treated schizophrenia patients that included two RCTsCitation19,Citation21 of topiramate augmentation. The pooled result showed that topiramate significantly lowered weight compared with the placebo (mean difference −5.20 kg).Citation28 However, these meta-analyses focused on topiramate augmentation as a strategy only for psychopathology or metabolic adverse events (including weight gain), and the number of topiramate trials included was small. In this updated systematic review and meta-analysis of topiramate-augmentation therapy for patients with schizophrenia and its related disorders, we have incorporated all relevant findings involving various antipsychotics from the 12 published RCTs.Citation15–Citation26 Because a meta-analysis can increase the statistical power for group comparisons and overcome the limitation of sample size when larger trials are lacking (http://www.cochrane.org), a systematic review and meta-analysis is considered to provide the “best evidence” for clinical practice. Moreover, safety outcomes are critical for adherence; therefore, we also evaluated topiramate-discontinuation rates and individual adverse events.
Materials and methods
Inclusion criteria, search strategy, data extraction, and outcomes
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.Citation29 We performed a systematic literature review according to the PICO (patients, intervention, comparison, outcome) strategy: patients, schizophrenia and schizophrenia-related disorders; intervention, topiramate in addition to an antipsychotic agent; comparison, versus placebo in addition to an antipsychotic agent or antipsychotic only; and outcome, primary outcome (improvement in overall symptoms) and secondary outcomes (improvement in positive and negative symptoms and Positive and Negative Syndrome Scale [PANSS] general subscale scores,Citation30 decreasing weight and body mass index [BMI], improvement in Clinical Global Impression – Severity [CGI-S],Citation31 and depressive symptoms, discontinuation rate, and individual adverse events). Only those RCTs involving the use of topiramate in patients with schizophrenia and its related disorders were included.
Relevant studies were identified through searches of PubMed, the Cochrane Library, and PsycINFO citations. There were no language restrictions, and we accepted studies retrieved using the keywords “topiramate” and “schizophrenia” published up to June 20, 2016. Additional eligible studies were sought by scrutiny of the reference lists from primary articles and relevant reviews. Two authors (YO and KO) checked the inclusion and exclusion criteria for each of the identified studies, and resolved discrepancies in coding by discussion. The same authors independently extracted, checked, and entered data into RevMan version 5.3 for Windows (Cochrane Collaboration, London, UK). When data required for the meta-analysis were missing, the first/corresponding authors were contacted for additional information. We also assessed the risk of bias in the trials using the Cochrane risk-of-bias criteria (domains of random-sequence generation, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias) (http://www.cochrane.org).
Data synthesis
The primary efficacy measure was overall symptom reduction as measured by PANSS total scores and Brief Psychiatric Rating Scale (BPRS)Citation32 total scores (). Secondary outcomes were as follows: 1) positive symptoms as measured by PANSS positive subscale scores and Scale for the Assessment of Positive SymptomsCitation33 (), 2) negative symptoms as measured by PANSS negative subscale scores and Scale for the Assessment of Negative SymptomsCitation34 (), 3) PANSS general subscale scores (), 4) CGI-SCitation31 (), 5) depressive symptoms as measured by the Calgary Depression Scale for SchizophreniaCitation35 or Montgomery–Åsberg Depression Rating ScaleCitation36 (), 6) weight (), 7) BMI (), and discontinuation rates due to 8) all causes, 9) inefficacy, and 10) adverse events.
Table 1 Data synthesis
In addition, we pooled the data for individual adverse events. With regard to tolerability, discontinuations due to worsening mental condition and unsatisfactory responseCitation17,Citation23 were considered “discontinuation due to inefficacy”, and discontinuation due to low leukocyte levelsCitation23 was considered “discontinuation due to adverse events”. For estimation of missing data from individual studies, we imputed the standard deviation (SD) of BPRS-change scores from baseline to end point in Ko et al,Citation19 from the data of Muscatello et al,Citation20 and SD of CGI-S and BMI-change scores from baseline to end point in the study of Ko et alCitation19 from the SD of Chengappa et al.Citation17 We converted weights from pounds to kilograms for Chengappa et al.Citation17 We assumed that the number of patients required for evaluating efficacy outcomes in Behdani et alCitation16 was the same as the number of patients required for evaluating safety outcomes and the number of patients required for evaluating efficacy outcomes in Afshar et alCitation15 was the same number of randomized patients for each treatment arm, because the trial used general linear model analysis. We used those estimations to increase sample size for analysis to gain as much statistical power as possible. The median highest topiramate dose for all studies except Ko et alCitation19 was 238 mg/day, so data from the 200 mg/day topiramate group but not the 100 mg/day topiramate group were used for meta-analysis. For individual adverse events, we considered memory disturbancesCitation23 and forgetfulnessCitation17 as “memory loss”, and sedationCitation17,Citation23 and drowsinessCitation16 as “somnolence”.
