Abstract
Tourette’s syndrome (TS) is a neurodevelopmental disorder that comprises vocal and motor tics associated with a high frequency of psychiatric comorbidities, which has an important impact on quality of life. The onset is mainly in childhood and the symptoms can either fade away or require pharmacological therapies associated with cognitive-behavior therapies. In rare cases, patients experience severe and disabling symptoms refractory to conventional treatments. In these cases, deep brain stimulation (DBS) can be considered as an interesting and effective option for symptomatic control. DBS has been studied in numerous trials as a therapy for movement disorders, and currently positive data supports that DBS is partially effective in reducing the motor and non-motor symptoms of TS. The average response, mostly from case series and prospective cohorts and only a few controlled studies, is around 40% improvement on tic severity scales. The ventromedial thalamus has been the preferred target, but more recently the globus pallidus internus has also gained some notoriety. The mechanism by which DBS is effective on tics and other symptoms in TS is not yet understood. As refractory TS is not common, even reference centers have difficulties in performing large controlled trials. However, studies that reproduce the current results in larger and multicenter randomized controlled trials to improve our knowledge so as to support the best target and stimulation settings are still lacking. This article will discuss the selection of the candidates, DBS targets and mechanisms on TS, and clinical evidence to date reviewing current literature about the use of DBS in the treatment of TS.
Introduction
Tourette’s syndrome (TS) is a neurobehavioral disease characterized by motor and phonic tics often associated with many behavioral comorbidities such as obsessive– compulsive disorder (OCD), attention-deficit hyperactivity syndrome, impulse control, and autism spectrum disorders.Citation1,Citation2 According to the last DSM-V criteria, TS is now classified as a movement disorder under neurodevelopmental disorders section and its diagnosis is based on the persistent occurrence of at least one vocal and two motor tics beginning before 18 years old and lasting longer than 1 year excluding other causes.Citation3 Tics are defined as sudden, short, intermittent, “semi-involuntary” movements and vocalizations (can be suppressed temporarily) that are preceded by a premonitory urge or impulse.Citation1,Citation4 The family history of tics or behavior disorders is often positive.Citation5 TS patients also present with other psychiatric comorbidities such as depression, anxiety and impulsivity, sleep disorders, learning disorders, and in some cases a self-injurious behavior.Citation2
Typically, between 15 and 17 of age, the majority of TS patients experience a decrease in frequency and severity of tics. By early adulthood, about three-quarters of children with TS will have considerable improvement in symptoms and about 32% will be tic-free. While it does not affect cognition and the intellect itself, this condition can cause significant functional and social burden, sometimes affecting normal development in school and professional activities. Treatment includes mostly behavioral therapy and oral medications, alone or in association. At present, there are a variety of psychoactive medications that interact with dopamine (typical but mostly atypical antipsychotic agents) and non-dopamine systems (such as α2 agonists), associated or not with behavioral therapy, and psychoeducative interventions with responses ranging from 30% to 85%.Citation6 Botulinum toxin injections can be effective in focal tics.Citation7 However, there are patients who do not benefit from medication either due to poor response or due to unpleasant side effects that further limit their use. This subset of patients can evolve with the persistence of tics,Citation2,Citation8 thus becoming treatment-refractory and severely disabled.Citation9 In this scenario, deep brain stimulation (DBS) can be considered as an additional therapeutic option for symptom control since the clinical benefits have been demonstrated and complications are at low rate.Citation9
DBS has become an established treatment for movement disorders including Parkinson’s disease, essential tremor, dys-tonia, and in some psychiatric disorders.Citation10 The first stereotactic surgical treatment with thalamotomy on the centromedian-parafascicular complex for TS was introduced in 1970,Citation11,Citation12 and Vanderwalle et al reported the first case of severe DBS in 1999.Citation13 Since then, many case series have been published,Citation14–Citation16 and also a few randomized clinical trials have been conducted in this area.Citation17–Citation19 While small and uncontrolled studies have demonstrated the positive effects of stimulation on motor symptoms in TS,Citation20–Citation24 its effects on Tourette psychiatric comorbidities remain uncertain and the published results are conflicting.Citation25–Citation27
The precise pathophysiology of TS is unknown, but collective concepts include it among “brain circuits” disorders. A closer look at system dysfunctions suggests an overactivity in the basal ganglia thalamo-cortical (BGTCC) loops that may involve various networks, apparently involving a wide range of parallel loops, from ventral and mesolimbic structures to the sensory-motor dorsolateral segments of the circuit.Citation28–Citation30 Overall, DBS brain targets currently used for the treatment of TS mostly resemble the targets that are earlier used in focal ablative procedures such as the thalamus, pallidum, and ventral striatum/ventral capsule (VS/VC). Targets aim at the control of motor and psychiatric symptoms.Citation35
This article provides evidence on the applicability of DBS in the treatment of therapy-refractory TS, discussing the best candidates for surgery and targets, and provides an overview of the mechanism behind the modulation of neural circuits in TS.
Indication criteria for DBS in TS (who?)
The current clinical indication criteria for DBS in a TS patient are based on clinical diagnosis, with high tic severity scores and the presence of symptoms, despite the use of at least three different pharmacological drugs: 1) an alpha-adrenergic agonist, 2) two dopamine antagonists, and 3) a drug from at least one additional class (eg, tetrabenazine or clonazepam).Citation31 Although some recommendations use age as an exclusion criterion (impeding DBS to subjects below 18 or 25), this should not be an absolutely strict criterion ( and ).Citation15,Citation30 However, it is recommended to consult a local ethics committee when considering surgery for patients younger than 18 years of age.
