Abstract
Objective
Recent advances have provided compelling evidence for the role of excessive complement activity in the pathophysiology of schizophrenia. In this study, we aimed to detect the association of the gene encoding complement factor H (CFH), a regulator in complement activation, with schizophrenia.
Materials and methods
A sample of 1783 individuals with or without schizophrenia was recruited for genetic analysis. Genomic DNA samples were extracted from peripheral blood cells using multiplex polymerase chain reaction and the SNaPshot assay. A Database for Schizophrenia Genetic Research (SZDB) was used to detect the association of brain CFH expression with schizophrenia. Next, we performed a genotype–phenotype analysis to identify the relationship between CFH Y402H polymorphism and clinical features of schizophrenia.
Results
There was a significant association of hippocampal CFH expression with schizophrenia (P=0.017), whereas this significance did not survive after adjusting for false discovery rate (P=0.105). Comparing the genotype and allele frequencies of the genotyped single-nucleotide polymorphisms between case and control groups showed no significant difference. There were significant differences in the scores of negative symptoms and delayed memory between the patients with C allele and those without C allele (P<0.01 and P=0.04 after Bonferroni correction, respectively). Furthermore, we observed a marginally significant association between the Y402H polymorphism and CFH expression in the hippocampus (P=0.051); however, this significance was lost after multiple testing correction (P=0.51, after Bonferroni correction).
Conclusion
Our findings provide suggestive evidence for the role of CFH in the development of negative symptoms and cognitive dysfunction in schizophrenia.
Introduction
Schizophrenia is a chronic, severe and devastating neuropsychiatric disorder with a lifetime risk of ~1% and characterized by positive and negative symptoms and cognitive dysfunction. Although intensive research has been done in the past decades, the biological mechanism of schizophrenia remains obscure.Citation1 Early literature indicated that both maternal bacterial and viral infections during pregnancy epidemiologically increase the risk of schizophrenia in offspring.Citation2 There is also evidence showing that patients with schizophrenia or certain autoimmune diseases share some key clinical, epidemiological and genetic features.Citation3 Therefore, it is believed today that immune alterations may be involved in the pathophysiology of schizophrenia.
Family, twin and adoption studies have demonstrated that schizophrenia is a familiar disorder with a complex mode of inheritance, and its heritability reaches upward of 80%.Citation4,Citation5 Hence, understanding the genetics involved in schizophrenia seems to provide a way to dissect the biological mechanism of this disorder.Citation1 Recent genome-wide association studies (GWASs) have identified several genes within the extended human major histocompatibility complex (MHC) region conferring susceptibility to schizophrenia across different ethnics.Citation6–Citation11 Given the best role of MHC in immunity, Sekar et alCitation12 reported a novel susceptibility gene encoding complement component 4 (C4) in schizophrenia, implying that excessive complement activity increases schizophrenia risk. In the activation of the complement system, complement factor H (CFH) acts as a major inhibitor of the alternative pathway in the complement cascade.Citation13 Abnormalities in the structure or function of CFH can accordingly unbalance the normal homeostasis of the complement system, resulting in “bystander” damage to healthy tissues. Our previous work has reported that the gene encoding CFH (CFH) increases the risk for major depressive disorder (MDD) in Han Chinese.Citation14 In clinics, patients with schizophrenia or MDD might share some symptoms such as loss of interests, sad mood, insomnia, energy and cognitive dysfunction.Citation15,Citation16 In genetics, there are quite a few studies indicating that both diseases might share some polygenic basis.Citation17 Collectively, this study aimed to verify whether CFH has some potential associations with schizophrenia in Han Chinese.
Here, we first used a public database to detect whether CFH is differentially expressed in brain between patients with schizophrenia and healthy controls. Then, we genotyped a total of 11 single-nucleotide polymorphisms (SNPs), which were screened for a good coverage of this region in DNA samples of 1783 individuals with or without schizophrenia, in order to characterize the association between genetic variations within CFH and the risk of developing schizophrenia in Han Chinese. It has been well established that schizophrenia is a heterogeneous disease and clinical phenotype would be more close to certain susceptibility genes rather than the whole spectrum of schizophrenia. Hence, investigating the genotype–phenotype correlations of schizophrenia may lead to a more detailed understanding of this disease.Citation18,Citation19 A non-synonymous SNP rs1061170 (Y402H) was reported to have a significant association with MDD,Citation14 and we hypothesized that this functional polymorphism may have a genotype–phenotype correlation with schizophrenia symptoms. In the third step, we analyzed the relationship between Y402H and clinical features of schizophrenia.
