Abstract
Objective
There is a significant interindividual variability in treatment outcomes in methadone maintenance treatment (MMT) for opioid use disorder (OUD). This prospective cohort study examines the impact of comorbid psychiatric disorders on continued illicit opioid use in patients receiving MMT for OUD.
Methods
Data were collected from 935 patients receiving MMT in outpatient clinics between June 2011 and June 2015. Using linear regression analysis, we evaluated the impact of having a comorbid psychiatric disorder on continued illicit opioid use during MMT, adjusting for important confounders. The main outcome measure was percentage of opioid-positive urine screens for 6 months. We conducted a subgroup analysis to determine the influence of specific comorbid psychiatric disorders, including substance use disorders, on continued illicit opioid use.
Results
Approximately 80% of participants had at least one comorbid psychiatric disorder in addition to OUD, and 42% of participants had a comorbid substance use disorder. There was no significant association between having a psychiatric comorbidity and continuing opioid use (P=0.248). Results from subgroup analysis, however, suggest that comorbid tranquilizer (β=20.781, P<0.001) and cocaine (β=6.344, P=0.031) use disorders are associated with increased rates of continuing opioid use.
Conclusion
Results from our study may serve to guide future MMT guidelines. Specifically, we find that cocaine or tranquilizer use disorder, comorbid with OUD, places patients at high risk for poor MMT outcomes. Treatment centers may choose to gear more intensive therapy toward such populations.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Opioid use disorder (OUD) is the largest contributor to disability-adjusted life years caused by drug use worldwide.Citation1 Efforts to maximize treatment efficacy and reduce opioid abuse are timely as the prevalence of OUD is on the rise,Citation2,Citation3 with its global burden increasing by 74% between 1990 and 2010.Citation4
Methadone maintenance treatment (MMT) is a widely implemented and effective treatment for OUD,Citation5 yet there is significant interindividual variability in treatment effectiveness and outcomes.Citation6–Citation8 To improve treatment, research groups are actively investigating the factors that influence MMT outcomes.Citation9–Citation15 Well-documented predictors of treatment outcome include length of time in treatment,Citation11–Citation13 methadone dose,Citation14 and injecting behavior.Citation15 The impact of comorbid psychiatric disorders on MMT outcome, however, remains unclear.
Comorbid psychiatric disorders place individuals using recreational or prescription opioids at an increased risk for developing OUD.Citation16–Citation18 Similarly, patients with OUD have a higher lifetime prevalence of psychiatric disorders, including other substance use disorders (SUDs), compared to that in the general population.Citation19,Citation20 As psychiatric comorbidity is associated with increased risk for opioid dependence, one may infer that it similarly impacts MMT outcomes.Citation21–Citation30 However, current literature investigating the relationship between psychiatric comorbidity and MMT outcomes is inconclusive, with some studies reporting lack of association,Citation21–Citation24 while others document adverse impactCitation25–Citation28 or protective effects of psychiatric comorbidity.Citation29–Citation30
In this prospective cohort study, we investigated the influence of comorbid psychiatric disorders on MMT outcome during a 6-month duration in patients with OUD. Our objective was to determine the impact of a patient’s psychiatric profile (ie, the presence of comorbid psychiatric disorders and use of psychotropic medications) on response to MMT treatment.
Specifically, we examined the following:
The prevalence of comorbid psychiatric disorders in a cohort of patients with OUD receiving MMT.
The impact of comorbid psychiatric disorders on continued opioid use during MMT as assessed by urine drug screen.
The association between specific comorbid psychiatric and SUDs and continued opioid use during MMT.
Methods
We used observational data from the Genetics of Opioid Addiction (GENOA) program in this study.Citation31 We collected data from patients with OUD receiving MMT at 13 community-based outpatient methadone clinics across Southern Ontario, Canada, between June 2011 and June 2015. Ethics approval was obtained from the Hamilton Integrated Research Ethics Board (project ID 11-056), and written informed consent was obtained from each study participant. Further details related to GENOA study methods have been previously reported.Citation31–Citation34 This study is reported in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.Citation35
Participants
Using the following inclusion criteria, we screened and recruited study participants: males and females aged 18 years or older, diagnosed with OUD as per the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)Citation36 criteria, and receiving MMT for their OUD. Participants must have also been able to provide written informed consent.
Participants receiving opioid substitution therapy other than methadone were excluded from the study, as were those unable to communicate in English. All treatment centers included in our study are managed centrally and follow the same management protocols.