Figure 1 Diagram showing some of the possible clinical evolution that can be interpreted as natural history of TS or outcome from non-surgical therapeutic interventions based on YGTSS as a severity measure. This diagram intends to illustrate the current indications for DBS according to earlier and latest criteria. (A) Clinical resolution of TS symptoms. (B) Presence of tics that do not resolve spontaneously or are kept stable under non-surgical treatments. (C) Classical indication for DBS based on the severity of disease and age (18 years). (D) Latest proposed indication for DBS based on severity as a determinant factor even before 18 years of age.
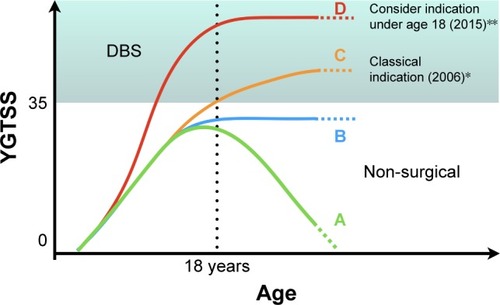
Table 1 Clinical criteria for the indication of DBS in Tourette’s syndrome
Table 2 Summary of the studies: level III evidence
Table 3 Summary of studies: level IV evidence
The exclusion criteria comprise major unstable and non-treated psychiatric disorders, suicidal ideation or psychiatric hospitalization preceding 6 months of surgery, active dependence on alcohol or drugs, and pregnancy and severe cognitive impairments. Importantly, a multidisciplinary specialized DBS team including a neurologist, psychiatrist, neurosurgeon, neuropsychologist, speech therapist, and physiotherapist should make all of these assessments. Other exclusion factors include significant structural lesion or abnormalities on MRI.Citation15 In addition, real expectations of motor outcome and social support are essential when referring patients for DBS.
Mechanisms of action of DBS in TS (how?)
The mechanism of action of DBS in movement disorders has not yet been fully elucidated. There are many theories that intended to explain how DBS interacts with specific brain structures modulating pathological oscillations on basal ganglia and related circuits. DBS is mostly based on focalized high-frequency stimulation (HF-DBS) in targets of basal ganglia and thalamus involved in the mechanism of movement disorders. The effect of HFS is classically described as focal “lesion-like” effect in most subcortical targets and stimulation of fibers, including the ones used for the treatment of TS. However, current concepts suggest that the effect of DBS may be more complex. In 2016, Florence et al published an article hypothesizing that the HF-DBS induces ionic changes focalized in the region surrounding the active tip, reversibly increasing extracellular concentrations of potassium, which in turn affects the dynamics of both cell bodies and axons. This would contribute to the intermittent excitation and inhibition of these elements, reversibly interrupting local abnormal pathological activity and consequently correcting circuit irregularities.Citation32
Regarding TS, when HF-DBS is applied in the anteromedial globus pallidus internus (GPi), it reduces the amplitude of tic-associated phasic changes in the GPi. An animal study reported that the suppression of the brain activity related to tics was linked to a temporal locking of spiking activity with the stimulation pulse, which induces different patterns of inhibition and excitation in affected cells.Citation33 As previously mentioned, dysfunction in the pathways related to the cortico-basal ganglia integrative network has been associated with vocal and motor tics; based on this, several surgical targets have been proposed for the control of motor and psychiatric symptoms.Citation34 Unilateral stimulation was found to be unsuccessful compared to bilateral stimulation in a double-blind study.Citation17
In TS, although acute effects of high frequency stimulation (HFS) in deep structures are observed, the major response after DBS, as observed in idiopathic dystonia, is in general delayed and gradually built-in.Citation35 This suggests that the mechanism of DBS in TS may be mediated by neuroplastic changes in the circuit components. Conversely, although a carry-over effect has been observed, tics recur in most refractory cases after DBS has been turned off, suggesting that the plastic changes are of short or intermediate term. After the DBS, as also observed in dystonia, the improvements following TS DBS are delayed and are gradually progressive.Citation35
The role of dopaminergic modulation
Although different psychopharmacologic agents are used to treat TS, the D2/3 receptor antagonists are among the most effective. Therefore, this suggests that the least the dopamine released in striatal target neurons, the best the symptom control in TS. In order to investigate this hypothesis, Vernalaken et al reported an on/off stimulation experiment using [18F] fallypride-positron emission tomography scan during the steady phase of DBS treatment in a TS patient showing a dramatic increase of endogenous dopamine during off condition. So, bilateral thalamic stimulation somehow induces a decrease in dopamine in striatum.Citation36 Corroborating this hypothesis, a similar study involving three patients also showed that DBS acts by modulating dopamine transmission.Citation37 It is possible that the stimulation of the centromedian nucleus and substantia periventricularis suppresses excitatory feedback projections to motor and limbic circuits of the striatum, thereby decreasing tics and consequently improving behavioral disorders.Citation13 Therefore, the chronic circuit abnormalities present in TS are probably related to the failure of cortical inhibition to the basal ganglia “filter”, which in turn will end-up in thalamic hyperactivity feeding the pathological loop, originating the Tics.