Materials and methods
Subjects
We recruited 878 patients with schizophrenia from three mental hospitals in Eastern China, including Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Jinhua Second Hospital and Wenzhou Kangning Hospital. All patients met the diagnoses of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) and had no other observable physical disease or other psychiatric disorders aside from schizophrenia. Among them, there were 254 schizophrenia patients under olanzapine monotherapy enrolled for evaluating clinical features, whose inclusion criteria were according to our previous publicationsCitation20–Citation22 as follows: 1) duration of illness <5 years; 2) a minimum education of primary middle school; 3) receiving atypical antipsychotic monotherapy; 4) maintained a stable condition for >6 months before entry into the study and 5) a Positive and Negative Syndrome Scale for Schizophrenia (PANSS) total score <60.
A total of 905 healthy controls were recruited from hospital staff and students of School of Medicine in Shanghai and then interviewed by a specialized psychiatrist using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition.
All the patients and control subjects were of Han Chinese origin. All procedures were reviewed and approved by the institutional review boards of Shanghai Mental Health Center, Jinhua Second Hospital and Wenzhou Kanging Hospital. This study was performed in accordance with the guidelines laid out in the Declaration of Helsinki as revised in 1989. All subjects provided written informed consent before any study-related procedures were performed.
Evaluation
The PANSS was employed to evaluate symptom severity.Citation22 The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was the primary outcome instrument for this study.Citation23 The 12-item RBANS consists of five subsets, corresponding to five domains of neuropsychological process: 1) immediate memory (list learning and story memory), 2) visuospatial/constructional (figure copy and line orientation), 3) language (picture naming and semantic fluency), 4) attention (digit span and coding) and 5) delayed memory (list learning free recall, list learning recognition, story memory free recall and figure free recall).
Brain eQTL (expression quantitative trait loci) analysis for CFH expression
It is known that schizophrenia originates from brain structural and functional abnormalities,Citation24 and dysregulation of gene expression has a key role in the pathogenesis of this disease. In this study, we performed an eQTL analysis to detect whether CFH is differentially expressed in brain between patients with schizophrenia and healthy controls, using SZDB database (http://www.szdb.org/), a newly developed comprehensive resource for schizophrenia research.Citation25
SNP selection
In our recent studies,Citation14,Citation26 we performed an extensive analysis of SNPs in CFH and selected a total of 11 SNPs with 80% coverage of the gene. We genotyped all these SNPs in this study, including nine tagging SNPs (rs800292, rs10801555, rs10922096, rs10733086, rs10737680, rs11582939, rs2019727, rs1410996 and rs426736) from the 5′ to 3′ regions of CFH that were selected from phase 2 of the HapMap projectCitation27 using the Tagger algorithm with an r2 cutoff of 0.8 (minor allele frequency >0.05) and two important functional variants rs1061170 (p.Y402H) and rs460184 (p.V1197A) that were previously reported to be associated with age-related macular degeneration and other human diseases.Citation28,Citation29 Detailed information of these selected SNPs is shown in our previous publications.Citation14,Citation26
Genotyping
Genomic DNA was isolated from whole blood using a Tiangen DNA isolation kit (Tiangen Biotech, Beijing, China). The 11 SNPs were detected using multiplex polymerase chain reaction and the SNaPshot assay, while details have been described in our previous work.Citation14,Citation26
Psychiatric Genomics Consortium data analysis
To further validate the association between the studied SNPs and schizophrenia, we extracted the schizophrenia genetic association data from the Psychiatric Genomics Consortium (PGC; http://www.broadinstitute.org/mpg/ricopili/) databaseCitation30 and reanalyzed the data set as an independent sample.