Statistical analysis
We based our data analyses on intention to treat, observed cases, and crossover studies. This meta-analysis was performed using RevMan. To combine studies, we used the random-effect model described by DerSimonian and Laird.Citation37 We used this conservative model to address the possibility that underlying effects differed across studies and populations were heterogeneous. For continuous data, we used standardized mean difference (SMD), combining effect size (Hedges’ g) data and 95% confidence interval (CI). For dichotomous data, relative risk (RR) was estimated along with the 95% CI. When the random-effect model revealed significant between-group differences in dichotomous outcomes, the number needed to harm (NNH) was calculated. Study heterogeneity was measured using χ2 and I2 statistics, with values of P<0.05 and I2≥50% indicating heterogeneity.Citation38 In cases where I2 values were ≥50% for primary outcome, sensitivity analyses were performed to determine the reasons for heterogeneity. Finally, funnel plots were visually inspected to explore the possibility of publication bias.
Results
Study characteristics
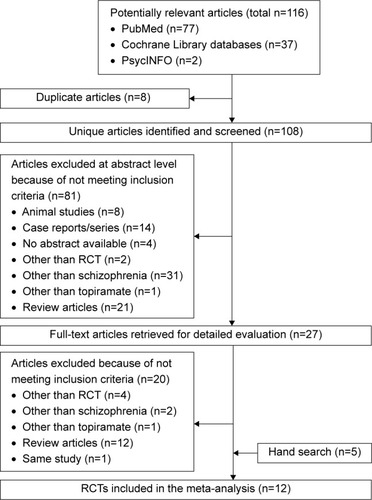
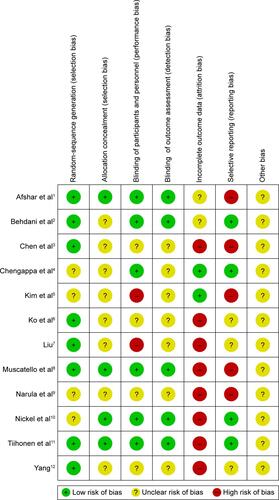
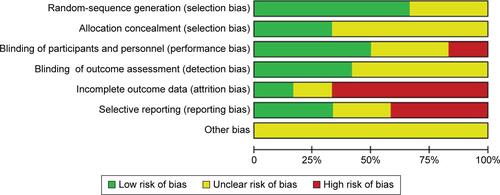
Searches of the PubMed, Cochrane Library, and PsycINFO databases yielded 116 hits. We excluded eight duplicate studies, 81 studies based on title or abstract review, and 20 studies after full-text reading. Five additional articlesCitation17,Citation22,Citation24–Citation26 were identified by manually searching the review articles.Citation17,Citation39 Finally, 12 eligible studiesCitation15–Citation26 were accepted (). Mean study duration was 13.4 (range 8–24) weeks, and 676 patients were included (schizophrenia 579, schizoaffective disorder 48, psychosis and unipolar or bipolar disorder 49). Patient numbers in each treatment arm were 358 for topiramate and 318 for control (consisting of 258 placebo-treated and 60 treatment as usual). The mean number of patients included in the individual studies was 56. Three studies were published in Chinese.Citation24–Citation26 We evaluated the methodological quality of the individual studies based on Cochrane risk-of-bias criteria ( and ). Three studies did not employ a placebo control.Citation18,Citation24,Citation25 One study was a crossover design.Citation23 Seven studies used a completer analysis.Citation19–Citation22,Citation24–Citation26 Five studies were at high risk of selective reporting bias.Citation15,Citation18,Citation20,Citation21,Citation24 One study was sponsored by the pharmaceutical industry.Citation15 The characteristics of the studies are summarized in .
