Abstract
Rationale
Long-acting injectable (LAI) paliperidone palmitate 1-month formulation (PP1M) has demonstrated acceptable tolerability and favorable clinical outcomes in Western and Asian patients with schizophrenia. Hence, analysis of the outcomes of long-term PP1M treatment specifically in Chinese patients is of interest.
Objective
The aim of this study is to evaluate the long-term safety and efficacy of PP1M treatment in Chinese patients with schizophrenia.
Methods
In this 25-week, open-label, Phase IV study, patients (18–65 years) diagnosed with schizophrenia and having a baseline Positive and Negative Syndrome Scale (PANSS) total score of 60–120 (inclusive) were enrolled. All patients received injections of PP1M 150 mg eq. (day 1) and 100 mg eq. (day 8), followed by a flexible once-monthly maintenance dosing (75, 100, or 150 mg eq.).
Results
Of the 353 patients, 234 (66.3%) completed the study treatment (mean age, 31.1 years; 52.7% men). The PANSS total score (primary end point) improved significantly over the 6-month treatment period (mean [standard deviation] change from baseline to end of treatment, −27.2 [18.30]; P<0.0001). The Clinical Global Impressions-Severity and Personal and Social Performance scores (secondary end points) also improved significantly (P<0.0001). At 6 months, PP1M had a positive impact on medication satisfaction, adherence, and increased preference for LAIs. Treatment-emergent adverse events (TEAEs) were reported by 181 (51.3%) patients (TEAEs ≥5%: extrapyramidal disorder [15.3%], akathisia [10.5%], blood prolactin increase [8.8%], insomnia [5.4%]). A total of 8 deaths were reported, including 4 completed suicides.
Conclusion
Long-term treatment with PP1M was efficacious, and no new safety concerns were identified in Chinese patients with schizophrenia. Overall, the results were comparable with observations from previous studies.
Introduction
Sustained symptomatic remission and prevention of relapses are key components of the pharmacotherapy of schizophrenia that positively impact treatment outcomes.Citation1–Citation3 Medication nonadherence is regarded as the strongest determinant of psychotic relapses that in turn impose enormous occupational and social impediment and considerably increase the risk of suicidal behavior.Citation4–Citation7 In Chinese patients with schizophrenia, the medication nonadherence rate is estimated to range from 20% to 38%.Citation8,Citation9 Among several other factors, poor medication compliance was reported to be the most important predictor of psychotic relapses in these patients.Citation9
Long-acting injectable (LAI) antipsychotics have the advantage of simplified treatment regimen, steady drug delivery, and freedom from daily dosing compared to their oral counterparts.Citation10,Citation11 LAIs also increase the feasibility of monitoring appointments for scheduled injections, enabling tracking of nonadherence.Citation11 Several clinical studies and meta-analyses have attributed LAIs with optimum control of symptoms, prevention of relapses, and reductions in rehospitalizations, likely through achieving improved medication adherence.Citation10,Citation12–Citation14 The atypical LAI, paliperidone palmitate 1-month formulation (PP1M), is approved for acute symptom control and maintenance treatment of schizophrenia in adults in most countries worldwide.Citation15,Citation16 Although LAIs are most likely used in cases of failure of oral antipsychotic treatment, exacerbations of symptoms, and frequent relapses, the unique pharmacokinetic profile of PP1M supports initiation of PP1M even in acute cases of early psychosis.Citation11,Citation15–Citation17
A large body of clinical data from global studies has substantiated the efficacy and acceptable tolerability of PP1M for short-term treatment of schizophrenia.Citation10,Citation18–Citation24 The efficacy of PP1M for acute treatment of schizophrenia in Chinese patients was demonstrated by 4 short-term (13-week) studies that reported PP1M to be well tolerated with similar safety findings as other populations.Citation17,Citation25–Citation27
The benefits of long-term maintenanceCitation28–Citation31 and recurrence preventionCitation32 in global patient populations with schizophrenia have also been shown across a flexible dose range of 50–150 mg eq. Two recently published long-term studies demonstrated the favorable efficacy and safety of PP1M in patients from the Asia-Pacific region (Chinese patients: n=100 in each study).Citation33,Citation34 The 18-month study of PP1M in patients with recent-onset schizophrenia also showed significant reduction in the number and duration of hospitalizations.Citation33 The second study was a global noninferiority study including patients from the People’s Republic of China that compared efficacy of PP1M to the newer paliperidone palmitate 3-month formulation.Citation34 Both studies established the acceptable tolerability of PP1M for maintenance therapy of schizophrenia and did not raise any safety concern specific to the Asian population.
The present study was conducted in response to a request from the China Food and Drug Administration to collect clinical data for PP1M in 500 Chinese patients. Previous clinical studies from the Asia-Pacific region provided data for 200 Chinese patients.Citation33,Citation34 Therefore, the aim of the present study is to evaluate the safety and efficacy of PP1M in routine clinical use over a 6-month treatment period in an additional 300 Chinese patients with recent-onset or chronic schizophrenia. The study also intends to explore outcomes that are close to real world such as treatment adherence and caregiver burden following treatment with PP1M in these patients.
Methods
Study design
This 25-week, multicenter, non-randomized, open-label, prospective Phase IV study was conducted between October 2013 and March 2015, across 13 centers in the People’s Republic of China (ClinicalTrials.gov identifier: NCT01947803). The study was divided into a screening phase (up to 1 week), a treatment phase (25 weeks), and a follow-up phase (30 days). The study protocol and amendments were reviewed by an Independent Ethics Committee at each study site: The Second Xiangya Hospital of Central South University, Nanjing Brain Hospital, First Hospital of Shanxi Medical University, First Affiliated Hospital of Kunming Medical University, Tianjin Anding Hospital, Guangzhou Brain Hospital, Mental Health Center of Wuhan City, First Affiliated Hospital of Medical College of Xi’an Jiaotong University, The Sixth People’s Hospital of Hebei Province, The First Hospital of Hebei Medical University, Mental Health Center of Xi’an City, Beijing Huilongguan Hospital, West China Hospital, and The First Affiliated Hospital of College of Medicine – Zhejiang University. All studies were conducted in compliance with the Declaration of Helsinki consistent with Good Clinical Practices and applicable regulatory requirements. Written informed consent was obtained from all patients before enrollment.