Data collection and instruments
Participants provided information on sociodemographic factors, medical history, current medications, daily methadone dose, and length of time in treatment through face-to-face interviews. Participants also completed the Mini-International Neuropsychiatric Interview (MINI) version 6.0,Citation37 administered by trained interviewers. The MINI has been validated against both the Structured Clinical Interview for DSM diagnoses (SCID)Citation38 and the Composite International Diagnostic Interview for ICD-10 (CIDI)Citation39 in English.Citation37
We administered all modules of the MINI 6.0, including mood disorder (major depression or bipolar affective disorder), anxiety disorder (generalized anxiety disorder, social phobia, posttraumatic stress disorder, obsessive compulsive disorder, and panic disorder with and without agoraphobia), psychotic disorder (schizophrenia, schizoaffective disorder, brief psychotic disorder, delusional disorder, substance-induced psychosis, and psychosis not otherwise specified [NOS]), antisocial personality disorder, eating disorder (anorexia nervosa and bulimia nervosa), alcohol use disorder, and SUD.Citation37
MINI diagnoses of anxiety disorders and eating disorders reflect only current symptomatology in the past month, whereas diagnoses of mood, psychotic, and antisocial personality disorders reflect past year and lifetime symptomatology.Citation37 Diagnoses of alcohol and substance abuse reflect 12-month history of symptomatology.Citation37 Substances assessed for abuse in the MINI include stimulants, cocaine, narcotics, hallucinogens, phencyclidine, inhalants, cannabis, tranquilizers, and “other” (ie, steroids, nonprescription sleep or diet pills, and cough medicine).Citation37
Urine toxicology analysis
In MMT clinical sites, methadone is provided to patients under supervision, and urine drug screens for illicit opioids are conducted at weekly or biweekly frequency. We collected information on illicit opioid use patterns through urine toxicology screening, using the iMDx™ Prep assay.Citation40 This assay detects opioids present in the urine at concentrations above 300 ng/mL and distinguishes between methadone, prescribed synthetic opioids, and naturally occurring opioids.Citation40 For the purpose of this study, an “opioid-positive” urine drug screen is defined by the presence of a nonmethadone opioid in a patient’s urine sample, with the exception of those receiving prescription opioids for reasons other than OUD (eg, chronic pain), confirmed by their documented medical chart.
Primary analysis and outcome measures
Our objective is to determine the association between presence of psychiatric comorbidity and MMT outcome. The primary outcome in this study is continued illicit opioid abuse during a 6-month period of MMT, as detected by opioid-positive urine samples. Results are measured as the percent-age of opioid-positive urine drug screens per total number of urine screens available over a 6-month duration.
Although we acknowledge that MMT success may be assessed through several treatment outcomes, including retention in treatment, risk taking behaviors, and social stability, the primary indication of MMT is to ameliorate withdrawal symptoms, reduce cravings for opioids, and ultimately promote abstinence from illicit opioids.Citation41,Citation42 Urine toxicology is the gold standard for detecting illicit opioid use during MMT, and many studies have used the absence of illicit opioids, as measured through urine drug screens, as a treatment outcome indicator for MMT.Citation41,Citation43
We compare demographic and clinical information of patients with psychiatric comorbidity, as diagnosed by MINI, to those with no comorbid psychiatric diagnoses. For the purposes of this study, we define “psychiatric comorbidity” as the presence of any MINI diagnosis in addition to OUD.
We constructed a multiple linear regression model, in which percentage of opioid-positive urine drug screens was the continuous-dependent variable. Covariates included psychiatric comorbidity (a bivariate variable, noting presence, or absence of any MINI diagnosis), age, sex, methadone dose, duration in MMT, and psychotropic medications. Results are reported in β coefficient and standard error. Positive β values indicate a higher percentage of opioid-positive urine drug screens and thus higher illicit opioid use.
To further assess the relationship between psychiatric comorbidity and MMT outcome, we conducted a sensitivity analysis in which we used the multiple linear regression model described above but excluded participants who did not have a MINI-diagnosed psychiatric comorbidity yet were treated with psychotropic medications (n=51) to avoid missing cases of psychiatric comorbidity.