Brain eQTL analysis for risk SNPs
The brain eQTL analysis was performed using the brain eQTL database (http://caprica.genetics.kcl.ac.uk/BRAINEAC/), a large exon-specific eQTL data set covering 10 human brain regions. More detailed information can be found in the original study.Citation31
Statistical analysis
Demographic data were analyzed using chi-squared or t-test as appropriate. For expression analyses, analysis of covariance (ANCOVA) was carried out with age, sex and smoking status as covariates controlled in the model to minimize the potential effect of these factors on the expression level of CFH messenger RNA. Hardy–Weinberg equilibrium testing and allele and genotype frequency analysis were conducted using SHEsis (http://analysis.bio-x.cn).Citation32 The pairwise linkage disequilibrium (LD) analysis for all pairs of SNPs was applied to detect the inter-marker relationship in case–control samples. The LD blocks were identified using the solid spine of LD method, with extended spine if D′>0.5 in Haploview (version 4.1). The possible genotype–phenotype correlation of Y402H with schizophrenia symptoms was examined using ANCOVA by comparing the mean PANSS and RBANS scores of each genotype. Variables that affect symptom severity (that is, age, sex, education and duration of illness) were included as covariates. Statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). All P-values were two-tailed, and P-values <0.05 were considered statistically significant after Bonferroni correction.
Results
We extracted brain CFH expression data between schizophrenia patients and healthy controls from SZDB database. showed that there is a significant association of hippocampal CFH expression with schizophrenia (P=0.017), whereas this significance did not survive after adjusting for false discovery rate (P=0.105). However, patients with schizophrenia seem to have higher levels of CFH expression in hippocampus than controls ().
Figure 1 Differential expression of CFH in the brain between patients with schizophrenia and healthy controls.
Abbreviations: CFH, complement factor H; SZDB, A Database for Schizophrenia Genetic Research.
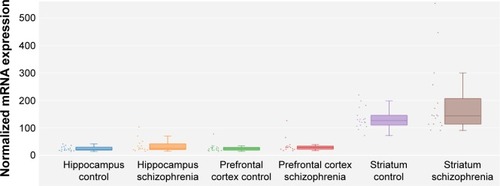
Table 1 CFH expression level in the brain between case and control groups
Genotype distributions revealed no deviation from Hardy–Weinberg equilibrium in controls, except for rs460184, and we excluded this SNP from the following study. The genotype and allele frequencies of these CFH SNPs are presented in . There was a significant difference in allelic distribution of SNP rs1061170 (Y402H) between the case and control groups (P=0.03). However, this significance did not remain after correcting for multiple testing (P=0.30, after Bonferroni correction). We further examined the genetic association between the 10 SNPs and schizophrenia in the PGC database. Although we did not find the data of rs1061170 in PGC database, showed that its tag SNP rs1061147 is not associated with schizophrenia in PGC GWAS (P=0.273). Thus, none of the SNPs exhibited significant association with schizophrenia.
Table 2 Comparison of genotypic and allelic distributions of CFH variants between case and control groups
Analysis of pairwise LD showed three strong LDs between rs1061170 and rs10801555, rs10922096 and rs2019727, as well as rs10733086 and rs10737680 (). In view of the strong LDs, we performed a 2-SNP haplotype analysis, analyzing only those common haplotypes with at least 3% of frequency in either case or control samples (P-values corresponding to the haplotypes are shown in ). However, no significant difference was found for any haplotype.
We further examined the relationship between the Y402H polymorphism and schizophrenia symptoms by comparing scores of the PANSS scale and RBANS with genotypes of the Y402H polymorphism. Taking into consideration the low frequency of C/C genotype in our sample, ANCOVA was carried out with the Y402H genotypes (C/C + C/T versus T/T) as the independent variables, the scores of PANSS scale and RBANS as the dependent variables and age, sex, years of education and duration of illness as the covariates. showed significant differences in the scores of negative symptoms and delayed memory between the patients with C allele and those without C allele (P<0.01 and P=0.04 after Bonferroni correction, respectively).
Table 3 Comparison of clinical characteristics among Y402H genotypic groups in schizophrenia
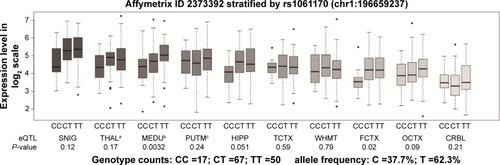
To detect the role of Y402H in hippocampal CFH expression, we performed an eQTL analysis. As shown in , we observed a marginally significant association between the Y402H polymorphism and CFH expression in the hip-pocampus (P=0.051); however, this significance was lost after multiple testing correction (P=0.51, after Bonferroni correction).
Figure 2 Association of rs1061170 with CFH expression level in 10 brain regions (Affymetrix ID 2373392).