Patients
Men and women aged 18–65 years (inclusive), diagnosed with schizophrenia (according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision [DSM-IV-TR]), having a Positive and Negative Syndrome Scale (PANSS) total score between 60 and 120 (inclusive), and currently receiving treatment with or without any antipsychotic were eligible. Enrolled patients included those who experienced recurrent episodes and those experiencing first psychotic episode.
Key exclusion criteria included active DSM-IV-TR diagnosis other than schizophrenia, history of substance dependence within 6 months before screening, suicide attempt within 12 months before screening or being at an imminent risk of suicidal or violent behavior, history of nonresponsiveness to risperidone or paliperidone, and prior treatment with clozapine for refractory schizophrenia.
Study treatments
Doses of PP1M given to the patients are expressed herein as mg eq. of paliperidone, the active fraction of PP1M.Citation19 During the treatment phase, eligible patients received deltoid injections of paliperidone palmitate 150 mg eq. on day 1 and 100 mg eq. on day 8 followed by a monthly flexible dosing (75, 100, or 150 mg eq.), based on patient’s tolerability and/or efficacy, administered as either deltoid or gluteal injection. Patients without documented exposure to oral risperidone, paliperidone extended-release (ER), risperidone LAI, or PP1M were recommended for oral tolerability testing and received paliperidone ER (3–6 mg/day) or oral risperidone (1–3 mg/day) for 2–3 days during the screening phase.
Concomitant medications
Mood stabilizers or antidepressants (except nonselective or irreversible monoamine oxidase inhibitors, β-blockers, sedative hypnotics, and anticholinergic antiparkinsonian medications) were allowed. Benzodiazepines (−10 consecutive days within 1 month) were permitted in cases of emergencies during the study. Use of lorazepam (≤6 mg/day orally) or any other equivalent short-acting benzodiazepine to control agitation, irritability, restlessness, and hostility was allowed. All antipsychotics other than PP1M were discontinued before initiation of study treatment on day 1. Any other psychoactive prescription medications, herbal agents, and over-the-counter drugs were not allowed during the treatment phase. Electroconvulsive therapy and transcranial magnetic stimulation were also prohibited during the course of this study.
Efficacy measures
The primary efficacy end point was changes in PANSS total scoreCitation35 from baseline to days 8, 36, 92, and 176. The secondary efficacy analysis included changes in Clinical Global Impressions-Severity (CGI-S) scoresCitation36 and Personal and Social Performance (PSP) scoresCitation37 from baseline to days 8, 36, 92, and 176. Medication satisfaction was evaluated based on changes from baseline to days 64 and 176 in Medication Satisfaction Questionnaire (MSQ) score. The MSQ was a single-item questionnaire that was offered to patients or caregivers who rated the treatment between 1 (extremely dissatisfied) and 7 (extremely satisfied).Citation38,Citation39 Medication adherence was assessed using changes from baseline to days 64 and 176 in the 10-item questionnaire, Medication Adherence Rating Scale (MARS; total score <4 indicates nonadherence).Citation40,Citation41 The Attitude of Patient’s Preference for LAI (APL) measure was used to evaluate patient’s preference for LAI over oral antipsychotics and was calculated as the number and percentage of patients showing preference for either treatment and also investigated the reasons for preference. The Involvement Evaluation Questionnaire-31 (IEQ-31) was used to evaluate caregiver burden and was offered to all caregivers. Scoring was based on a 5-point Likert scale for 31 items on 4 dimensions (tension, supervision, worrying, and urging) and recorded during the previous 4 weeks as a recall period to assess caregiver consequences.Citation42
Safety measures
Evaluations of safety and tolerability included incidence of treatment-emergent adverse events (TEAEs) and changes from baseline in extrapyramidal symptom (EPS) rating scales (Abnormal Involuntary Movement Scale [AIMS],Citation43 Barnes Akathisia Rating Scale [BARS],Citation44 and Simpson Angus Scale [SAS]),Citation45 Columbia-Suicide Severity Rating Scale (C-SSRS; included after protocol amendment), clinical laboratory tests (including prolactin measurements), vital signs measurements, 12-lead electrocardiograms (ECGs), and physical examinations.
Statistical analysis
No formal sample size calculation was performed. Assuming a dropout rate of 15% based on experiences from 2 previous clinical studies of PP1M, 353 patients were expected to be enrolled in this study.Citation33,Citation34 This sample size provided ~95% chance of observing no less than 1% of incidence of TEAEs.
All efficacy analyses were performed on the full analysis set that included patients who received at least 1 injection of PP1M during the treatment phase and had at least 1 post-baseline efficacy assessment. The safety set that included patients who received at least 1 injection of PP1M during study duration was used for safety evaluations. IEQ-31 evaluation was performed on the pharmacoeconomic analysis set comprising caregivers for patients who received at least 1 injection of PP1M during the treatment phase and had at least 1 postbaseline evaluation. All efficacy end points were compared using paired t-test or Wilcoxon signed-rank test, at a 2-sided significance level of 5%, and no adjustments were made for multiplicity. Subgroup analyses based on disease duration (recent-onset schizophrenia: ≤5 years and chronic schizophrenia: >5 years of psychiatric history) were performed using analysis of covariance, and the values at baseline were used as covariates. The cut-off year for the subgroups was fixed at 5 years based on evidence suggestive of functional deterioration and decline in antipsychotic treatment responses within the 5 years of first psychiatric diagnosis.Citation46–Citation48 For all efficacy end points, the values and changes from baseline were summarized descriptively, and last observation carried forward data were used for missing data imputation. All safety measurements were summarized descriptively.