Table 2 Study, patient, and treatment characteristics of the included double-blinded and open label randomized controlled trials of topiramate augmentation therapy for antipsychotic-treated schizophrenia patients
Meta-analysis
Efficacy
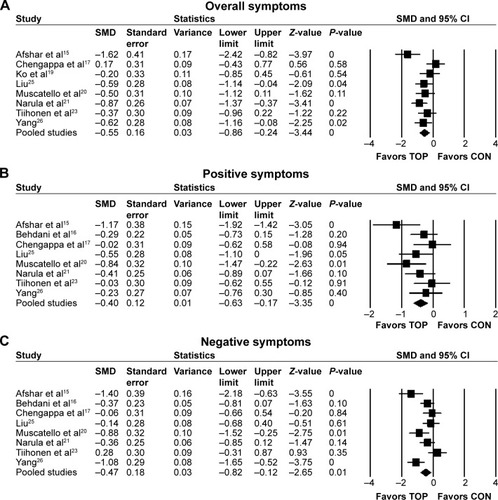
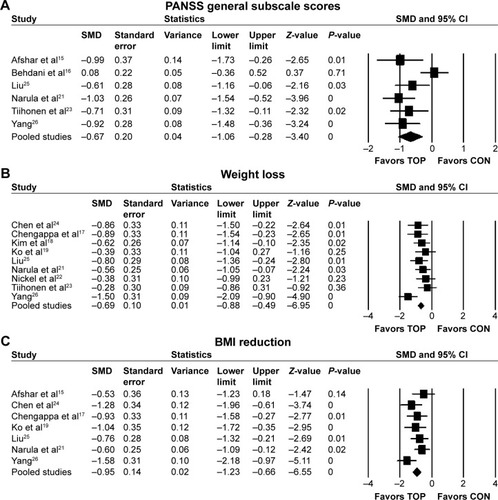
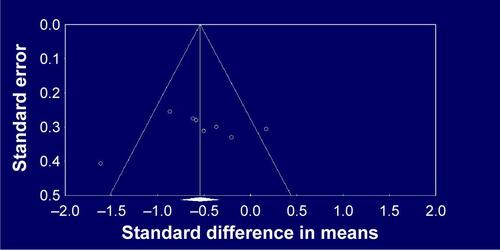
Topiramate as augmentation to antipsychotic therapy was superior to control (antipsychotic alone or placebo plus antipsychotic) for the reduction of overall symptoms (SMD −0.55, 95% CI −0.86 to −0.24; P=0.001; I2=55%, eight comparisons, n=380) (). Visual inspection of the funnel plot for primary outcome did not suggest publication bias (). Topiramate was superior to control for the following secondary outcomes: positive symptoms (SMD −0.4, 95% CI −0.63 to −0.17; P=0.001; I2=29%, eight comparisons, n=423) (), negative symptoms (SMD −0.47, 95% CI −0.82 to −0.12; P=0.01; I2=67%, eight comparisons, n=423) (), PANSS general subscale scores (SMD −0.67, 95% CI −1.06 to −0.28; P=0.001; I2=66%, six comparisons, n=332) (), weight loss (SMD −0.69, 95% CI −0.88 to −0.49; P<0.0001; I2=32%, nine comparisons, n=447) (), and BMI reduction (SMD −0.95, 95% CI −1.23 to −0.66; P<0.0001; I2=32%, seven comparisons, n=331) (). Topiramate was not different from control for depressive symptoms (SMD 0.15, 95% CI −0.71 to 1.01; P=0.73; I2=75%, two comparisons, n=91) or CGI-S (SMD −0.24, 95% CI −0.65 to 0.18; P=0.26; I2=31%, three comparisons, n=140).
Sensitivity/subgroup analysis
There was significant heterogeneity in overall symptoms among studies (I2=55%, P=0.03), so we performed 13 sensitivity analyses for primary outcome (antipsychotic class, blinding, analyzed population, region, control, study duration, topiramate dose, publication year, diagnosis, number of patients, patient status, language, and sponsorship) (). Significant heterogeneity in overall symptoms among the various-antipsychotic subgroup, open-label subgroup, Europe–US subgroup, and older publication year (before 2008) subgroup disappeared without significant difference when comparing topiramate to control. On the other hand, significant heterogeneity in the olanzapine subgroup, non-intention-to-treat population subgroup, Asia subgroup, the long-duration subgroup (13 weeks or more), low-dose topiramate subgroup (200 mg/day or less), newer publication year (after 2008) subgroup, diagnosis (schizophrenia) subgroup, not treatment-refractory subgroup, and large number of patients (more than 55) subgroup disappeared when comparing topiramate to control. One study was unique, because it was the only study that was sponsored by the pharmaceutical industry ().