Secondary analyses
We conducted a subgroup analysis to determine the association between specific comorbid psychiatric and SUDs and continued illicit opioid use. We used a multiple linear regression model in which percentage of opioid-positive urine drug screens was the dependent variable. Covariates included age, sex, methadone dose, and duration in MMT, as well as the following aforementioned psychiatric disorder groups: mood disorders, anxiety disorders, psychotic disorders, antisocial personality disorder, and alcohol use disorder. Due to low prevalence of eating disorders (n=7), we excluded eating disorders from our regression analyses. We also included comorbid SUD variables, grouped by the substance used: stimulants, cocaine, hallucinogens, phencyclidines, cannabis, tranquilizers, and other.
Statistical analysis
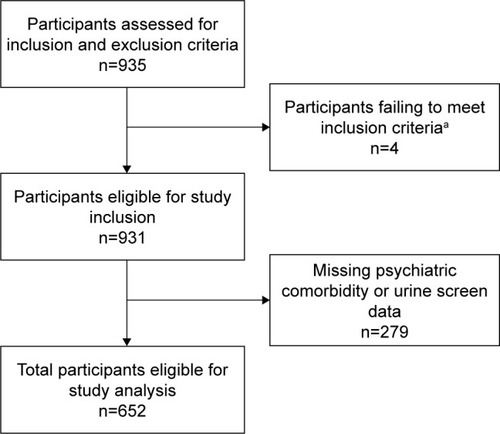
All statistical analyses were performed using STATA version 12.1 (StataCorp LP, College Station, TX, USA). We present patient demographic data by psychiatric comorbidity status, assessed using independent sample t-tests and Chi-square tests. Mean values and standard deviations (SDs) are reported for continuous variables, and percentages for categorical variables. Multivariable linear regression analyses were conducted as described above. We assessed for multicollinearity using variance inflation factor testing. The level of significance for hypothesis testing was set at α=0.05. After excluding 279 participants for not having MINI or urine drug screen data, we had 652 participants included in the analysis (, participants flow diagram). Our sample size of 652 participants was adequately powered to perform these analyses allowing for more than 10 participants per covariate.Citation44
Results
Characteristics of participants with and without psychiatric comorbidity
Our sample consisted of 78.53% of participants with a comorbid psychiatric disorder (n=512), of which 48.44% were females, with a mean age of 37.96 years (SD: 10.70; ).
Table 1 Characteristics of participants with and without psychiatric comorbidity receiving methadone treatment
Participants with psychiatric comorbidity were younger (P<0.001), more commonly males (P=0.012), and more likely to be unemployed (P=0.005). Participants with psychiatric comorbidity were also more commonly using a psychotropic medication (P=0.01). The mean proportion of opioid-positive urine drug screens obtained from participants with psychiatric comorbidity was 18.93% (SD: 27.42), compared to 17% (SD: 27.18) in those without a psychiatric comorbidity, which was not significantly different (P=0.46; ). In the psychiatric comorbidity group, 32.42% of participants had zero opioid-positive urine drug screens, as compared to 37.86% of participants without psychiatric comorbidity (P=0.228; ).
Prevalence of psychiatric comorbidity and use of psychotropic medication
Anxiety disorders were the most common comorbid psychiatric disorder identified, with 42.64% of participants meeting MINI diagnostic criteria for generalized anxiety disorder, social phobia, posttraumatic stress disorder, obsessive-compulsive disorder, or panic disorder (). We identified mood disorders in 41.41% of participants, and comorbid mood and anxiety disorders in 20.12% of participants ().
Table 2 Psychiatric comorbidity identified in participants receiving methadone treatmentTable Footnotea,Table Footnoteb
A comorbid SUD, in addition to OUD, was identified in 41.56% of participants. The most commonly abused substance was cannabis (27.91%) followed by cocaine (19.94%) and tranquilizers (4.29%; ).
Just under half (46.01%) of the participants were prescribed psychotropic medications in addition to methadone (; n=300), with 24.54% reporting the use of two or more psychotropic medications (data not shown). Antidepressants were the most commonly used psychotropic medication (27.45%), followed by benzodiazepines (23.93%), antipsychotics (12.73%), stimulants (4.14%), nonbenzodiazepine sedatives (2.91%), and mood stabilizers (1.84%; data not shown). Participants with psychiatric comorbidity were more commonly taking antidepressants and antipsychotics (data not shown). There was no significant difference between the two groups in their use of benzodiazepines, nonbenzodiazepine sedatives, stimulants, or mood stabilizers (data not shown).
Impact of psychiatric comorbidity on MMT outcome
Our primary analysis did not show a significant association between presence of a psychiatric comorbidity and continuing illicit opioid use (P=0.248; ). These results remained the same in our sensitivity analysis, in which we excluded participants without MINI-diagnosed psychiatric comorbidity who were nevertheless prescribed psychotropic medication (data not shown).