The role of pathological oscillations
The analysis of activity dynamics recorded from depth electrodes suggests that prominent oscillatory brain activity at low frequencies (2–7 Hz) and in alpha band (8–13 Hz), associated with decreased thalamic beta activity, may be an important component in the pathophysiology of TS.Citation38–Citation41 Comparisons of the effect of “on” and “off” stimulation in the dynamics of these frequencies suggested that HF-DBS is able to suppress the abnormal oscillatory activity within the motor cortico-basal ganglia network.Citation38,Citation39,Citation41 Notable increases in normalized gamma-band power activity (25–45 Hz) were also observed, which indicate clinical benefit. Correlation analysis showed that the power of the gamma oscillations was inversely associated with the degree of the TS symptoms, as measured by the Yale Global Tic Severity Scale (YGTSS).Citation42 All of these information are fundamental to the development of advanced treatment strategies such as closed-loop deep brain stimulation, also called adaptive DBS (aDBS).Citation43
The functional brain (cortical) modulatory effects
The pathophysiology of TS is still under investigation, but some studies suggest overactivity in the BGTCC.Citation29,Citation44 A functional study showed that TS patients have a decrease in the fractional anisotropy (FA) in many cortical areas, including the pars opercularis of the left inferior frontal gyrus, the medial frontal gyrus, and the right cingulate gyrus. There was a positive correlation between tic severity and FA scores in the corpus callosum, thalamus, temporal gyrus, and parahippocampal gyrus. Overall, the findings advocated that tics are mostly produced by alterations in prefrontal areas, thalamus, and putamen.Citation30
Regarding the effects of DBS in TS, few functional studies have explored the white matter pathways and the projections activated by stimulation in animal models and patients, and, in general, they support that good motor outcomes are related to the activation of several fiber pathways and brain cortical regions.Citation39,Citation45 The effect of DBS in TS, as in other conditions, seems to be related to local brain changes and also to the modulation of multiple cortical distance areas (through structural and functional connectivities).Citation40
The closed-loop stimulation
Adaptive stimulation from closed-loop devices (aDBS) depends on functional neural feedback through variables recorded by DBS electrodes (such as abnormal electro-graphic discharges or more recently on neurochemical feedback).Citation40,Citation43,Citation46 The term “adaptative stimulation” was created with the concept that some implantable generators are not passive devices any more. Instead of only creating and delivering monotonous trains of electrical pulses, they perform recording and analysis of neural signals and can be programmed to deliver, stop, or change stimulation parameters when a certain neural pattern takes place. Although the studies that correlate recordings of deep brain activity and simultaneous occurrence of symptoms are still in the beginning stage, it seems that rather than rapid activity related to every behavioral event (tics), studies found changes in background activity that correlates with periods of increased tics, which helps to predict when those events will arise. Therefore, detected changes in oscillatory activity can lead to automatic responses from the stimulator intended to suppress tic onset. When used in a more dynamic way, the aDBS can adjust stimulation parameters based on a feedback information, leading to a more individualized treatment.Citation47,Citation48
The main targets (where?)
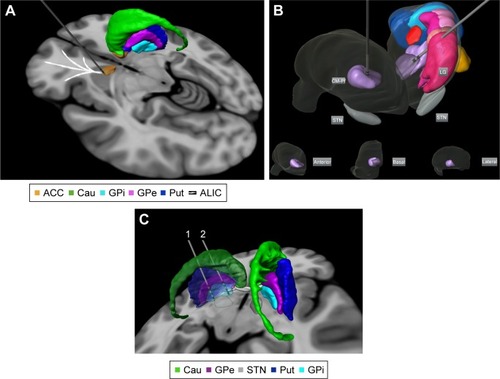
A variety of brain targets have been proposed as potential therapeutic targets for DBS in TS, along the BGTCC circuit. In recent years, the centers of DBS worldwide explored at least nine brain targets for the treatment of TS: centromedian-parafascicular-thalamic complex (CM-Pf), the intersection point between centromedian nucleus, periventricular substance, and inferior ventro-oral nucleus in the thalamus (CM-Spv-Voi), the posterior ventro-oral nucleus, the anterior ventro-oral, and Voi, the GPi anteromedial or posteroventral, the nucleus accumbens (NA), the anterior internal capsule (ALIC), subthalamic nucleus (STN), and globus pallidus externus (GPe) ( and and ).Citation35,Citation49–Citation51 If TS is considered a complex disease between movement and psychiatric disorders (with anxiety and compulsive symptoms), then both sensory-motor and associative/limbic areas may be used as targets. Therefore, of these options, regions of the medial thalamus and the GPi are the most frequently used targets probably because of historical reasons and their involvement in motor and limbic pathways. However, because of the close interconnection of basal ganglia structures, the effects of stimulation would block pathological signals in their local network as well as reduce aberrant signals in other connected structures associated with the mechanism of TS.Citation52–Citation55 Other authors have suggested that combining targets can provide additional benefits.Citation56
Figure 2 Targets proposed for DBS treatment in Tourette’s syndrome: (A) anterior limb of internal capsule/accumbens; (B) bilateral centromedian-parafascicular complex targeted in an anterolateral thalamic view (basal, anterior, and lateral thalamic views of the right thalamus are displayed for localization within the thalamus); and (C) different parts of GPi. Electrode 1 is located in the posteroventrolateral GPi and electrode 2 is located in the anteromedial GPi. The 3D representations are histological postmortem reconstructions of the nuclei from the University of São Paulo – Würzburg Atlas of the Human Brain (Alho et al, 2018Citation96).