Results
Patient disposition and characteristics
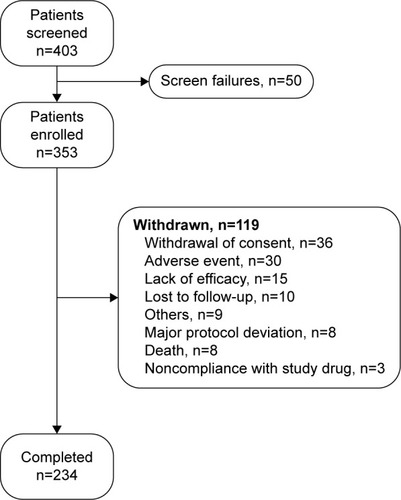
A total of 403 patients were screened for eligibility of which 353 with mean (standard deviation, SD) age of 31.1 (10.52) years entered the treatment phase. The majority (68.6%, n=242) of patients had recent-onset schizophrenia (). The demographic characteristics were consistent across the 2 subgroups except mean (SD) body weight (64.0 [12.28] vs 66.3 [12.29] kg), body mass index (23.1 [3.80] vs 24.4 [3.95] kg/m2), and age (28.3 [9.40] vs 37.2 [10.32] years) that were numerically lower in patients with psychiatric history of ≤5 years than those with history of >5 years. The baseline clinical characteristics were similar between the 2 subgroups. The commonly prescribed prior antipsychotic and concomitant medications included trihexyphenidyl hydrochloride (24.4%), risperidone (11.3%), paliperidone (8.2%), lorazepam (7.4%), and alprazolam (5.9%).
Table 1 Demographic and baseline characteristics (safety set)
Of the 353 enrolled patients, 234 (66.3%) completed the treatment at day 176. The most common reasons for study discontinuation were withdrawal of consent (36 [10.2%]), TEAEs (30 [8.5%]), and lack of efficacy (15 [4.2%]) (). All patients were included in the safety set (100%), whereas the full analysis set had 345 (97.7%) patients and the pharmacoeconomic analysis set had 301 (85.3%) patients. Of the 234 patients who completed the 25-week study, 230 completed the follow-up period at day 206. The mean (SD) dose of PP1M (excluding the dose on day 1 and day 8) in the entire patient population was 118.7 (24.60) mg eq. (recent-onset subgroup, 117.3 [24.43]; chronic schizophrenia subgroup, 121.8 [24.82]) during the study.
Primary efficacy
A significant (P<0.0001) improvement in mean (SD) PANSS total score was observed from baseline to day 8 (−5.8 [7.97]), day 36 (−13.9 [13.02]), day 92 (−21.5 [16.46]), and day 176 (end of the 6-month treatment period; −27.2 [18.30]) (). The subgroup analyses revealed that the magnitude of reductions in PANSS total scores was greater in patients with psychiatric history of ≤5 years as compared with those with a history of >5 years at all time points measured ().
Table 2 Changes from baseline in primary efficacy scores (full analysis set; LOCF)
Secondary efficacy
Significant (P<0.0001) changes in mean (SD) CGI-S scores from baseline on day 8 (−0.2 [0.55]), day 36 (−0.7 [0.88]), day 92 (−1.2 [1.06]), and day 176 (−1.6 [1.22]) were observed (). Mean (SD) changes in PSP scores also showed significant improvement on day 8 (3.3 [7.26]), day 36 (7.4 [10.24]), day 92 (11.0 [13.09]), and day 176 (14.9 [15.04]) (P<0.0001). Clinically meaningful and significant changes in mean (SD) MSQ scores on day 64 (0.6 [1.51], P<0.0001) and day 176 (0.8 [1.62], P<0.0001) represented treatment satisfaction among patients. Subgroup analysis did not indicate any significant differences in the extent of improvement for CGI-S, PSP, and MSQ scores between patients with a psychiatric history of ≤5 and >5 years.
Improved medication adherence was observed as indicated by significant mean (SD) changes from baseline in MARS scores on day 64 (1.7 [3.06], P<0.0001) and day 176 (2.2 [3.28], P<0.0001). Overall, the treatment adherence rate for all medications increased from 46.9% at baseline to 75.0% on day 64 and 82.3% on day 176 (). Based on the APL, the percentage of patients preferring LAI over oral antipsychotics was greater consistently throughout the study: 70.4% (baseline), 79.2% (day 64), and 76.9% (day 176) (). Among patients preferring LAIs, >70% favored deltoid injections over gluteal injections at all investigated study points. The reasons for this preference were simpler procedure and less embarrassment than receiving gluteal injections. A smaller proportion of patients preferred gluteal injections and claimed that these were less painful and simpler than deltoid injections. Simplified regimen, freedom from taking oral antipsychotics, and better symptom remission were among the top reasons for the higher preference toward LAIs. Significant (P<0.0001) mean (SD) reduction from baseline (31.8 [15.52]) in IEQ-31 scores was observed on day 64 (−6.7 [14.30]) and day 176 (−8.2 [15.84]), indicating meaningful relief in caregiver burden. Lowering of caregiver burden was significant (P<0.0001) for both subgroups on day 176 (psychiatric history of ≤5 years, −8.6 [16.98]; psychiatric history of >5 years, −7.2 [13.01]).