Abbreviations: BRAINEAC, The Brain eQTL Almanac; CFH, complement factor H; eQTL, expression quantitative trait locus; SNIG, substantia nigra; THAL, thalamus; MEDU, inferior olivary nucleus; PUTM, putamen; HIPP, hippocampus; TCTX, temporal cortex; WHMT, intralobular white matter; FCTX, frontal cortex; OCTX, occipital cortex; CRBL, cerebellar cortex.
Discussion
A recent cross-disorder genome-wide analysis showed that a broad set of common variants has cross-disorder effects for all the adult-onset disorders (MDD and schizophrenia and bipolar disorder).Citation33 This group also calculated the genetic correlation of different psychiatric disorders using common SNPs and found that there was a moderate value (0.47±0.06 standard error [s.e.]) between MDD and schizophrenia.Citation34 It is suggested that some similar brain pathology may be shared by the two psychiatric disorders. In the past decades, a body of literature has supported the implication of immune alterations in MDD and schizophrenia.Citation35 Our previous work indicated that CFH plays a major role in the development of MDD.Citation14 On this premise, we attempted to investigate the role of CFH in schizophrenia. We investigated 11 SNPs within CFH and carried out the PGC analysis for further validation. Although the data of rs1061170 (Y402H) were not found in the PGC database, its tag SNP rs1061147 that has perfect LD (r2=1.0) with rs1061170 shows no significant association with schizophrenia in PGC GWAS. The frequency of A allele of rs1061147 is similar to that of C allele of rs1061170 either in Caucasian (36%) or in Han Chinese (7%) populations. Therefore, our results implied that there is no significant association of CFH with schizophrenia in either Chinese Han or Caucasian populations. However, we found that hippocampal CFH expression may be enriched in patients with schizophrenia than healthy controls through an eQTL analysis using the SZDB database.
CFH is a major inhibitor of the alternative complement pathway, which regulates complement activation in tissue inflammation during degeneration.Citation36 Previous literature has reported that increased serum CFH level is associated with Alzheimer’s disease, a neurodegenerative disorder.Citation37,Citation38 Thus, increased CFH expression may be implicated with the development of neurodegeneration. On the other side, it has been well documented that the largest magnitude of subcortical brain volume abnormalities in schizophrenia is in the hippocampus, which can be seen in both the early and chronic stages of this disorder.Citation39,Citation40 Hippocampus is hypothesized to underlie the neuropsychological deficits and symptoms observed in schizophrenia.Citation41,Citation42 Thereby, the role of CFH in schizophrenia could not be excluded, even though we did not detect any association of CFH with schizophrenia at molecular level. The finding that hippocampal CFH expression alters in schizophrenia implied that CFH may be involved in certain specific symptoms of this disorder. The Y402H polymorphism is a non-synonymous SNP and is of particular interest because it is located within the region of short consensus repeat domains 7 binding heparin and C-reactive protein.Citation43 The base transition of thymine to cytosine occurs in the exon 9 of the gene and leads to a tyrosine–histidine substitution in the protein.Citation44 Previous studies demonstrated that this variant exerts allelic differences on the binding affinity to C-reactive proteins, with the risk allele showing reduced affinity.Citation45 In doing so, this could influence complement activation, host immune status and inflammation process and hence account for ~17% of age-related macular degeneration liability.Citation46 Hence, we further examined whether Y402H polymorphism is associated with clinical dimensions of schizophrenia.
In general, patients with schizophrenia performed worse in cognitive function than healthy controls in almost all the cognitive domains.Citation47,Citation48 Among the case group, we observed a positive association of Y402H polymorphism with the severity of negative symptoms and delayed memory. Negative symptoms are deficits of normal emotional responses, including avolition, affective flattening and social withdrawal.Citation49 There is considerable conceptual overlap between the negative symptoms and cognitive dysfunction.Citation50 The postmortem studies are consistent with the neuroimaging findings showing an association of altered structure and function of hippocampus with schizophrenia.Citation51 There is evidence from functional magnetic resonance imaging study showing an association of hippocampal neural activity with amygdala activity and emotional memory,Citation52 suggesting an involvement of hippocampus in emotional processing. A recent functional magnetic resonance imaging study showed hippocampal hypoactivity in patients with schizophrenia during facial emotional processing tasks.Citation53 On the other side, the hippocampus has been well established to be necessary for learning and memory.Citation54 A line of MRI scans have indicated that hippocampus plays a critical role in cognitive dysfunction in schizophrenia.Citation55 Therefore, hippocampus is likely to be a crucial brain region in the development of negative symptoms and cognitive dysfunction in schizophrenia. To detect the association of Y402H polymorphism and CFH expression in hippocampus, we performed an eQTL analysis. Our results implied that the Y402H polymorphism has a possible modulatory effect on CFH expression in hippocampus. As such, these findings suggested that Y402H polymorphism may give risk to the alternation of CFH expression in hippocampus and influence the severity of negative symptoms and cognitive dysfunction in schizophrenia. We noticed in SZDB database that patients with schizophrenia had higher levels of hippocampal CFH expression than controls. However, in the brain eQTL analysis for risk SNP, hippocampal CFH expression seemed lower in individuals with the risk C/C genotype than those with C/T or T/T genotypes. The counterintuitive results may be caused by small sample size and different ethnic origins. Therefore, further investigations are warranted to address this issue.