Thalamus
Several studies and clinical trials of thalamic DBS indicated that bilateral CM-Pf and Voi stimulation provide a beneficial therapeutic role in TS for both tic severity (motor via CM) and psychiatric symptoms (limbic via Pf).Citation14,Citation57,Citation58 This target was introduced by the ablative surgery of Hassler and Dieckmann in 1970.Citation11 Based on this, Vandewalle et al (1999)Citation13 published the first report of thalamic DBS for a 42-year-old man with refractory TS. They applied high-frequency continuous bilateral stimulation (4 V, 130 Hz, 450 µs). Preoperatively, he had 38 tics per minute; at 4 months, after 12 hours in the off-stimulation condition, only eight tics per minute were counted; all tics subsided 5 minutes after the stimulation was switched on except for some excessive eye-blinking. After 1 year, stimulation of 1.5 V was sufficient to abolish his tics. In long term (5 years), the results of these patients were published in 2003, together with two more cases, and the results showed an average improvement of 72. 2%–90% with no serious complications. Obsessive–compulsive and self-injurious behaviors completely disappeared in all patients.Citation21 Ten years after surgery, patient 1 showed sustained improvement in tic frequency with no change in cognition.Citation59
The first blinded trial on thalamic stimulation for TS was conducted by Maciunas et al with five TS patients in 2007. Three of the five presented with 50% reduction in tics severity after open-label stimulation at 3 months. There was a marked improvement according to all primary (modified Rush Video-Based Rating Scale) and secondary outcome measures (OCD, depression, and anxiety scales).Citation17 Also, this study showed that unilateral stimulation did not appear to be beneficial. Bajwa et al reported a patient who showed improved total tic score and Yale-Brown Obsessive-Compulsive Scale (YBOCS) by a mean of 66%, evaluated 24 months after the surgery.Citation59
In a larger series of study conducted by Servello et al, 15 of 18 patients showed YGTSS improvement between 24% and 79%, with improvement in comorbidities. Stimulation was performed with current between 1 and 5 mA, 100 Hz, and a pulse width of 60 µs.Citation60 This same group of authors later published their long follow-up results of 36 TS patients with different DBS targets. Most of the patients had thalamic DBS, and significant improvements were documented. Servello et al also published their results of a cohort of 48 TS DBS patients. In 40 of them, the thalamus was the target chosen. The target was different than that chosen by Vandewalle et al (1999)Citation117 because it is located 2 mm more anteriorly. The authors stated that this can stimulate the limbic fibers and, consequently, act on the behavioral components of TS. The patients had a mean improvement of 47.5% in YGTSS after DBS and kept at 35% improvement at the final followup. After 2 years of thalamic DBS, Porta et al reported a clinical follow-up of 15 patients, whose YGTSS scores decreased from 76.5 to 36.6. The neuropsychiatric scales also improved.Citation61 The same group of authors published a longer follow-up study (5–6 years) of the same cohort and showed a mean YGTSS improvement of 73% and YBOCS of 42%. However, compared with the results at 2 years, they demonstrated some long-term difficulties.Citation62
Similarly, in a 2-year follow-up study, Rossi et al showed that a 30% improvement in the total YGTSS scores (range 10%–58%) was observed in four CM-Pf DBS cases across the cohort.Citation56 In 2011, Ackermans et al studied six TS patients in a double-blind randomized trial in which chronic stimulation was delivered bilaterally in the CM-Spv-Voi complex (1–6 mA, 130 Hz, 60 µs). The authors reported improvements in the YGTSS scores during the on- vs off-stimulation conditions. The YGTSS improved by 37% and remained after 1-year open-label follow-up, with a 49% improvement reported.Citation18 In 2012, Savica et al described three patients with TS who underwent CM-Pf DBS with an excellent clinical outcome (mean reduction in the YGTSS of 70%) at 1-year follow-up.Citation23 Recently, two other prospective trials presented five and eight intractable TS patients.Citation25,Citation63 The first study indicated that bilateral CM-Pf DBS provided treatment for medically refractory TS with concomitant improvement in depression and anxiety with no neuropsychological morbidity.Citation27 In the second study the patients were treated with DBS of the Voa-Vop, indicating a significant beneficial effect on psychiatric and motor symptoms of TS. In addition, the presence of compulsive behavior, anxiety, and emotional deregulation before surgery appeared to be significant predictors of good outcome after DBS.Citation63
Globus pallidus internus
Posteroventral GPi (pGPi)
The GPi stimulation affects both motor and limbic pathways; however, this specific target has been used for motor symptoms especially for Parkinson’s disease and dystonia. Accordingly, pGPi as a target for DBS has been considered for the treatment of hyperkinetic movements as well as in TS. There are a number of case reports and trials using this target in TS. The first pallidal stimulation in TS was reported by Van der Linden et al.Citation64 The patient underwent both pGPi and thalamic DBS and showed 80% reduction in tics with thalamic stimulation and 95% with pallidal stimulation maintained for 6 months. In 2005, Diederich et al reported progressive improvements in tic frequency reaching 73% within 14-month follow-up after pGPi together with improvement in depressive and anxiety symptoms.Citation65 Dehning et al reported 87% improvement on YGTSS 1 year after bilateral pGPi electrodes in four patients with refractory TS with maintenance of the benefit for 4 years. The authors observed that the patients who improved after DBS had also shown prior response to electroconvulsive therapy.Citation66,Citation67 More recently, pGPi stimulation for the treatment of TS has been performed more frequently with substantial motor tics.Citation24,Citation25,Citation68 The youngest TS patient ever treated by DBS received leads in the pGPi (Shahed et al’s study), who showed 84% improvement on YGTSS after 6 months.Citation27 That patient was followed for 5 years and later reported, with other two patients (followed for 4 and 2 years), to show good results. Over the longitudinal evaluation, stimulation parameters were considered high (mean values 4.9 V, 198 ms, 168 Hz) and rechargeable batteries were eventually used. Transient reduction and gradual retitration of stimulation parameters were sometimes required after the battery exchange. Overall, clinical improvement was maintained over the treatment period. The authors demonstrated that the benefits over symptom could be maintained for up to 5 years.Citation27 There are also other series of cases reported in the literature with positive results.Citation69–Citation72
Anteromedial GPi (aGPi)
The GPi is functionally divided into an anteromedial region that is part of the associative/limbic part of the BGCTCC circuit.Citation73 There are studies that report good outcomes in stimulating the aGPi (the limbic subregion). This involves the limbic loops in tic expression.Citation74,Citation75 More recently, Akbarian-Tefaghi described 15 patients with aGPi DBS for severe TS and explored whether a specific anatomical location within the aGPi correlated with motor outcome for tics, obsessive-ompulsive behavior (OCB), and mood. They demonstrated that the region within the ventral limbic GPi – specifically on the medial medullary lamina in the pallidum at the level of the anterior comissure-posterior comissure line (AC-PC Line) – was significantly associated with improved tics, but not mood or OCB outcome.Citation46 Another recent randomized clinical trial by Welter et al involved 19 patients and showed that aGPi DBS was insufficient to decrease tic severity after 3 months. Future research is warranted to explore the effectiveness of aGPi DBS over longer follow-up and optimal stimulation parameters as well as to study potential predictors of the therapeutic response.Citation76
Comparative studies
Gpi vs thalamus/Gpi and thalamus
In the search for an optimal surgical target, a few studies have compared the outcomes of stimulation in the limbic regions of the GPi and medial thalamus.Citation77,Citation78 A randomized blinded study evaluated the efficacy of stimulating the CM-Pf vs the ventromedial GPi in patients with TS refractory to medical treatment. Bilateral stimulation of the GPi reduced tic severity by 65%, 96%, and 74% in patients 1, 2, and 3, respectively, whereas CM-Pf DBS reduced tic severity by 64%, 30%, and 40%, respectively. The association of thalamic and pallidal stimulation showed no further reduction in tic severity. The tics returned during the sham condition.Citation77
aGpi vs pGpi/aGpi vs pGpi
Martinez-Fernandez et al studied five TS DBS patients – three of them target-implanted in the pGPi and the other two in the aGPi. All patients experienced improvements in tic severity but to variable extents. The YGTSS scores reduced by 29% (before = 77.8, after = 54.2) and the YBOCS reduced by 34% (before = 16.3, after = 10.8) – this effect was sustained until the last follow-up. The authors stated that the anteromedial part of GPi appeared to be a more effective target.Citation75
Other targets
The STN, GPe, ALIC, and NA also referred as VS/VC can act as alternative targets for TS stimulation, and a few reports have been reported on this topic.