Table 3 Changes in MSQ scores, MARS scores, and APL from baseline (full analysis set)
Safety
Overall, 181 (51.3%) patients experienced TEAEs during the study. The most frequently reported TEAEs (in ≥5% patients) were extrapyramidal disorder (54 [15.3%]), akathisia (37 [10.5%]), blood prolactin increase (31 [8.8%]), and insomnia (19 [5.4%]) (). The majority of TEAEs were rated as mild or moderate. There were 24 (6.8%) serious TEAEs; exacerbation of psychotic symptoms (11 [3.1%]) was the most common serious TEAE. In total, 31 (8.8%) patients prematurely discontinued the study due to TEAEs; worsening of schizophrenia (10 [2.8%]) was the most frequent TEAE responsible for discontinuation. In all, 96 (27.2%) patients experienced EPS-related TEAEs. Extrapyramidal disorder (54 [15.3%]), akathisia (37 [10.5%]), restlessness (3 [0.8%]), musculoskeletal stiffness (3 [0.8%]), Parkinsonism (2 [0.6%]), and tremor (2 [0.6%]) were the common (n≥2) EPS events. Changes in EPS-rating scales (AIMS/BARS/SAS) indicated that >90% of patients had normal movements (severity of abnormal movement = none) throughout the study (based on AIMS assessment of severity of dyskinesia) and that the majority (≥75%) of patients had no akathisia, and none reported severe akathisia throughout the study (BARS scores). No trend suggestive of Parkinsonism was identified throughout the study as indicated by the median SAS total score (0.00) at the end of treatment phase. Prolactin-related TEAEs were experienced by 41 (11.6%) patients, and the more common (n≥3) events included increased blood prolactin levels (31 [8.8%]), amenorrhea, menstrual disorder, and galactorrhea (3 [0.8%] each). Glucose-related TEAEs (blood glucose increase) were reported in 2 (0.6%) patients. Clinically relevant weight gain (>7% increase in body weight) was reported in 28.6% of patients at the end of the follow-up period (day 206). Post-baseline changes in hematological parameters, vital signs, or ECGs were not clinically meaningful.
Table 4 Treatment-emergent adverse events experienced by at least 1% of patients (safety set)
Deaths were reported in 8 (2.3%) patients (completed suicide, n=4; deaths, n=2; unexplained deaths, n=2). One of the 2 deaths was reported as a “sudden cardiac death” since the autopsy results did not find any other cause for the death, such as trauma or poisoning. However, the consideration of the death as a “sudden cardiac death” was speculative as the death was not witnessed and no ECG monitoring was performed. The reason for the second death (found dead in river) was not reported; however, the possibility of a suicide could not be ruled out. Patients who died were in the age group of 22–45 years (completed suicides, 22–45 years; other deaths, 30–43 years); mostly women (completed suicides: men =2, women =2; other deaths: men =1, women =3) and 6 of the 8 patients had a psychiatric history of >5 years (completed suicides and other deaths, n=3 each). As compared with patients who survived (n=345), the patients who died had a longer duration of schizophrenia (mean [SD] duration, 4.6 [5.79] vs 7.5 [4.92] years). The mean (SD) MARS total score at baseline was 4.6 (2.26) in patients who died (n=8) and 4.3 (2.89) in patients who survived (n=341). Among the suicidal events, 2 of 3 suicide attempters and 2 of 4 suicide completers were women, and all 7 events occurred in younger patients (19–45 years). Two of the 4 suicide completers had documented prior nonfatal suicide attempt, and recent hospital admissions were reported in 3 of the 4 fatal suicide cases.
A protocol amendment was initiated to collect additional safety data, to include suicide risk evaluation (C-SSRS) and additional 12-lead ECG examination at all weekly visits (in addition to baseline and day 176 assessments, up to day 206 of the follow-up). After the amendment, evaluation of suicide risk based on C-SSRS scores was conducted in 28 of 353 patients. There were no reported suicidal attempts or completed suicides after amendment. No clinically notable cardiovascular abnormalities were observed based on additional ECG assessments.
Discussion
The present study evaluated the long-term safety and efficacy of PP1M in a large population of Chinese patients (n=353) with schizophrenia. In contrast to randomized controlled trials, the patient sample included in this study was representative of patients treated in everyday clinical practice, thus improving the external validity of these results. In addition, this study was designed to simulate real-world clinical situations in which patients are treated with flexible dosing of PP1M and evaluate pragmatic outcomes that potentially predict treatment responses. To our knowledge, such findings have not been reported earlier in a Chinese population with schizophrenia.
The efficacy findings from this study demonstrated that monthly flexible dosing (75, 100, or 150 mg eq.) of PP1M over a period of 25 weeks achieved favorable and significant (P=0.0001) reductions from baseline in the PANSS total scores (primary efficacy measure) for both total population and subgroups (patients with psychiatric history of ≤5 years and >5 years). Significant improvements in secondary efficacy end points, CGI-S and PSP scores, indicated progressive treatment response and improvements in psychosocial functioning in these patients.
Overall, PP1M showed greater symptom improvement in patients with recent-onset schizophrenia (psychiatric history of ≤5 years). This finding adds to available evidence that values the use of PP1M treatment in early schizophrenia, challenging the practice of reserving LAIs for chronic cases and treatment failures.Citation17,Citation48,Citation49 The efficacy findings from this study are consistent with the outcomes from earlier global studies of PP1M and studies in Asian patients with schizophrenia.Citation21,Citation22,Citation25,Citation27,Citation31,Citation33
The safety findings from this study also corroborated the outcomes reported in other studies of PP1M in patients from the Asia-Pacific region.Citation17,Citation25–Citation27 The rates of TEAEs (51.3%) reported herein were consistent with the results of 2 long-term studies of PP1M in patients from the Asia-Pacific region (66.0% and 82.3%)Citation33,Citation34 and global studies of PP1M,Citation30,Citation31,Citation50,Citation51 and were comparable with the TEAE rates generally observed with oral atypical antipsychotic treatment (64%–70%).Citation52 Worsening of schizophrenia (2.8%) was the most common TEAE that led to treatment discontinuation.