Table 3 Sensitivity analysis for overall symptoms of topiramate-augmentation therapy
Safety
Topiramate and control did not differ regarding discontinuation rate due to all causes (RR 1.19, 95% CI 0.79–1.79; P=0.4; I2=1%, ten comparisons, n=561), discontinuation rate due to inefficacy (RR 1.71, 95% CI 0.52–5.66; P=0.38; I2=0, seven comparisons, n=377), or discontinuation rate due to adverse events (RR 1.09, 95% CI 0.14–8.49; P=0.93; I2=0, seven comparisons, n=377). Although topiramate was associated with a lower incidence of weight gain (RR 0.31, 95% CI 0.15–0.64; P=0.002; I2=62%, NNH −2, three comparisons, n=165) (), ≥7% weight gain (RR 0.25, 95% CI 0.12–0.51; P=0.0002; I2=0, NNH −2, two comparisons, n=97) (), and increased appetite (RR 0.26, 95% CI 0.1–0.66; P=0.005; I2=0, NNH −4, two comparisons, n=120) () than the controls, it was associated with a higher incidence of paresthesia (RR 2.67, 95% CI 1.15–6.19; P=0.02; I2=0, NNH not significant, four comparisons, n=248) () and attention difficulty (RR 8.97, 95% CI 1.17–68.63; P=0.03; I2=0, NNH 8, two comparisons, n=120) () than the controls. There were no significant differences in individual adverse events reported (asthenia, constipation, diarrhea, dizziness, dry mouth, fatigue, headache, insomnia, memory loss, muscle weakness, nausea, psychomotor slowing, psychosis exacerbation, or somnolence) between topiramate and control ().
Figure 4 Forest plots of individual adverse events.
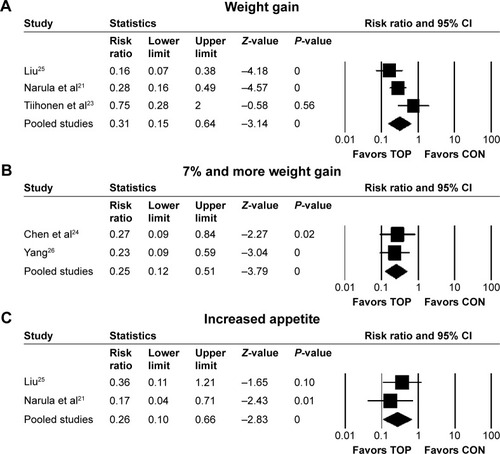
Figure 5 Forest plots of individual adverse events.
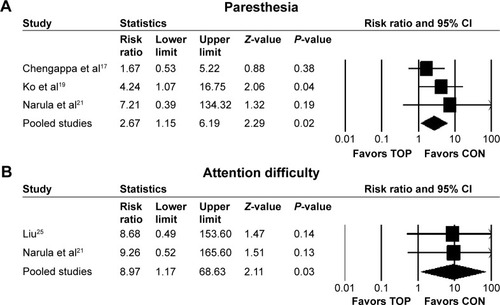
Table 4 Discontinuation rates and individual adverse events
Discussion
To the best of our knowledge, the current study is the largest comprehensive systematic review and meta-analysis of topiramate-augmentation therapy for schizophrenia and related diseases. Topiramate augmentation was more efficacious than control (antipsychotic alone or antipsychotic plus placebo) for reducing overall, positive, and negative symptoms, PANSS general subscale scores, weight, and BMI. We did not find any cause for the heterogeneity among individual studies, despite performing 13 sensitivity analyses of overall symptoms. Based on the results of the sensitivity analyses, low-dose topiramate (≤200 mg/day)-augmentation therapy for olanzapine may be useful. The prevalence of metabolic syndrome is at least twice as high in schizophrenia patients compared with the age-adjusted population.Citation40 Given the importance of maintaining medication adherence for schizophrenia, it is often necessary to use a safer but less efficacious antipsychotic. Augmentation therapy should be considered before changing from the safer antipsychotic to one with greater potential efficacy but also higher metabolic risk.Citation41,Citation42 In a prospective study, 27% of patients with schizophrenia eventually developed poor adherence.Citation43 Moritz et alCitation44 studied the reasons for antipsychotic nonadherence, and concluded that side effects were the most common reason. Weight gain is also a well-known side effect, especially with second-generation antipsychotics, which are more widely used throughout the world than first-generation antipsychotics for treating schizophrenia.Citation45 Furthermore, augmentation therapy for clozapine may be particularly important, since clozapine is thought to be the final option for treatment-resistant schizophrenia (alongside modified electroconvulsive therapy). If clozapine efficacy is still insufficient, effective augmentation drugs should be considered.Citation27
However, we cannot apply the results of this study in daily clinical practice because of the following. There are some limitations to this meta-analysis. The main limitation is the paucity of RCTs, despite updating from previous meta-analyses.Citation27,Citation28 All the trials included were small, and it is well documented that small trials exaggerate effects. Second, although we utilized a funnel plot to assess potential publication bias, these are generally used only if ten or more studies are included. The third limitation is the short follow-up period (8–24 weeks). Fourth, we did not investigate the optimal topiramate dose for augmentation therapy. Future research should investigate the long-term efficacy of multiple doses and generate more safety data using larger samples. Fifth, although several of our included studies were from China, it has been reported that many Chinese studies are problematic.Citation46 Some studies included in the meta-analysis did not report detailed information about sequence generation or allocation concealment. Moreover, seven studies used a completer analysis.