Table 3 Influence of psychiatric comorbidity on percentage of opioid-positive urine drug screens (N=652)
The subgroup analysis revealed the influence of the specific psychiatric and substance use comorbidities on MMT treatment outcome (). Having a comorbid tranquilizer use disorder (β=20.781; 95% confidence interval [CI]: 10.006, 31.555; P<0.001; ) or cocaine use disorder (β=6.344; 95% CI: 0.592, 12.095; P=0.031; ) was associated with continued illicit opioid use, with positive β-values indicating higher percentage of opioid-positive urine drug screens (). No other comorbid psychiatric disorders were significantly associated with the presence of opioid-positive urine drug screens ().
Table 4 Influence of specific psychiatric comorbidities on percentage of opioid-positive urine drug screens (N=652)
Discussion
Implications for treatment
In this large prospective study, we found a high proportion of patients with OUD to have other psychiatric disorders and SUDs, most commonly anxiety and mood disorders. The most commonly used substances in this population were cannabis and cocaine. Approximately two-thirds of patients in this study continued to use illicit opioids while receiving MMT.
MMT is not without risks, and the possibility of overdose and death is of key concern to clinicians.Citation41,Citation45,Citation46 In particular, continued use of illicit opioids during methadone treatment carries significant risk of overdose.Citation47 Thus, urine screens for illicit opioids are an important measure of methadone treatment safety and efficacy.
We found that participants with comorbid tranquilizer and cocaine use disorders were more likely to show continued illicit opioid use while in MMT. The association with tranquilizer use disorder was particularly significant. Polysubstance use has been identified as a major contributing factor to decreased abstinence and retention in treatment,Citation12 as well as methadone-related deaths, particularly early in the course of MMT.Citation47,Citation48 Cocaine, amphetamines, benzodiazepines, and alcohol are especially implicated in polysubstance use leading to death.Citation49
In the MINI, “tranquilizers” are considered to include benzodiazepines, benzodiazepine-derivatives, barbiturates, and gamma-hydroxybutyrate.Citation37 Benzodiazepines, in particular, are medications commonly prescribed for anxiety or insomnia that have well-known abuse liabilityCitation50 and require careful consideration as to the risks associated with their use in MMT.Citation51 Numerous studies have documented adverse effects of ongoing benzodiazepine use during MMT, negatively impacting abstinence,Citation11 retention in treatment,Citation12 and risk of relapse to opioid use.Citation52–Citation54
An investigation by Chen et alCitation55 revealed that half of the patients using benzodiazepines during MMT began using them, or increased use, upon commencing treatment for OUD. We found that approximately 25% of our study participants were taking routinely prescribed benzodiazepines – and there was no significant difference in rate of use between participants with and without MINI-diagnosed psychiatric comorbidity. Further, our study is in agreement with a study by White et al,Citation56 which revealed that only nonprescription benzodiazepines were associated with negative consequences on MMT outcomes. We found that when participants reported tranquilizers as drugs of abuse, this was associated with continued illicit opioid use, whereas prescribed benzodiazepine use was not associated with the same (data not shown).
It has been shown that misuse of sedative medications, including benzodiazepines, is not improved by treatment in MMT.Citation53 Understanding the reason for benzodiazepine misuse in MMT will be important for improving treatment outcomes. At the same time, treating anxiety or insomnia in patients who are dependent on opioids requires careful clinical consideration and recognizing which patients will benefit from benzodiazepine treatment for their psychiatric comorbidity is integral.Citation57
In keeping with previous evidence,Citation12,Citation58,Citation59 our study showed that cocaine use is also associated with continued opioid use while on MMT. Conversely, decreased cocaine use has been associated with cessation of illicit opioid use.Citation60 Cocaine use, however, does not appear to be significantly altered by treatment in MMT,Citation53,Citation61 and though occasional cocaine use may be reduced, there is little impact on regular use.Citation62
Cannabis use disorder was the most prevalent substance use comorbidity we identified, though it had no association with continued illicit opioid use. Previous literature has been inconclusive regarding its impact on MMT outcome, with both beneficialCitation63 and detrimental associations demonstrated.Citation64 As such, the association between cannabis use and MMT outcomes is an important area of ongoing study.Citation65
Knowing factors associated with reduced abstinence from illicit opioids while on MMT is an opportunity for clinicians to improve outcomes and minimize serious adverse events such as drug–drug interactions, overdose, and mortality. Yet, knowledge of these risks, without appropriate intervention, does not suffice, as in many cases of methadone-related deaths, clinicians are aware of the patient’s history of polysubstance abuse or dependence.Citation48 The issue of comorbid substances is not uniquely addressed in MMT, and no specialized treatment plans for these patients are defined and implemented.Citation66 Our study adds to the knowledge of comorbid substance abuse during MMT and calls for vigilance in monitoring patients’ drug use other than opioids.