The clinical benefits of PP1M with regard to treatment satisfaction (MSQ scores), adherence to antipsychotic medications (MARS), and preference for LAI treatments over oral equivalents (APL) were also demonstrated by this study. These efficacy measures are indicators of antipsychotic treatment success in the real world that impact long-term management of schizophrenia, ultimately achieving prevention of relapses and reductions in hospitalizations.Citation4,Citation11,Citation53 Several studies have regarded positive patient attitude as a key outcome driver and a reliable predictor of treatment acceptance that favorably impacts long-term medication adherence and clinical responses.Citation54–Citation59 Improved patient satisfaction and positive attitude toward LAIs have been considered as important attributes that influence treatment choice among physicians.Citation54,Citation56 In the present study, a higher number of patients preferred LAIs throughout the course of the 6-month treatment with PP1M. These results, along with data from a systematic review that observed a greater preference for LAIs among patients previously treated with LAIs versus LAI-naïve patients, represent a progressive trend and changing attitude towards LAIs among patients, as well as prescribing physicians.Citation55,Citation59 The higher patient preference for deltoid over gluteal injections noted in this study is consistent with results from other reports, although gluteal injections have been associated with better tolerability.Citation50,Citation54 Thus, the flexibility of PP1M administration as both deltoid and gluteal injections offers an additional advantage. These results add to the current knowledge about patient and physician experiences that favor the use of LAI antipsychotics in routine clinical practice at different stages of schizophrenia.Citation54,Citation60,Citation61
A significant reduction in the magnitude of caregiver burden following the 6-month treatment with PP1M was also observed. Alleviations in caregiver burden reflect meaningful changes in various aspects of life such as social functioning, personal relationships, daily routine activities, leisure, and physical and mental health, all suggestive of improving the quality of life. In most cases, family members are the caregivers, and reducing the burden positively influences their role in offering intensive patient support and care and ensuring treatment adherence.Citation62,Citation63
A total of 8 deaths were reported in the present study, and half of those deaths were reportedly due to suicide. A greater risk of suicidality has been observed in patients with psychiatric illness as compared with the general population, with the estimates for lifetime risk of suicide attempt ranging from 1% to 4.7% in the non-afflicted population, escalating to 5%–22% in patients with schizophrenia.Citation64–Citation67 The prevalence of schizophrenia in the People’s Republic of China is generally similar to that of Western and other Asian populations; however, there are some different demographic and sociocultural risk factors for suicide in China. For example, in rural China, women are considered to be at greater risk than men for suicide.Citation68–Citation71
Some common predictors of suicides associated with schizophrenia include prior suicide attempt, comorbid depression or higher depressive symptoms, early onset of illness, frequent psychiatric hospitalizations, and unemployment.Citation65,Citation72–Citation76 In the present study, most patients who completed suicide were documented as having multiple known risk factors for suicide, including younger age at onset of illness and a history of prior suicide attempts, which is a strong clinical correlate of an eventual successful suicide attempt.Citation75,Citation77
Overall, the crude incidence rate for completed suicide noted in this study (28.42 per 1,000 patient-years; 95% confidence interval [CI]: 7.74–72.76) was substantially higher than the pooled incidence data for PP1M from the 4 studies (NCT01685931, NCT01051531, NCT01515423, and current study) conducted in the People’s Republic of China (7.06 per 1,000 patient-years; 95% CI: 2.29–16.47; ) (unpublished data on file). Treatment with atypical antipsychotics has been favorably associated with reduction in suicidal intentions and a potential for protection against suicide via attenuation of symptom severity.Citation73,Citation78 Further, based on cumulative experience from controlled Phase III studies of PP1M in Chinese patients with schizophrenia, the rates for completed suicide in the PP1M active treatment arms have been observed to be lower in comparison with placebo and risperidone LAI treatment arms. From case-level analysis, patients who completed suicides during the present study had a range of documented suicide-related risk factors. Thus, it is likely that the suicide rate in this study was related to patient selection.
Table 5 Death and suicide rates in clinical studies of paliperidone palmitate 1-month formulation conducted in the People’s Republic of China
The remaining 4 of the 8 deaths that occurred in the study were of unclear etiology. Two were classified as “unexplained”, and 1 was classified as being “of cardiac origin” but this result was speculative since the autopsy failed to yield any other clear cause. Unfortunately, none of the 4 deaths were witnessed; all 4 patients were found dead (1 each in a “rental room”, “by a river”, “in bed”, and “at home”). According to a previous study, mortality rates in schizophrenia were significantly greater than in the general population.Citation79 Cardiovascular disorders were the most common causes of sudden death in schizophrenia patients admitted to a psychiatric hospital, and sudden cardiac death occurred at a 0.8% rate in a psychiatric hospital, which is well above general population rates.Citation80 In the present study, 2 of 4 deaths of unclear etiology reported abnormal ECG at baseline suggestive of a possible cardiovascular involvement. Recent population-based studies from the People’s Republic of China have presented age-stratified mortality rates among patients with schizophreniaCitation81 and noted higher mortality rates in younger patients that diminished greatly with increased age (standard mortality ratio, 18–29 years: 120.89; 70+ years: 4.95).Citation81,Citation82 Similar to observations among patients who had fatal suicides in this study, the patients who died (all-cause) were young and had prolonged history of schizophrenia (≥5 years) as compared with patients who survived.
The crude incidence rates for deaths (all-cause) observed in this study were also considerably higher than the pooled data from 4 Chinese studies (including the current study) of PP1M (16.94 per 1,000 patient-years; 95% CI: 8.75–29.59; ) and global studies (7.1 per 1,000 patient-years);Citation83 the pooled death rates from 3 studies excluding the present study range from 5.86 to 8.24 per 1,000 person-years (unpublished data on file). The all-cause mortality rates in the present study were also higher than that reported in a large observational (ZODIAC) study (13 per 1,000 patient-years) for other antipsychotics (ziprasidone and olanzapine).Citation84
Based on the limited data from the 8 deaths reported during this study, the high crude incidence rate of completed suicides and deaths does not appear to indicate a definite association with PP1M treatment. The high suicide and all-cause mortality rates may be attributable to an interplay of preexisting pertinent risk factors,Citation72,Citation81,Citation82 limitations in analysis, and other study-related variables such as small sample size, low number of observed cases (completed suicides and suicide attempts), and wide CIs that limit the assessment of the validity of these findings. Furthermore, there is insufficient information to ascertain if there was a systematic bias involved in selection of high-risk patients. Thus, the findings from the current study do not represent a new identified risk for PP1M, and the benefit–risk assessment of PP1M remains positive and unchanged for the approved indications.