STN: A case report was published in 2009 of a patient who had Parkinson’s disease (PD) and TS and who received STN DBS; the patient showed a 97% improvement in both tics and parkinsonian symptoms after stimulation.Citation79
VS/VC: Stimulation of VS/VC has been used as a main target in treatment-resistant OCD; it has also been proposed as a treatment for disorders that are highly associated with psychiatric comorbidities, such as TS. Based on this, a few studies have reported that stimulation of VS/VC target moderately improved motor severity and significantly improved OCD.Citation80–Citation82 However, clinical evidences from these targets rely on case reports and small series since there are no controlled studies yet.
In 2005, a study showed that a TS subject who was treated with ALIC DBS presented with only 23% improvement on the YGTSS.Citation82 For this reason and also due to device problems, the authors opted to change the target to thalamus, which resulted in more satisfactory outcomes with a 46% decrease in the symptoms. In 2007, Kuhn et al described another case of TS/OCD which also improved YGTSS scores.Citation81 Two years later, Neuner et al reported a follow-up of 36 months after VS/VC DBS and documented close to 50% improvement in YGSTS and significant reduction in the YBOCS.Citation83 Burdick et al also shared their experience about an OCD/TS patient who was implanted in the VS/VC target; their study revealed no objective assessment improvement, despite the positive opinion of the patient.Citation84
GPe: Only case reports are available for GPe stimulation in TS, and all of them have shown good outcomes. In 2007, Vilela Filho et al reported GPe DBS for TS with a double-blind assessment design. The authors reported 81% reduction in tic scores and 84% reduction in OCD scores, 23 months after the procedure.Citation85 Later, Piedmonte et al also described a case of GPe stimulation for TS and showed a 70.5% improvement on average in anxiety and motor symptoms.Citation86
Non-motor symptoms effects
Although most studies focus on the effects of motor tic, some also have reported neuropsychological correlates of DBS in TS.Citation26,Citation87–Citation89 Besides the most used targets (GPi and thalamus), DBS in the VS/VC, STN, and GPe have also recorded beneficial effects in OCD components and other psychiatric comorbidities.
In a recent study of 15 severe TS patients with long-term aGPi DBS, Akbarian-Tefaghi et al investigated whether a specific anatomical site within the aGPi correlated with optimal clinical outcome for the measures of tics, OCB, and mood changes. The authors observed that a region within the ventral limbic GPi, specifically on the medial medullary lamina in the pallidum at the level of the AC-PC, was significantly associated with improved tics, but changes in the mood and OCB were less significant.Citation46 Cury et al reported that a 23-year-old TS patient treated with CM-Pf DBS showed very severe scores and high anxiety rate with 70.5% improvement on YGTSS and also a significant improvement in the anxiety scores (53%), with clinical global impression “much improved” (from 1 to 6) after 18 months of follow-up.Citation26
Adverse effects and complications
DBS for TS is overall considered a safe procedure; however, some facts must be pointed out. A recent publication from the prospective International Deep Brain Stimulation Database and Registry presented 185 patients with refractory TS who underwent DBS implantation from January 2012 to December 2016, at 31 institutions in 10 countries worldwide. Thirty-five percent reported a total of 160 adverse events during the first year of follow-up, including dysarthria that was reported 17 times in 10 of 158 patients (6.3%), and paresthesia that was reported 15 times in 13 of 158 patients (8.2%). All of these events were stimulation-induced and transitory without major complications, and no deaths were reported. The infection rate was reported to be 2.5% (4/158), the hemorrhage rate was 1.3% (2/158), and total explant rate at 1 year was 0.6% (1 of 158).Citation31
Hemorrhage was described as a serious surgical complication only in a few cases.Citation90,Citation91 Servello et al in 2011 showed a higher rate of postoperative infections of extracranial cables and generator pockets in TS patients compared with other movement-disorder patients (18% vs 3.7%).Citation92
Other side effects probably stimulation-related effects such as fatigue, apathy, lethargy, and also maniac symptoms have been reported occasionally with several targets.Citation20,Citation21,Citation66,Citation72,Citation83,Citation93 Sedative effects have been reported mainly at high-amplitude stimulation. There are also reports of stimulation-induced changes in sexual behavior.Citation72,Citation94 Duits et al have hypothesized that the surgical procedure or stimulation may have caused an imbalance in the limbic and associative cortico-basal ganglia-thalamocortical loops, thus leading to psychiatric symptoms.Citation93 Recently, in a long-term follow-up of seven TS patients who underwent bilateral DBS (CM-Pf-Voi), the authors showed that a possible imbalance between beneficial and adverse effects at long term can lead to either switching the stimulator off or a proposal for an implant in a different target.Citation95
Conclusion
TS is a relatively rare neurodevelopmental diorder that probably originates due to dysfunction in motor-limbic brain circuitry linking exacerbated anxiety to the triggering of recurring behaviors and tics; however, the precise mechanisms are still largely unknown. Mostly, TS starts in teenagers, improves with conventional treatment, and tends to disappear toward adulthood. Only severe cases, which are uncommon, really need additional treatment. Although DBS is not an approved therapy for TS in most countries, positive evidence from several case and series reports and some comparative studies together suggest that DBS is partially effective in alleviating symptoms in severe and medication-resistant cases of TS. Generally, clinical evidence has been produced by applying chronic bilateral DBS more frequently in the CM-Pf complex but also in the pGPi (motor GPi) or aGPi (limbic GPi) and less frequently in VS/VC and STN targets. This multiplicity of targets in the literature reflects the fact that there is no consensus on which target is the most effective. Also, there are no defined predictors of outcome; however, high scores in tic severity scales may be indirectly related to better response after the DBS.