Further, the presence of other comorbid psychiatric disorders must be acknowledged in MMT, and treatment should be tailored to maximize the likelihood of positive treatment outcomes. Approximately 80% of our participants in MMT met MINI criteria for one or more psychiatric disorders. Whether these patients had been previously recognized to have psychiatric comorbidity is unknown, and it is possible that without structured screening some patients may not receive appropriate diagnosis, treatment, and support. The high prevalence of psychiatric comorbidity in OUD suggests that a thorough review of past psychiatric history and current symptomatology is warranted for each patient entering MMT.
We found that many participants with no psychiatric comorbidity were regularly taking a psychotropic medication. For those patients with no identifiable psychiatric comorbidity, care must be taken to prevent polypharmacy and overmedication. On the other hand, it may be that these participants had a psychiatric disorder not captured by the MINI time frame for some disorders, or, alternatively, that the medications were managing their symptoms such that they were not captured on screening. Although these participants did not score to meet diagnostic criteria in the MINI, because they were prescribed psychotropic medications, they may have a psychiatric comorbidity. To account for this, we conducted a sensitivity analysis in which we excluded those participants from our main analysis and found no change in results: psychiatric comorbidity was not significantly associated with increased illicit opioid use while in MMT.
Despite finding no association between psychiatric comorbidity and abstinence from illicit opioid use, the impact on other MMT outcomes or quality of life measures was not evaluated. Psychiatric comorbidity in MMT patients has previously been associated with severely diminished quality of life.Citation67 Improving appropriate diagnosis and treatment of comorbid psychiatric illness should meaningfully impact outcomes both in MMT and in other measures of well-being. Management of psychiatric comorbidity through on-site and integrated psychiatric care has been shown to improve psychiatric outcomes but may not impact abstinence or substance abuse outcomes.Citation68
Strengths and limitations
To our knowledge, this is the largest study to date to detail the prevalence of comorbid psychiatric disorders and their influence on MMT outcome in patients receiving MMT for OUD.
Our study is strengthened by real-time diagnosis of psychiatric comorbidity, thus averting the need to use less reliable historical data in past medical records. However, a significant limitation associated with the MINI is the inability to distinguish current and lifetime comorbidity for each psychiatric disorder, as certain diagnoses reflect only 30-day symptomatology, whereas others are based on the presence of 12-month or lifetime symptoms.Citation38 Therefore, it was not possible to distinguish current versus lifetime prevalence of psychiatric comorbidity in this study. We also lack data on the longitudinal progression of psychiatric symptomatology for each patient.
All data were collected from clinics in Southern Ontario, Canada. How well these results would generalize to other areas of the world must also be questioned. Finally, the average length of time in treatment for those with psychiatric comorbidity was 50.18 months, or greater than 4 years, thus the majority of patients had been receiving lengthy treatment. We have no data on patients who dropped out of MMT, however, which may result in a healthy adherer or compliance bias in our results.Citation69,Citation70 Future research should explore the association between psychiatric comorbidity and outcomes in patients newly entering MMT for OUD, as the findings may differ from our population that had already received lengthy treatment.
Conclusion
Psychiatric comorbidity, on the whole, was not significantly associated with MMT outcome, as evidenced by abstinence from illicit opioid use. However, comorbid cocaine and tranquilizer use disorders were significantly associated with increased use of illicit opioids. This study highlights a target population for whom more intensive treatment may be required to achieve optimal results in MMT for OUD.