When interpreting the results of this study, we would be remiss in not noting some limitations. First, cross-sectional association studies always have the potential for population stratification. Although the subjects were all of Han Chinese origin and collected from Eastern China, we could not fully exclude the possibility of a population structure effect in our sample. Second, this study details an exploratory study performed on a subset of the general Chinese Han population. The sample size is modest and precludes us from making any definitive statements on the associations between CFH and schizophrenia in Han Chinese. Third, all the patients had received antipsychotic treatment and maintained stable conditions for >6 months prior to this study. It is known that antipsychotic treatment would bias symptomatology, and therefore, we could not completely conclude that CFH is associated with negative symptoms and cognitive dysfunction in schizophrenia. Accordingly, our findings should be considered only preliminary and exploratory. Further investigations need to validate our results in independent populations and more fully explain any potential relationship or lack thereof.
Conclusion
We performed a comprehensive analysis for the association between CFH and schizophrenia in Han Chinese. Our findings provided suggestive evidence for CFH’s role in the development of negative symptoms and cognitive dysfunction in schizophrenia. Further investigations are required to evaluate this association in a larger and independent sample across various ethnicities.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors are deeply grateful to all participants. This work was supported by the National Natural Science Foundation of China (81471358 and 81671326), the Shanghai Science and Technology Commission Foundation (14411969000), the Shanghai Municipal Education Commission – Gaofeng Clinical Medicine Grant Support (20152530), the Shanghai Municipal Commission of Health and Family Planning Foundation (201540029) and the Shanghai Mental Health Center Foundation (2014-FX-03).
Supplementary materials
Figure S1 Association of rs1061147 with schizophrenia.
Note: Data from the Psychiatric Genomics Consortium (PGC; http://www.broadinstitute.org/mpg/ricopili/) database.
Abbreviations: PGC, Psychiatric Genomics Consortium; SCZ, schizophrenia; GWAS, genome-wide association studies; Sept., September.
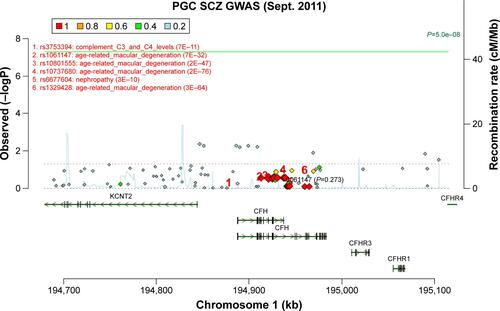
Figure S2 LD plot consisting of 10 SNPs at the CFH gene and its region plot.
Notes: Pairwise LD was computed for all possible combinations of the 10 SNPs using the values of D′ and r2. The individual square showed the 100× D′ (or r2) value for each SNP pair. SNP rs460184 was not included due to the deviation from HWE.
Abbreviations: LD, linkage disequilibrium; SNP, single-nucleotide polymorphism; CFH, complement factor H; HWE, Hardy–Weinberg equilibrium.
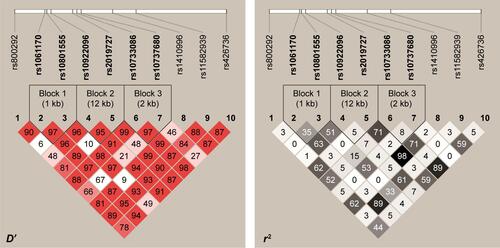
Table S1 Results of the pairwise haplotype test of the case and control groups
Disclosure
The authors report no conflicts of interest in this work.