The higher occurrence of EPS-related events (27.2%) during this study was similar to that reported in earlier studies of PP1M in Chinese patients with schizophrenia.Citation25,Citation26 Extrapyramidal disorder (15.3%) showed the highest incidence among the EPS-related events, although the EPS-rating scale (SAS; median score: 0.0) did not indicate any trend of Parkinsonism. Further, the higher incidences of EPS events did not appear to deter medication adherence or the patient’s preferences for LAI in this study. The rates of other clinically significant TEAEs, including prolactin-related TEAEs and glucose-related TEAEs, were also generally comparable with previous studies of PP1M.Citation25,Citation27,Citation31,Citation33,Citation48
The open-label design and absence of a comparator in the current study may have introduced an observational bias on the physicians, and the knowledge of treatment allocation may have resulted in reporting bias for subjective outcomes such as patient-reported end points.
To summarize, treatment with PP1M showed symptom improvement, and no new safety concerns were identified over a 6-month treatment period in a real-world setting. These results were comparable with available evidence, validating the long-term benefits of PP1M in Chinese patients with schizophrenia.
Author contributions
Drs Lili Zhang, Shangli Cai, Yu Feng, Weihong Liu, and Huafei Lu were responsible for the design, conduct, and data analysis of the study. Drs Jingping Zhao, Lehua Li, Jianguo Shi, Yi Li, Xiufeng Xu, and Keqing Li were the principal investigators of the study, contributed data, and participated in the development of the manuscript. Jianmin Zhuo was responsible for the statistical analyses and design/interpretation of study results. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. All authors meet ICMJE criteria, and all those who fulfilled those criteria are listed as authors.
Acknowledgments
The authors thank Priya Ganpathy, ISMPP CMPP™ (SIRO Clinpharm Pvt. Ltd., India), for writing assistance and Dr Ellen Baum (Janssen Research & Development, LLC) for additional editorial assistance in the preparation of this manuscript. The authors also thank the study participants, without whom this study would not have been accomplished, and the following investigators for their contributions to this study: Drs Xueyi Wang, Fude Yang, Kerang Zhang, Jie Li, Wei Deng, Yi Xu, Chengge Gao, and Jianxiong Fan. This study was funded by Xi’an Janssen Pharmaceutical Ltd., People’s Republic of China. The sponsor also provided a formal review of the manuscript.
Disclosure
Drs Jingping Zhao, Lehua Li, Jianguo Shi, Yi Li, Xiufeng Xu, and Keqing Li were the principal investigators of this study and have received research support or fees as consultants from Xi’an Janssen Pharmaceutical Ltd., People’s Republic of China. Drs Lili Zhang, Yu Feng, Jianmin Zhuo, Weihong Liu, and Huafei Lu are employees of Xi’an Janssen Pharmaceutical Ltd., People’s Republic of China, which is a Johnson & Johnson company. Dr Shangli Cai was an employee of Xi’an Janssen Pharmaceutical Ltd., People’s Republic of China, at the time of study. The authors report no other conflicts of interest in this work.
References
- EmsleyRChilizaBAsmalLHarveyBHThe nature of relapse in schizophreniaBMC Psychiatry2013135023394123
- KarsonCDuffyRAEramoANylanderAGOffordSJLong-term outcomes of antipsychotic treatment in patients with first-episode schizophrenia: a systematic reviewNeuropsychiatr Dis Treat201612576726792993
- De HertMSermonJGeertsPVansteelandtKPeuskensJDetrauxJThe use of continuous treatment versus placebo or intermittent treatment strategies in stabilized patients with schizophrenia: a systematic review and meta-analysis of randomized controlled trials with first- and second-generation antipsychoticsCNS Drugs201529863765826293744
- MorkenGWidenJHGraweRWNon-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophreniaBMC Psychiatry200883218447935
- Ascher-SvanumHFariesDEZhuBErnstFRSwartzMSSwansonJWMedication adherence and long-term functional outcomes in the treatment of schizophrenia in usual careJ Clin Psychiatry200667345346016649833
- SendtKVTracyDKBhattacharyyaSA systematic review of factors influencing adherence to antipsychotic medication in schizophrenia-spectrum disordersPsychiatry Res20152251–2143025466227
- SchennachRObermeierMMeyerSPredictors of relapse in the year after hospital discharge among patients with schizophreniaPsychiatr Serv2012631879022227766
- WangXZhangWMaNAdherence to antipsychotic medication by community-based patients with schizophrenia in China: a cross-sectional studyPsychiatr Serv201667443143726725294
- XiaoJMiWLiLShiYZhangHHigh relapse rate and poor medication adherence in the Chinese population with schizophrenia: results from an observational survey in the People’s Republic of ChinaNeuropsychiatr Dis Treat2015111161116726056450
- AlphsLBossieCASliwaJKFuDJMaYWHulihanJPaliperidone palmitate and risperidone long-acting injectable in subjects with schizophrenia recently treated with oral risperidone or other oral antipsychoticsNeuropsychiatr Dis Treat2013934135023493643
- AgidOFoussiasGRemingtonGLong-acting injectable antipsychotics in the treatment of schizophrenia: their role in relapse preventionExpert Opin Pharmacother201011142301231720586707
- TiihonenJHaukkaJTaylorMHaddadPMPatelMXKorhonenPA nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophreniaAm J Psychiatry2011168660360921362741
- LeuchtCHeresSKaneJMKisslingWDavisJMLeuchtSOral versus depot antipsychotic drugs for schizophrenia – a critical systematic review and meta-analysis of randomised long-term trialsSchizophr Res20111271–3839221257294
- SchreinerAAadamsooKAltamuraCA randomized, active-controlled rater-blinded 2-year study of paliperidone palmitate versus investigators’ choice of oral antipsychotic monotherapy in patients with schizophrenia (prosipal)Eur Psychiatry201429Suppl 1124119631