Conclusion
Our results indicate that topiramate-augmentation therapy is well tolerated, can improve the psychopathology of schizophrenia, and reduces weight gain associated with antipsychotics. Since there were some limitations in this study, we could not apply the results of this study in daily clinical practice. Future research should investigate long-term efficacy and generate more safety data for schizophrenia patients receiving topiramate augmentation of antipsychotics.
Supplementary materials
References
- AfsharHRoohafzaHMousaviGTopiramate add-on treatment in schizophrenia: a randomised, double-blind, placebo-controlled clinical trialJ Psychopharmacol200923215716218515465
- BehdaniFHebraniPArdaniARRafeeEEffect of topiramate augmentation in chronic schizophrenia: a placebo-controlled trialArch Iran Med201114427027521726104
- ChenZXXiaMLXiaWJHuangHGJinLTopiramatefor prevention of olanzapine associated weight gainModern Pract Med201224979980 Chinese
- ChengappaKNKupferDJParepallyHA placebo-controlled, random-assignment, parallel-group pilot study of adjunctive topiramate for patients with schizoaffective disorder, bipolar typeBipolar Disord20079660961717845276
- KimJHYimSJNamJHA 12-week, randomized, open-label, parallel-group trial of topiramate in limiting weight gain during olanzapine treatment in patients with schizophreniaSchizophr Res200682111511716326074
- KoYHJoeSHJungIKKimSHTopiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gainClin Neuropharmacol200528416917516062095
- LiuJJThe efficacy of topiramate in the treatment of antipsychotic-induced weight gain and lipid disordersModern Pract Med201123665667 Chinese
- MuscatelloMRBrunoAPandolfoGTopiramate augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled studyJ Psychopharmacol201125566767420615930
- NarulaPKRehanHSUnniKEGuptaNTopiramate for prevention of olanzapine associated weight gain and metabolic dysfunction in schizophrenia: a double-blind, placebo-controlled trialSchizophr Res20101181–321822320207521
- NickelMKNickelCMuehlbacherMInfluence of topiramate on olanzapine-related adiposity in women: a random, double-blind, placebo-controlled studyJ Clin Psychopharmacol200525321121715876898
- TiihonenJHalonenPWahlbeckKTopiramate add-on in treatment-resistant schizophrenia: a randomized, double-blind, placebo-controlled, crossover trialJ Clin Psychiatry20056681012101516086616
- YangYThe studying about the effects of topiramate in preventing olanzapine-induced weight gain and glucose and lipid metabolic dysfunction [dissertation]ChongqingChongqing Medical University2008 Available at: http://cdmd.cnki.com.cn/Article/CDMD-10631-2008176780.htm.dissertation
Disclosure
The authors report no conflicts of interest in this work, but report the following interests: Dr Okuyama has received speaker’s honoraria from Janssen; Dr Oya has received honoraria from Eisai, Eli Lilly, Janssen, Meiji, Otsuka, and Tanabe-Mitsubishi; Dr Matsunaga has received honoraria from Eisai, Janssen, Novartis, Daiichi Sankyo, Ono, Eli Lilly, Takeda, and Otsuka and has a Fujita Health University School of Medicine research grant and Grant-in-Aid for Young Scientists (B); Dr Kishi has received speaker’s honoraria from Abbvie, Astellas, Daiichi Sankyo, Dainippon Sumitomo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Mochida, Shionogi, Tanabe-Mitsubishi, Tsumura, Novartis, and Pfizer and has a Fujita Health University School of Medicine research grant and Grant-in-Aid for Young Scientists (B); and Dr Iwata has received honoraria from Astellas, Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Shionogi, Novartis, and Pfizer.