References
- DhindsaRSGoldsteinDBSchizophrenia: from genetics to physiology at lastNature2016530758916216326814972
- BrownASDerkitsEJPrenatal infection and schizophrenia: a review of epidemiologic and translational studiesAm J Psychiatry2010167326128020123911
- BenrosMENielsenPRNordentoftMEatonWWDaltonSOMortensenPBAutoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register studyAm J Psychiatry2011168121303131022193673
- GoldmanALPezawasLMattayVSHeritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation studyBiol Psychiatry200863547548317727823
- SullivanPFKendlerKSNealeMCSchizophrenia as a complex trait: evidence from a meta-analysis of twin studiesArch Gen Psychiatry200360121187119214662550
- PurcellSMWrayNRStoneJLCommon polygenic variation contributes to risk of schizophrenia and bipolar disorderNature2009460725674875219571811
- StefanssonHOphoffRASteinbergSCommon variants conferring risk of schizophreniaNature2009460725674474719571808
- ShiJLevinsonDFDuanJCommon variants on chromosome 6p22.1 are associated with schizophreniaNature2009460725675375719571809
- IkedaMAleksicBKinoshitaYGenome-wide association study of schizophrenia in a Japanese populationBiol Psychiatry201169547247820832056
- ZhangYLuTLYanHReplication of association between schizophrenia and chromosome 6p21–6p22.1 polymorphisms in Chinese Han populationPLoS One201382e5673223437227
- YueWHWangHFSunLDGenome-wide association study identifies a susceptibility locus for schizophrenia in Han Chinese at 11p11.2Nat Genet201143121228128422037552
- SekarABialasARde RiveraHSchizophrenia risk from complex variation of complement component 4Nature2016530758917718326814963
- RipocheJDayAJHarrisTJSimRBThe complete amino acid sequence of human complement factor HBiochem J198824925936022963625
- ZhangCZhangDFWuZGComplement factor H and susceptibility to major depressive disorder in Han ChineseBr J Psychiatry2016208544645226941266
- CraddockNFortyLGenetics of affective (mood) disordersEur J Hum Genet200614666066816721402
- WangMChenJHeKThe NVL gene confers risk for both major depressive disorder and schizophrenia in the Han Chinese populationProg Neuropsychopharmacol Biol Psychiatry20156271325891250
- ChenJWangMWaheed KhanRAThe GSK3B gene confers risk for both major depressive disorder and schizophrenia in the Han Chinese populationJ Affect Disord201518514915526186530
- PapiolSMalzahnDKastnerADissociation of accumulated genetic risk and disease severity in patients with schizophreniaTransl Psychiatry20111e4522833191
- ZhangXYChenDCTanYLBDNF polymorphisms are associated with schizophrenia onset and positive symptomsSchizophr Res20161701414726603468
- CaiJZhuYZhangWWangYZhangCComprehensive family therapy: an effective approach for cognitive rehabilitation in schizophreniaNeuropsychiatr Dis Treat2015111247125326056456
- ZhangCLiZShaoYAssociation study of tryptophan hydroxylase-2 gene in schizophrenia and its clinical features in Chinese Han populationJ Mol Neurosci201143340641120938755
- KaySRFiszbeinAOplerLAThe positive and negative syndrome scale (PANSS) for schizophreniaSchizophr Bull19871322612763616518
- RandolphCTierneyMCMohrEChaseTNThe repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validityJ Clin Exp Neuropsychol19982033103199845158
- FornitoABullmoreETReconciling abnormalities of brain network structure and function in schizophreniaCurr Opin Neurobiol201530445025238608
- WuYYaoYGLuoXJSZDB: a database for schizophrenia genetic researchSchizophr Bull Epub201672210.1093/schbul/sbw102
- ZhangDFWangDLiYYYaoYGMapping genetic variants in the CFH gene for association with leprosy in Han ChineseGenes Immun201415750651025030427
- International HapMap ConsortiumThe International HapMap ProjectNature2003426696878979614685227
- ChenHLiuKChenLJHouPChenWPangCPGenetic associations in polypoidal choroidal vasculopathy: a systematic review and meta-analysisMol Vis20121881682922509112
- HuangLLiYGuoSDifferent hereditary contribution of the CFH gene between polypoidal choroidal vasculopathy and age-related macular degeneration in Chinese Han PeopleInvest Ophthalmol Vis Sci20145542534253824692129
- Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) ConsortiumGenome-wide association study identifies five new schizophrenia lociNat Genet2011431096997621926974
- RamasamyATrabzuniDGuelfiSGenetic variability in the regulation of gene expression in ten regions of the human brainNat Neurosci201417101418142825174004
- ShiYYHeLSHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism lociCell Res2005152979815740637
- Cross-Disorder Group of the Psychiatric Genomics ConsortiumIdentification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysisLancet201338198751371137923453885
- LeeSHRipkeSNealeBMInternational Inflammatory Bowel Disease Genetics Consortium (IIBDGC)Genetic relationship between five psychiatric disorders estimated from genome-wide SNPsNat Genet201345998499423933821
- LisboaSFGomesFVGuimaraesFSCamposACMicroglial cells as a link between cannabinoids and the immune hypothesis of psychiatric disordersFront Neurol20167526858686
- Langford-SmithAKeenanTDClarkSJBishopPNDayAJThe role of complement in age-related macular degeneration: heparan sulphate, a ZIP code for complement factor H?J Innate Immun20146440741624335201
- HyeALynhamSThambisettyMProteome-based plasma bio-markers for Alzheimer’s diseaseBrain2006129pt 113042305017071923
- HyeARiddoch-ContrerasJBairdALPlasma proteins predict conversion to dementia from prodromal diseaseAlzheimers Dement2014106799.e807.e25012867
- AdrianoFCaltagironeCSpallettaGHippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysisNeuroscientist201218218020021531988
- van ErpTGHibarDPRasmussenJMSubcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortiumMol Psychiatry201621454755326033243
- HýžaMKuhnMČeškováEUstohalLKašpárekTHippocampal volume in first-episode schizophrenia and longitudinal course of the illnessWorld J Biol Psychiatry201617642943827403591
- HoNFIglesiasJESumMYProgression from selective to general involvement of hippocampal subfields in schizophreniaMol Psychiatry201722114215226903271
- KleinRJZeissCChewEYComplement factor H polymorphism in age-related macular degenerationScience2005308572038538915761122
- Rodriguez de CordobaSEsparza-GordilloJGoicoechea de JorgeELopez-TrascasaMSanchez-CorralPThe human complement factor H: functional roles, genetic variations and disease associationsMol Immunol200441435536715163532
- ChenHYuKDXuGZAssociation between variant Y402H in age-related macular degeneration (AMD) susceptibility gene CFH and treatment response of AMD: a meta-analysisPLoS One201278e4246422905135
- RaychaudhuriSIartchoukOChinKA rare penetrant mutation in CFH confers high risk of age-related macular degenerationNat Genet201143121232123622019782
- HanMHuangXFChenDCGender differences in cognitive function of patients with chronic schizophreniaProg Neuropsychopharmacol Biol Psychiatry201239235836322820676
- ZhangXYChenDCXiuMHCognitive and serum BDNF correlates of BDNF Val66Met gene polymorphism in patients with schizophrenia and normal controlsHum Genet201213171187119522362486
- KirkpatrickBFentonWSCarpenterWTJrMarderSRThe NIMH-MATRICS consensus statement on negative symptomsSchizophr Bull200632221421916481659
- FervahaGTakeuchiHFoussiasGAgidORemingtonGUsing poverty of speech as a case study to explore the overlap between negative symptoms and cognitive dysfunctionSchizophr Res20161762–341141627242067
- HeckersSKonradiCGABAergic mechanisms of hippocampal hyperactivity in schizophreniaSchizophr Res20151671–341125449711
- PhelpsEAHuman emotion and memory: interactions of the amygdala and hippocampal complexCurr Opin Neurobiol200414219820215082325
- JiEWeickertCSLenrootRAdjunctive selective estrogen receptor modulator increases neural activity in the hippocampus and inferior frontal gyrus during emotional face recognition in schizophreniaTransl Psychiatry20166e79527138794
- ZhangCFangYXuLGlutamate receptor 1 phosphorylation at serine 845 contributes to the therapeutic effect of olanzapine on schizophrenia-like cognitive impairmentsSchizophr Res20141592–337638425219486
- KoolschijnPCvan HarenNECahnWHippocampal volume change in schizophreniaJ Clin Psychiatry201071673774420492835