Future research involving the clinical phenomenology, structural and functional neuroimaging together with data from intraoperative multi unit neuronal and multi target local field potential recordings in TS patients will probably allow better understanding the pathophysiology if this complex disease, guiding interventions such as conventional or adaptative DBS, leading to an individualized treatment. Severe and refractory TS is, in fact, a rare disease. In these circumstances, it is unlikely that large controlled trials will be performed in order to determine the efficacy of each DBS target. It is more likely that data from registry cohorts will provide less-qualified evidence that will lead to a more forgiving and humanitarian approval as it has occurred with OCD in most countries.
Many questions are still left with no specific answers: Is there a best DBS target for TS? Are there specific clinical subsets of TS that would preferentially improve with this or that target? If so, who are the best candidates for each target? Is adaptative DBS better than continuous stimulation?
Disclosure
Rubens G Cury has received honoraria from Medtronic, TEVA, UCB, and Roche for lecturing and scientific board services. Erich Talamoni Fonoff has received honoraria for lecturing and technical assistance, grants, personal fees, and non-financial support from Boston Scientific. The other authors report no conflicts of interest in this work.
References
- JankovicJTourette’s syndromeN Engl J Med20013451184119210.1056/NEJMra01003211642235
- EapenVCavannaAERobertsonMMComorbidities, social impact, and quality of life in Tourette syndromeFront Psychiatry201679710.3389/fpsyt.2016.0009727375503
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders: DSM-55th edWashington, (DC)American Psychiatric Association2013
- LeckmanJFTourette’s syndromeLancet20023601577158612443611
- BlochMHLeckmanJFClinical course of Tourette syndromeJ Psychosom Res20096749750110.1016/j.jpsychores.2009.09.00219913654
- EddyCMRickardsHECavannaAETreatment strategies for tics in Tourette syndromeTher Adv Neurol Disord20114254510.1177/175628561039026121339906
- ShprecherDKurlanRThe management of ticsMov Disord200924152410.1002/mds.2265619170198
- FreemanRDFastDKBurdLKerbeshianJRobertsonMMSandorPAn international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countriesDev Med Child Neurol20004243644710.1017/S001216220000083910972415
- MalatyIAAkbarUUpdates in medical and surgical therapies for Tourette syndromeCurr Neurol Neurosci Rep20141445810.1007/s11910-014-0458-424871966
- FasanoALozanoAMDeep brain stimulation for movement disorders: 2015 and beyondCurr Opin Neurol20152842343610.1097/WCO.000000000000022626110808
- RickardsHWoodCCavannaAEHassler and Dieckmann’s seminal paper on stereotactic thalamotomy for Gilles de la Tourette syndrome: translation and critical reappraisalMov Disord2008231966197210.1002/mds.v23:1418792123
- HasslerRDieckmannGStereotaxic treatment of tics and inarticulate cries or coprolalia considered as motor obsessional phenomena in Gilles de la Tourette’s diseaseRev Neurol (Paris)1970123891004932913
- VandewalleVvan der LindenCGroenewegenHJCaemaertJStereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamusLancet199935372410.1016/S0140-6736(98)09449-5
- Jimenez-ShahedJDesign challenges for stimulation trials of Tourette’s syndromeLancet Neurol20151456356510.1016/S1474-4422(15)00043-525882028
- SchrockLEMinkJWWoodsDWTourette syndrome deep brain stimulation: a review and updated recommendationsMov Disord20153044847110.1002/mds.2609425476818
- MartinezJAEArangoGJFonoffETDeep brain stimulation of the globus pallidus internus or ventralis intermedius nucleus of thalamus for Holmes tremorNeurosurg Rev20153875376310.1007/s10143-015-0636-025990341
- MaciunasRJMadduxBNRileyDEProspective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndromeJ Neurosurg20071071004101410.3171/JNS-07/11/100417977274
- AckermansLDuitsAvan der LindenCDouble-blind clinical trial of thalamic stimulation in patients with Tourette syndromeBrain201113483284410.1093/brain/awr04421354977
- KefalopoulouZZrinzoLJahanshahiMBilateral globus pallidus stimulation for severe Tourette’s syndrome: a double-blind, randomised crossover trialLancet Neurol20151459560510.1016/S1474-4422(15)00008-325882029
- Visser-VandewalleVTemelYBoonPChronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three casesJ Neurosurg2003991094110010.3171/jns.2003.99.6.109414705742
- MassanoJSousaCFoltynieTZrinzoLHarizMVazRSuccessful pallidal deep brain stimulation in 15-year-old with Tourette syndrome: 2-year follow-upJ Neurol20132602417241910.1007/s00415-012-6683-323884714
- SachdevPSMohanACannonEDeep brain stimulation of the antero-medial globus pallidus interna for Tourette syndromePLoS One20149e10492610.1371/journal.pone.010492625136825