Acknowledgments
The authors would like to acknowledge the contributions of Sheelagh Rutherford for her valuable assistance in data collection, the CATC staff and management team for their ongoing collaboration and facilitation of this study, The GENOA team members and volunteers for their countless hours and efforts that made this project possible. The authors would also like to acknowledge the significant contribution of the following study team members: Meha Bhatt, Laura Zielinski, Natalia Mouravska, and Guillaume Pare for their role in study design, acquisition of data, critically revising the manuscript, and approval of the manuscript final version; Jacqueline Hudson for data acquisition, entry, and quality management; Andrew Worster for critical revising the manuscript and approval of the final draft; Michael Varenbut for study design, data acquisition, approval of final draft, and accountability for all aspects of the study; Jeff Daiter for data acquisition and contribution to the study concept. The authors are most grateful to the study participants who volunteered their time, data, and efforts to complete this study that would not have been possible without their generosity. Tea Rosic is funded by the Mach-Gaensslen Foundation summer studentship. This work was supported by research grants from the Canadian Institute for Health Research (CIHR), Ottawa, ON, Canada, The Hamilton Academic Health Sciences Organization, Hamilton, ON, Canada, The Chanchlani Research Centre at the Population Genomics Program, Hamilton, ON, Canada, and Peter Boris Centre for Addictions Research, Hamilton, ON, Canada. The funding bodies have no role in the design of the study, the analysis of data, or publication of results.
Disclosure
The authors report no conflicts of interest in this work.
References
- DegenhardtLWhitefordHAFerrariAJGlobal burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010Lancet201338299041564157423993281
- ComptonWMVolkowNDMajor increases in opioid analgesic abuse in the United States: concerns and strategiesDrug Alcohol Depend200681210310716023304
- OkieSA flood of opioids, a rising tide of deathsN Engl J Med2010363211981198521083382
- DegenhardtLWhitefordHHallWDThe Global Burden of Disease projects: what have we learned about illicit drug use and dependence and their contribution to the global burden of disease?Drug Alcohol Rev201433141224251757
- Effective Medical Treatment of Opiate AddictionNational consensus development panel on effective medical treatment of opiate addictionJAMA199828022193619439851480
- MancinoMCurranGHanCAlleeEHumphreysKBoothBMPredictors of attrition from a national sample of methadone maintenance patientsAm J Drug Alcohol Abuse201036315516020465373
- DeckDCarlsonMJRetention in publicly funded methadone maintenance treatment in two Western statesJ Behav Health Serv Res2005321436015632797
- BellJBurrellTIndigDGilmourSCycling in and out of treatment: participation in methadone treatment in NSW, 1990–2002Drug Alcohol Depend2006811556115993552
- BrewerDCatalanoRFHaggertyKGaineyRRFlemingCBA meta-analysis of predictors of continued drug use during and after treatment for opiate addictionAddiction199893173929624713
- PrendergastMLPodusDChangEProgram factors and treatment outcomes in drug dependence treatment: an examination using meta-analysisSubst Use Misuse20003512–141931196511138713
- KamalFFlavinSCampbellFBehanCFaganJSmythRFactors affecting the outcome of methadone maintenance treatment in opiate dependenceIr Med J2007100339339717491538
- PelesESchreiberSAdelsonMFactors predicting retention in treatment: 10-year experience of a methadone maintenance treatment (MMT) clinic in IsraelDrug Alcohol Depend200682321121716219428
- VillafrancaSWMcKellarJDTraftonJAHumphreysKPredictors of retention in methadone programs: a signal detection analysisDrug Alcohol Depend200683321822416384657
- FaggionoFVigna-TagliantiuFVersinoELemmaPMethadone maintenance at different dosages for opioid dependenceCochrane Database Syst Rev20033CD002208
- NajiLDennisBBaworMA prospective study to investigate predictors of relapse among patients with opioid use disorder treated with methadoneSubst Abuse20161091827103815
- MichnaERossELHynesWLPredicting aberrant drug behavior in patients treated for chronic pain: importance of abuse historyJ Pain Symptom Manage200428325025815336337
- MartinsSSKeyesKMStorrCLZhuHChilcoatHDPathways between nonmedical opioid use/dependence and psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related ConditionsDrug Alcohol Depend20091031–2162419414225