References
- MacDonaldAWSchulzSCWhat we know: findings that every theory of schizophrenia should explainSchizophr Bull200935349350819329559
- WiersmaDNienhuisFJSlooffCJGielRNatural course of schizophrenic disorders: a 15-year followup of a Dutch incidence cohortSchizophr Bull199824175859502547
- LeuchtSTardyMKomossaKAntipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysisLancet201237998312063207122560607
- ValensteinMCopelandLABlowFCPharmacy data identify poorly adherent patients with schizophrenia at increased risk for admissionMed Care200240863063912187177
- MoritzSFavrodJAndreouCBeyond the usual suspects: positive attitudes towards positive symptoms is associated with medication noncompliance in psychosisSchizophr Bull201339491792222337789
- HaddadPMBrainCScottJNonadherence with antipsychotic medication in schizophrenia: challenges and management strategiesPatient Relat Outcome Meas20145436225061342
- LeuchtSCiprianiASpineliLComparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysisLancet2013382989695196223810019
- Rummel-KlugeCKomossaKSchwarzSHead-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysisSchizophr Res20101232–322523320692814
- Rummel-KlugeCKomossaKSchwarzSSecond-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisonsSchizophr Bull201238116717720513652
- National Institute for Health and Care ExcellencePsychosis and Schizophrenia in Adults: Treatment and ManagementLondonNICE2014
- Topamax (topiramate) [package insert]Beerse, BelgiumJanssen Pharmaceutica2005
- HahnMKCohnTTeoCRemingtonGTopiramate in schizophrenia: a review of effects on psychopathology and metabolic parametersClin Schizophr Relat Psychoses20136418619623302448
- WilkesJJNelsonEOsborneMDemarestKTOlefskyJMTopiramate is an insulin-sensitizing compound in vivo with direct effects on adipocytes in female ZDF ratsAm J Physiol Endocrinol Metab20052883E617E62415536205
- BerelowitzMMatthewsJPimstoneBLKronheimSSacksHImmunoreactive somatostatin in rat cerebral cortical and hypothalamic synaptosomesMetabolism1978279 Suppl 111711173682979
- AfsharHRoohafzaHMousaviGTopiramate add-on treatment in schizophrenia: a randomised, double-blind, placebo-controlled clinical trialJ Psychopharmacol200923215716218515465
- BehdaniFHebraniPArdaniARRafeeEEffect of topiramate augmentation in chronic schizophrenia: a placebo-controlled trialArch Iran Med201114427027521726104
- ChengappaKNKupferDJParepallyHA placebo-controlled, random-assignment, parallel-group pilot study of adjunctive topiramate for patients with schizoaffective disorder, bipolar typeBipolar Disord20079660961717845276
- KimJHYimSJNamJHA 12-week, randomized, open-label, parallel-group trial of topiramate in limiting weight gain during olanzapine treatment in patients with schizophreniaSchizophr Res200682111511716326074
- KoYHJoeSHJungIKKimSHTopiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gainClin Neuropharmacol200528416917516062095
- MuscatelloMRBrunoAPandolfoGTopiramate augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled studyJ Psychopharmacol201125566767420615930
- NarulaPKRehanHSUnniKEGuptaNTopiramate for prevention of olanzapine associated weight gain and metabolic dysfunction in schizophrenia: a double-blind, placebo-controlled trialSchizophr Res20101181–321822320207521
- NickelMKNickelCMuehlbacherMInfluence of topiramate on olanzapine-related adiposity in women: a random, double-blind, placebo-controlled studyJ Clin Psychopharmacol200525321121715876898
- TiihonenJHalonenPWahlbeckKTopiramate add-on in treatment-resistant schizophrenia: a randomized, double-blind, placebo-controlled, crossover trialJ Clin Psychiatry20056681012101516086616