- SavicaRSteadMMackKJLeeKHKlassenBTDeep brain stimulation in Tourette syndrome: a description of 3 patients with excellent outcomeMayo Clin Proc201287596210.1016/j.mayocp.2011.08.00522212969
- ZhangJ-GGeYSteadMLong-term outcome of globus pallidus internus deep brain stimulation in patients with Tourette syndromeMayo Clin Proc2014891506151410.1016/j.mayocp.2014.05.01925444487
- SchoenbergMRMadduxBNRileyDEFive-months-postoperative neuropsychological outcome from a pilot prospective randomized clinical trial of thalamic deep brain stimulation for Tourette syndromeNeuromodulation2015189710410.1111/ner.1223325250712
- CuryRGLopezWOCdos Santos GhilardiMGParallel improvement in anxiety and tics after DBS for medically intractable Tourette syndrome: A long-term follow-upClin Neurol Neurosurg2016144333510.1016/j.clineuro.2016.02.03026963088
- ShahedJPoyskyJKenneyCSimpsonRJankovicJGPi deep brain stimulation for Tourette syndrome improves tics and psychiatric comorbiditiesNeurology20076815916010.1212/01.wnl.0000250354.81556.9017210901
- NordstromEJBittnerKCMcGrathMJParksCRBurtonFH“Hyperglutamatergic cortico-striato-thalamo-cortical circuit” breaker drugs alleviate tics in a transgenic circuit model of Tourette’s syndromeBrain Res20151629385310.1016/j.brainres.2015.09.03226453289
- Da CunhaCBoschenSLGómezAAToward sophisticated basal ganglia neuromodulation: review on basal ganglia deep brain stimulationNeurosci Biobehav Rev20155818621010.1016/j.neubiorev.2015.02.00325684727
- Müller-VahlKRGrosskreutzJPrellTKaufmannJBodammerNPeschelTTics are caused by alterations in prefrontal areas, thalamus and putamen, while changes in the cingulate gyrus reflect secondary compensatory mechanismsBMC Neurosci201415610.1186/1471-2202-15-624397347
- Martinez-RamirezDJimenez-ShahedJLeckmanJFEfficacy and safety of deep brain stimulation in Tourette syndrome: the international Tourette syndrome deep brain stimulation public database and registryJAMA Neurol20187535335910.1001/jamaneurol.2018.262829340590
- FlorenceGSameshimaKFonoffETHamaniCDeep brain stimulation: more complex than the inhibition of cells and excitation of fibersNeuroscientist20162233234510.1177/107385841559196426150316
- McCairnKWIrikiAIsodaMDeep brain stimulation reduces Tic-related neural activity via temporal locking with stimulus pulsesJ Neurosci2013336581659310.1523/JNEUROSCI.3846-13.201323575855
- ViswanathanAJimenez-ShahedJBaizabal CarvalloJFJankovicJDeep brain stimulation for Tourette syndrome: target selectionStereotact Funct Neurosurg20129021322410.1159/00033777622699684
- KupschATagliatiMVidailhetMEarly postoperative management of DBS in dystonia: programming, response to stimulation, adverse events, medication changes, evaluations, and troubleshootingMov Disord201126Suppl 1S37S5310.1002/mds.2362421692111
- VernalekenIKuhnJLenartzDBithalamical deep brain stimulation in Tourette syndrome is associated with reduction in dopaminergic transmissionBiol Psychiatry200966e15e1710.1016/j.biopsych.2009.06.02519709645
- KuhnJJanouschekHRaptisMIn vivo evidence of deep brain stimulation-induced dopaminergic modulation in Tourette’s syndromeBiol Psychiatry201271e11e1310.1016/j.biopsych.2011.09.03522129758
- ZauberSEAhnSWorthRMRubchinskyLLOscillatory neural activity of anteromedial globus pallidus internus in Tourette syndromeClin Neurophysiol20141251923192410.1016/j.clinph.2014.01.00324484872
- ShuteJBOkunMSOpriEThalamocortical network activity enables chronic tic detection in humans with Tourette syndromeNeuroimage Clin20161216517210.1016/j.nicl.2016.06.01527419067
- MalingNHashemiyoonRFooteKDOkunMSSanchezJCIncreased thalamic gamma band activity correlates with symptom relief following deep brain stimulation in humans with Tourette’s syndromePLoS One20127e4421510.1371/journal.pone.004421522970181
- BarowENeumannW-JBrückeCDeep brain stimulation suppresses pallidal low frequency activity in patients with phasic dystonic movementsBrain20141373012302410.1093/brain/awu25825212852
- MarcegliaSRosaMServelloDAdaptive Deep Brain Stimulation (aDBS) for Tourette SyndromeBrain Sci20178410.3390/brainsci8010004
- KimJPMinH-KKnightEJCentromedian-parafascicular deep brain stimulation induces differential functional inhibition of the motor, associative, and limbic circuits in large animalsBiol Psychiatry20137491792610.1016/j.biopsych.2013.06.02423993641
- SingerHSMinzerKNeurobiology of Tourette’s syndrome: concepts of neuroanatomic localization and neurochemical abnormalitiesBrain Dev200325Suppl 1S70S8410.1016/S0387-7604(03)90012-X14980376
- HartmannCJLujanJLChaturvediATractography activation patterns in dorsolateral prefrontal cortex suggest better clinical responses in OCD DBSFront Neurosci2015951926834544
- Akbarian-TefaghiLAkramHJohanssonJRefining the deep brain stimulation target within the limbic globus pallidus internus for Tourette syndromeStereotact Funct Neurosurg20179525125810.1159/00047827328787721