- ConwayKPComptonWStinsonFSGrantBFLifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related ConditionsJ Clin Psychiatry200667224725716566620
- StrainECAssessment and treatment of comorbid psychiatric disorders in opioid-dependent patientsClin J Pain2002184 supplS14S2712479251
- EdlundMJSteffickDHudsonTHarrisKMSullivanMRisk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer painPain2007129335536217449178
- RounsavilleBJKostenTRWeissmanMMKleberHDPrognostic significance of psychopathology in treated opiate addicts. A 2.5-year follow-up studyArch Gen Psychiatry19864387397453729668
- AstalsMDiazLDomingo-SalvanyAMartin-SantosRBulbenaATorrensMImpact of co-occurring psychiatric disorders on retention in a methadone maintenance program: an 18-month follow-up studyInt J Environ Res Public Health20096112822283220049227
- TraftonJAMinkelJHumphreysKOpioid substitution treatment reduces substance use equivalently in patients with and without posttraumatic stress disorderJ Stud Alcohol200667222823516562404
- CacciolaJSAltermanAIRutherfordMJMcKayJRMulvaneyFDThe relationship between psychiatric comorbidity to treatment outcomes in methadone maintained patientsDrug Alcohol Depend200161327128011164691
- RounsavilleBJWeissmanMMCrits-ChristophKWilberCKleberHDiagnosis and symptoms of depression in opiate addicts. Course and relationship to treatment outcomeArch Gen Psychiatry19823921511567065829
- OuimettePCFinneyJWMoosRHTwo-year posttreatment functioning and coping of substance abuse patients with posttraumatic stress disorderPsychol Addict Behav199913105114
- KingVLKidorfMSStollerKBCarterJABroonerRKInfluence of antisocial personality subtypes on drug abuse treatment responseJ Nerv Ment Dis2001189959360111580002
- VertheinUDegkwitzPHaasenCKrauszMSignificance of comorbidity for the long-term course of opiate dependenceEur Addict Res2005111152115608467
- NgoHTTaitRJHulseGKHospital psychiatric comorbidity and its role in heroin dependence treatment outcomes using naltrexone implant or methadone maintenanceJ Psychopharmacol201125677478220360157
- GelkopfMWeizmanTMelamedYAdelsonMBleichADoes psychiatric comorbidity affect drug abuse treatment outcome? A prospective assessment of drug abuse, treatment tenure and infectious diseases in an Israeli methadone maintenance clinicIsr J Psychiatry Relat Sci200643212613616910375
- SamaanZBaworMDennisBBGenetic influence on methadone treatment outcomes in patients undergoing methadone maintenance treatment for opioid addiction: a pilot studyNeuropsychiatr Dis Treat2014101503150825187714
- BaworMDennisBBSamaanMCMethadone induces testosterone suppression in patients with opioid addictionSci Rep20144618925155550
- DennisBBSamaanMCBaworMEvaluation of clinical and inflammatory profile in opioid addiction patients with comorbid pain: results from a multicenter investigationNeuropsychiatr Dis Treat2014102239224725429222
- BaworMDennisBBVarenbutMSex differences in substance use, health, and social functioning among opioid users receiving methadone treatment: a multicenter cohort studyBiol Sex Differ201562126557977
- von ElmEAltmanDGEggerMSTROBE InitiativeThe Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studiesLancet200737095961453145718064739
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders4th text rev edWashington DCAmerican Psychiatric Publishing2000
- SheehanDVLecrubierYSheehanKHThe Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10J Clin Psychiatry199859suppl 202233 quiz 34–57
- FirstMBSpitzerRLGibbonMWilliamsJBStructured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient EditionNew York: (SCID-I/P) New YorkBiometrics Research, New York State Psychiatric Institute2002
- RobinsLNWingJWittchenHUThe Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different culturesArch Gen Psychiatry19884512106910772848472
- NOVX Systemsinventor iMDx™ Available from: http://www.novxsystems.com/www.novxsystems.com/what-we-do/index.htmlAccessed March 22, 2017
- MattickRPBreenCKimberJDavoliMMethadone maintenance therapy versus no opioid replacement therapy for opioid dependenceCochrane Database Syst Rev20093CD002209
- GossopMBradleyBPhillipsGTAn investigation of withdrawal symptoms shown by opiate addicts during and subsequent to a 21-day in-patient methadone detoxification procedureAddict Behav1987121163565107
- WassermanDAKorchaRHavassyBEHallSMDetection of illicit opioid and cocaine use in methadone maintenance treatmentAm J Drug Alcohol Abuse199925356157110473015