- CitromeLPaliperidone palmitate – review of the efficacy, safety and cost of a new second-generation depot antipsychotic medicationInt J Clin Pract201064221623919886879
- NewtonRHustigHLakshmanaRPractical guidelines on the use of paliperidone palmitate in schizophreniaCurr Med Res Opin201228455956722321007
- LiHTurkozIZhangFEfficacy and safety of once-monthly injection of paliperidone palmitate in hospitalized Asian patients with acute exacerbated schizophrenia: an open-label, prospective, noncomparative studyNeuropsychiatr Dis Treat201512152426730193
- AlphsLBossieCASliwaJKMaYWTurnerNOnset of efficacy with acute long-acting injectable paliperidone palmitate treatment in markedly to severely ill patients with schizophrenia: post hoc analysis of a randomized, double-blind clinical trialAnn Gen Psychiatry20111011221481243
- BossieCAFuDJSliwaJKMaYWAlphsLTolerability of initiation doses of once-monthly paliperidone palmitate in patients with recently diagnosed schizophrenia in an acute treatment trialTher Adv Psychopharmacol20111411112423983935
- FuDJBossieCASliwaJKMaYWAlphsLPaliperidone palmitate versus oral risperidone and risperidone long-acting injection in patients with recently diagnosed schizophrenia: a tolerability and efficacy comparisonInt Clin Psychopharmacol2014291455524113628
- KramerMLitmanRHoughDPaliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety studyInt J Neuropsychopharmacol201013563564719941696
- PandinaGLaneRGopalSA double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophreniaProg Neuropsychopharmacol Biol Psychiatry201135121822621092748
- PandinaGJLindenmayerJPLullJA randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophreniaJ Clin Psychopharmacol201030323524420473057
- GopalSHoughDWXuHEfficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response studyInt Clin Psychopharmacol201025524725620389255
- LiHRuiQNingXXuHGuNA comparative study of paliperidone palmitate and risperidone long-acting injectable therapy in schizophreniaProg Neuropsychopharmacol Biol Psychiatry20113541002100821315787
- SiTZhangKTangJEfficacy and safety of flexibly dosed paliperidone palmitate in Chinese patients with acute schizophrenia: an open-label, single-arm, prospective, interventional studyNeuropsychiatr Dis Treat2015111483149226150719
- TakahashiNTakahashiMSaitoTRandomized, placebo-controlled, double-blind study assessing the efficacy and safety of paliperidone palmitate in Asian patients with schizophreniaNeuropsychiatr Dis Treat2013818891898
- SliwaJKBossieCAFuDJTurkozIAlphsLLong-term toler-ability of once-monthly injectable paliperidone palmitate in subjects with recently diagnosed schizophreniaNeuropsychiatr Dis Treat2012837538522956873
- AttardAOlofinjanaOCorneliusVCurtisVTaylorDPaliperidone palmitate long-acting injection – prospective year-long follow-up of use in clinical practiceActa Psychiatr Scand20141301465124117209
- CoppolaDLiuYGopalSA one-year prospective study of the safety, tolerability and pharmacokinetics of the highest available dose of paliperidone palmitate in patients with schizophreniaBMC Psychiatry2012122622455454
- GopalSVijapurkarULimPMorozovaMEerdekensMHoughDA 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophreniaJ Psychopharmacol201125568569720615933
- HoughDGopalSVijapurkarULimPMorozovaMEerdekensMPaliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled studySchizophr Res20101162–310711719959339
- ZhangFSiTChiouCFEfficacy, safety, and impact on hospitalizations of paliperidone palmitate in recent-onset schizophreniaNeuropsychiatr Dis Treat20151165766825792835
- SavitzAJXuHGopalSEfficacy and safety of paliperidone palmitate 3-month formulation for patients with schizophrenia: a randomized, multicenter, double-blind, noninferiority studyInt J Neuropsychopharmacol2016197
- MarderSRDavisJMChouinardGThe effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trialsJ Clin Psychiatry199758125385469448657
- BusnerJTargumSDThe clinical global impressions scale: applying a research tool in clinical practicePsychiatry (Edgmont)2007472837
- MorosiniPLMaglianoLBrambillaLUgoliniSPioliRDevelopment, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioningActa Psychiatr Scand2000101432332910782554
- GharabawiGMGreenspanARupnowMFReduction in psychotic symptoms as a predictor of patient satisfaction with antipsychotic medication in schizophrenia: data from a randomized double-blind trialBMC Psychiatry200664517054789
- VernonMKRevickiDAAwadAGPsychometric evaluation of the Medication Satisfaction Questionnaire (MSQ) to assess satisfaction with antipsychotic medication among schizophrenia patientsSchizophr Res20101181–327127820172695
- ThompsonKKulkarniJSergejewAAReliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychosesSchizophr Res200042324124710785582
- FialkoLGaretyPAKuipersEA large-scale validation study of the Medication Adherence Rating Scale (MARS)Schizophr Res20081001–3535918083007
- van WijngaardenBScheneAKoeterMPeople with schizophrenia in five countries: conceptual similarities and intercultural differences in family caregivingSchizophr Bull200329357358614609250
- GuyWEarly Clinical Drug Evaluation Unit (ECDEU) Assessment Manual for PsychopharmacologyRockville, MDNational Institute of Mental Health1976
- BarnesTRA rating scale for drug-induced akathisiaBr J Psychiatry19891546726762574607
- SimpsonGMAngusJWA rating scale for extrapyramidal side effectsActa Psychiatr Scand Suppl197021211194917967
- LiebermanJJodyDGeislerSTime course and biologic correlates of treatment response in first-episode schizophreniaArch Gen Psychiatry19935053693768098203
- McGlashanTHJohannessenJOEarly detection and intervention with schizophrenia: rationaleSchizophr Bull19962222012228782282
- AlphsLBossieCMaoLLeeEStarrHLTreatment effect with paliperidone palmitate compared with oral antipsychotics in patients with recent-onset versus more chronic schizophrenia and a history of criminal justice system involvementEarly Interv Psychiatry Epub2015925
- SubotnikKLCasausLRVenturaJLong-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trialJAMA Psychiatry201572882282926107752