- ChenZXXiaMLXiaWJHuangHGJinLTopiramatefor prevention of olanzapine associated weight gainModern Pract Med201224979980 Chinese
- LiuJJThe efficacy of topiramate in the treatment of antipsychotic-induced weight gain and lipid disordersModern Pract Med201123665667 Chinese
- YangYThe studying about the effects of topiramate in preventing olanzapine-induced weight gain and glucose and lipid metabolic dysfunction [dissertation]ChongqingChongqing Medical University2008 Available at: http://cdmd.cnki.com.cn/Article/CDMD-10631-2008176780.htm.dissertation
- SommerIEBegemannMJTemmermanALeuchtSPharmacological augmentation strategies for schizophrenia patients with insufficient response to clozapine: a quantitative literature reviewSchizophr Bull20123851003101121422107
- MizunoYSuzukiTNakagawaAPharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysisSchizophr Bull20144061385140324636967
- MoherDLiberatiATetzlaffJAltmanDGPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementBMJ2009339b253519622551
- KaySRFiszbeinAOplerLAThe positive and negative syndrome scale (PANSS) for schizophreniaSchizophr Bull19871322612763616518
- GuyWBonatoRRManual for the ECDEU Assessment Battery2nd edChevy Chase (MD)National Institute of Mental Health1970
- OverallJGorhamDThe brief psychiatric rating scalePsychol Rep196210799812
- AndreasenNScale for the Assessment of Positive Symptoms (SAPS)Iowa CityUniversity of Iowa1984
- AndreasenNScale for the Assessment of Negative Symptoms (SANS)Iowa CityUniversity of Iowa1984
- AddingtonDAddingtonJSchisselBA depression rating scale for schizophrenicsSchizophr Res1990342472512278986
- MontgomerySAAsbergMA new depression scale designed to be sensitive to changeBr J Psychiatry1979134382389444788
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- LiangHLiHHuYLiSLuLSongXEffects of topiramate for atypical antipsychotic-induced body weight gain and metabolic adversities: a systematic review and meta-analysisZhonghua Yi Xue Za Zhi2016963216223 Chinese26879726
- De HertMAvan WinkelRVan EyckDPrevalence of the metabolic syndrome in patients with schizophrenia treated with antipsychotic medicationSchizophr Res2006831879316481149
- KishiTMukaiTMatsudaYMoriwakiMIwataNEfficacy and safety of noradrenalin reuptake inhibitor augmentation therapy for schizophrenia: a meta-analysis of double-blind randomized placebo-controlled trialsJ Psychiatr Res201347111557156323899496
- KishiTMeltzerHYIwataNAugmentation of antipsychotic drug action by azapirone 5-HT1A receptor partial agonists: a meta-analysisInt J Neuropsychopharmacol20131661259126623551924
- BrainCSamebyBAllerbyKTwelve months of electronic monitoring (MEMS) in the Swedish COAST-study: a comparison of methods for the measurement of adherence in schizophreniaEur Neuropsychopharmacol201424221522224359935
- MoritzSHünscheALincolnTMNonadherence to antipsychotics: the role of positive attitudes towards positive symptomsEur Neuropsychopharmacol201424111745175225444234
- LeuchtSCorvesCArbterDEngelRRLiCDavisJMSecond-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysisLancet20093739657314119058842
- WoodheadM80% of China’s clinical trial data are fraudulent, investigation findsBMJ2016355i539627707716
- CorrellCUMaayanLKaneJHertMDCohenDEfficacy for psychopathology and body weight and safety of topiramate-antipsychotic cotreatment in patients with schizophrenia spectrum disorders: results from a meta-analysis of randomized controlled trialsJ Clin Psychiatry201677e746e75627337425