- PeduzziPConcatoJKemperEHolfordTRFeinsteinARA simulation study of the number of events per variable in logistic regression analysisJ Clin Epidemiol19964912137313798970487
- CousinsGTeljeurCMotterliniNMcCowanCDimitrovBDFaheyTRisk of drug-related mortality during periods of transition in methadone maintenance treatment: a cohort studyJ Subst Abuse Treat201141325226021696913
- Jimenez-TreviñoLSaizPAGarcía-PortillaMPA 25-year follow-up of patients admitted to methadone treatment for the first time: mortality and gender differencesAddict Behav201136121184119021835551
- HuangCLLeeCWFactors associated with mortality among heroin users after seeking treatment with methadone: a population-based cohort study in TaiwanJ Subst Abuse Treat201344329530023021097
- ZadorDSunjicSDeaths in methadone maintenance treatment in New South Wales, Australia 1990–1995Addiction2000951778410723832
- United Nations Office on Drugs and CrimeWorld Drug Report 2015Herndon, VAUnited Nations Publication2015 Sales No. E.15.XI.6
- O’brienCPBenzodiazepine use, abuse, and dependenceJ Clin Psychiatry200666suppl 22833
- BleichAGelkopfMSchmidtVHaywardRBodnerGAdelsonMCorrelates of benzodiazepine abuse in methadone maintenance treatment. A 1 year prospective study in an Israeli clinicAddiction199994101533154010790905
- BrandsBBlakeJMarshDCSprouleBJeyapalanRLiSThe impact of benzodiazepine use on methadone maintenance treatment outcomesJ Addict Dis20082733748
- DeMariaPAJrSterlingRWeinsteinSPThe effect of stimulant and sedative use on treatment outcome of patients admitted to methadone maintenance treatmentAm J Addict20009214515310934576
- DarkeSRossJMillsKTeesonMWilliamsonAHavardABenzodiazepine use among heroin users: baseline use, current use and clinical outcomeDrug Alcohol Rev201029325025520565516
- ChenKWBergerCCFordeDPD’AdamoCWeintraubEGandhiDBenzodiazepine use and misuse among patients in a methadone programBMC Psychiatry2011119021595945
- WhiteWLCampbellMDSpenceRDHoffmanHACrissmanBDuPontRLPatterns of abstinence or continued drug use among methadone maintenance patients and their relation to treatment retentionJ Psychoactive Drugs201446211412225052787
- CirauloDANaceEPBenzodiazepine treatment of anxiety or insomnia in substance abuse patientsAm J Addict200094276279 Discussion 280–28411155783
- ProctorSLCopelandALKopakAMHoffmannNGHerschmanPLPolukhinaNPredictors of patient retention in methadone maintenance treatmentPsychol Addict Behav201529490691726098127
- SenbanioRWolffKMarshallEJStrangJPersistence of heroin use despite methadone maintenance treatment: poor coping self-efficacy predicts continued heroin useDrug Alcohol Rev200928660861519930013
- MaremmaniIPaniPPMelliniAAlcohol and cocaine use and abuse among opioid addicts engaged in a methadone maintenance treatment programJ Addict Dis20072616170
- HubbardRLCraddockSGAndersonJOverview of 5-year followup outcomes in the drug abuse treatment outcome studies (DATOS)J Subst Abuse Treat200325312513414670518
- RouxPLionsCVilotitchACorrelates of cocaine use during methadone treatment: implications for screening and clinical management (ANRS Methaville study)Harm Reduct J2016131227048152
- ScavoneJLSterlingRCWeinsteinSPVan BockstaeleEJImpact of cannabis use during stabilization on methadone maintenance treatmentAm J Addict201322434435123795873
- EpsteinDHPrestonKLDoes cannabis use predict poor outcome for heroin-dependent patients on maintenance treatment? A review of past findings, and more evidence againstAddiction200398326927912603227
- ZielinskiLBhattMEisenRAssociation between cannabis use and treatment outcomes in patients receiving methadone maintenance treatment: a systematic review protocolSyst Rev2016513927530914
- The College of Physicians and Surgeons of OntarioMethadone Maintenance Treatment Program Standards and Clinical GuidelinesToronto, ONThe College of Physicians and Surgeons of Ontario2011
- CarpentierPJKrabbePFvan GoghMTKnapenLJBuitelaarJKde JongCAPsychiatric comorbidity reduces quality of life in chronic methadone maintained patientsAm J Addict200918647048019874168
- BroonerRKKidorfMSKingVLManaging psychiatric comorbidity within versus outside of methadone treatment settings: a randomized and controlled evaluationAddiction2013108111942195123734943
- ShrankWHPatrickARBrookhartMAHealth user and related biases in observational studies of preventative interventions: a primer for physiciansJ Gen Intern Med2001265546550
- Delgado-RodríguezMLlorcaJBiasJ Epidemiol Community Health200458863564115252064