- HoughDLindenmayerJPGopalSSafety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophreniaProg Neuropsychopharmacol Biol Psychiatry20093361022103119481579
- HargarterLCherubinPBergmansPIntramuscular long-acting paliperidone palmitate in acute patients with schizophrenia unsuccessfully treated with oral antipsychoticsProg Neuropsychopharmacol Biol Psychiatry2015581725448776
- LiebermanJAStroupTSMcEvoyJPClinical Antipsychotic Trials of Intervention Effectiveness (CATIE) InvestigatorsEffectiveness of antipsychotic drugs in patients with chronic schizophreniaN Engl J Med2005353121209122316172203
- MarcusSCZummoJPettitARStoddardJDoshiJAAntipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital dischargeJ Manag Care Spec Pharm201521975476826308223
- GeertsPMartinezGSchreinerAAttitudes towards the administration of long-acting antipsychotics: a survey of physicians and nursesBMC Psychiatry2013135823414331
- WaddellLTaylorMAttitudes of patients and mental health staff to antipsychotic long-acting injections: systematic reviewBr J Psychiatry Suppl200952S43S5019880916
- HeresSReichhartTHamannJMendelRLeuchtSKisslingWPsychiatrists’ attitude to antipsychotic depot treatment in patients with first-episode schizophreniaEur Psychiatry201126529730120570493
- PatelMXHaddadPMChaudhryIBMcLoughlinSHusainNDavidASPsychiatrists’ use, knowledge and attitudes to first- and second-generation antipsychotic long-acting injections: comparisons over 5 yearsJ Psychopharmacol201024101473148219477883
- KwonJSKimSNHanJSatisfaction of immediate or delayed switch to paliperidone palmitate in patients unsatisfied with current oral atypical antipsychoticsInt Clin Psychopharmacol201530632032826196188
- KaplanGCasoyJZummoJImpact of long-acting injectable antipsychotics on medication adherence and clinical, functional, and economic outcomes of schizophreniaPatient Prefer Adherence201371171118024265549
- StevensGLDawsonGZummoJClinical benefits and impact of early use of long-acting injectable antipsychotics for schizophreniaEarly Interv Psychiatry201610536537726403538
- González-RodríguezACatálanRPenadésRProfile of paliperidone palmitate once-monthly long-acting injectable in the management of schizophrenia: long-term safety, efficacy, and patient acceptability – a reviewPatient Prefer Adherence2015969570626082620
- Caqueo-UrízarARus-CalafellMUrzúaAEscuderoJGutiérrez-MaldonadoJThe role of family therapy in the management of schizophrenia: challenges and solutionsNeuropsychiatr Dis Treat20151114515125609970
- ShulerKMApproaches to improve adherence to pharmacotherapy in patients with schizophreniaPatient Prefer Adherence2014870171424868149
- PalmerBAPankratzVSBostwickJMThe lifetime risk of suicide in schizophrenia: a reexaminationArch Gen Psychiatry200562324725315753237
- HorKTaylorMSuicide and schizophrenia: a systematic review of rates and risk factorsJ Psychopharmacol2010244 Suppl819020923923
- NockMKBorgesGBrometEJCross-national prevalence and risk factors for suicidal ideation, plans and attemptsBr J Psychiatry200819229810518245022
- KesslerRCBorgesGWaltersEEPrevalence of and risk factors for lifetime suicide attempts in the National Comorbidity SurveyArch Gen Psychiatry199956761762610401507
- VijayakumarLSuicide and mental disorders in AsiaInt Rev Psychiatry200517210911416194780
- PhillipsMRYangGLiSLiYSuicide and the unique prevalence pattern of schizophrenia in mainland China: a retrospective observational studyLancet2004364943910621068
- XiangYTWengYZLeungCMTangWKUngvariGSSociodemographic and clinical correlates of lifetime suicide attempts and their impact on quality of life in Chinese schizophrenia patientsJ Psychiatr Res200842649550217663994
- LyuJZhangJCharacteristics of schizophrenia suicides compared with suicides by other diagnosed psychiatric disorders and those without a psychiatric disorderSchizophr Res20141551–3596524657011
- PhillipsMRYangGZhangYWangLJiHZhouMRisk factors for suicide in China: a national case-control psychological autopsy studyLancet200236093471728173612480425
- LuiSYRisk factors for deliberate self-harm and completed suicide in young Chinese people with schizophreniaAust N Z J Psychiatry200943325225919221914
- SirisSGSuicide and schizophreniaJ Psychopharmacol200115212713511448086
- ZhangXYAl JurdiRKZoghbiAWPrevalence, demographic and clinical correlates of suicide attempts in Chinese medicated chronic inpatients with schizophreniaJ Psychiatr Res201347101370137523791457
- MontrossLPKasckowJGolshanSSolorzanoELehmanDZisookSSuicidal ideation and suicide attempts among middle-aged and older patients with schizophrenia spectrum disorders and concurrent subsyn-dromal depressionJ Nerv Ment Dis20081961288489019077855
- SuominenKIsometsäESuokasJHaukkaJAchteKLönnqvistJCompleted suicide after a suicide attempt: a 37-year follow-up studyAm J Psychiatry2004161356256314992984
- MeltzerHYAlphsLGreenAIInternational Suicide Prevention Trial Study GroupClozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT)Arch Gen Psychiatry2003601829112511175
- SahaSChantDMcGrathJA systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time?Arch Gen Psychiatry200764101123113117909124
- IfteniPCorrellCUBurteaVKaneJMManuPSudden unexpected death in schizophrenia: autopsy findings in psychiatric inpatientsSchizophr Res20141551–3727624704220
- LiuTSongXChenGParadisADZhengXPrevalence of schizophrenia disability and associated mortality among Chinese men and womenPsychiatry Res20142201–218118725113924
- RanMSMaoWJChanCLChenEYConwellYGender differences in outcomes in people with schizophrenia in rural China: 14-year follow-up studyBr J Psychiatry2015206428328825573398
- PiercePGopalSSavitzAPaliperidone palmitate: Japanese postmarketing mortality results in patients with schizophreniaCurr Med Res Opin201619
- StromBLEngSMFaichGComparative mortality associated with ziprasidone and olanzapine in real-world use among 18,154 patients with schizophrenia: the Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC)Am J Psychiatry2011168219320121041245