- MolinaROkunMSShuteJBReport of a patient undergoing chronic responsive deep brain stimulation for Tourette syndrome: proof of conceptJ Neurosurg20172917
- ChangS-YKimbleCJKimIDevelopment of the Mayo investigational neuromodulation control system: toward a closed-loop electrochemical feedback system for deep brain stimulationJ Neurosurg20131191556156510.3171/2013.8.JNS12214224116724
- AckermansLKuhnJNeunerITemelYVisser-VandewalleVSurgery for Tourette syndromeWorld Neurosurg201380S29.e15S29.e2210.1016/j.wneu.2012.06.017
- PiedadJCPRickardsHECavannaAEWhat patients with gilles de la Tourette syndrome should be treated with deep brain stimulation and what is the best target?Neurosurgery20127117319210.1227/NEU.0b013e3182535a00
- HamaniCFlorenceGHeinsenHSubthalamic nucleus deep brain stimulation: basic concepts and novel perspectiveseNeuro20174:ENEURO014010.1523/ENEURO.0140-17.2017
- MontgomeryEBEffects of GPi stimulation on human thalamic neuronal activityClin Neurophysiol20061172691270210.1016/j.clinph.2006.08.01117029953
- AndersonJSDhattHSFergusonMAFunctional connectivity targeting for deep brain stimulation in essential tremorAJNR Am J Neuroradiol2011321963196810.3174/ajnr.A263821885716
- HashimotoTElderCMOkunMSPatrickSKVitekJLStimulation of the subthalamic nucleus changes the firing pattern of pallidal neuronsJ Neurosci2003231916192310.1523/JNEUROSCI.23-05-01916.200312629196
- HarizMIRobertsonMMGilles de la Tourette syndrome and deep brain stimulationEur J Neurosci2010321128113410.1111/j.1460-9568.2010.07519.x21039952
- RossiPJOpriEShuteJBScheduled, intermittent stimulation of the thalamus reduces tics in Tourette syndromeParkinsonism Relat Disord201629354110.1016/j.parkreldis.2016.05.03327297737
- Ramirez-ZamoraAGiordanoJJGunduzAEvolving applications, technological challenges and future opportunities in neuromodulation: proceedings of the fifth annual deep brain stimulation think TankFront Neurosci20171173410.3389/fnins.2017.0073429416498
- AckermansLDuitsATemelYLong-term outcome of thalamic deep brain stimulation in two patients with Tourette syndromeJ Neurol Neurosurg Psychiatr2010811068107210.1136/jnnp.2009.176859
- BajwaRJde LotbinièreAJKingRADeep brain stimulation in Tourette’s syndromeMov Disord2007221346135010.1002/mds.2123417580320
- ServelloDPortaMSassiMBrambillaARobertsonMMDeep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulationJ Neurol Neurosurg Psychiatry20087913614210.1136/jnnp.2007.12495817846115
- PortaMBrambillaACavannaAEThalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcomeNeurology2009731375138010.1212/WNL.0b013e3181bd809b19858459
- PortaMServelloDZanaboniCDeep brain stimulation for treatment of refractory Tourette syndrome: long-term follow-upActa Neurochir (Wien)20121542029204110.1007/s00701-012-1497-822961243
- HuysDBartschCKoesterPMotor improvement and emotional stabilization in patients with Tourette syndrome after deep brain stimulation of the ventral anterior and ventrolateral motor part of the thalamusBiol Psychiatry20167939240110.1016/j.biopsych.2014.05.01425034948
- Van der LindenCColleHVandewalleVAlessiGRijckaertDDe WaeleLSuccessful treatment of tics with bilateral internal pallidum (GPi) stimulation in a 27-year-old male patient with Gilles de la Tourette’s syndrome (GTS)Mov Disord200217suppl 5S341
- DiederichNJKalteisKStamenkovicMPieriVAleschFEfficient internal pallidal stimulation in Gilles de la Tourette syndrome: a case reportMov Disord2005201496149910.1002/mds.v20:1116037913
- DehningSMehrkensJ-HMüllerNBötzelKTherapy-refractory Tourette syndrome: beneficial outcome with globus pallidus internus deep brain stimulationMov Disord2008231300130210.1002/mds.2193018528896
- DehningSLeitnerBSchennachRFunctional outcome and quality of life in Tourette’s syndrome after deep brain stimulation of the posteroventrolateral globus pallidus internus: long-term follow-upWorld J Biol Psychiatry201415667510.3109/15622975.2013.84900424304122
- SmeetsAYJMDuitsAAPlantingaBRDeep brain stimulation of the internal globus pallidus in refractory Tourette syndromeClin Neurol Neurosurg2016142545910.1016/j.clineuro.2016.01.02026811866
- WelterMLHouetoJLTezenas du MontcelSClinical predictive factors of subthalamic stimulation in Parkinson’s diseaseBrain200212557558311872614
- DongSZhuangPZhangX-HLiJ-YLiY-JUnilateral deep brain stimulation of the right globus pallidus internus in patients with Tourette’s syndrome: two cases with outcomes after 1 year and a brief review of the literatureJ Int Med Res2012402021202810.1177/03000605120400054523206487
- DehningSFeddersenBCeroveckiABötzelKMüllerNMehrkensJ-HGlobus pallidus internus-deep brain stimulation in Tourette’s syndrome: can clinical symptoms predict response?Mov Disord2011262440244110.1002/mds.2389221953770
- MotlaghMGSmithMELanderos-WeisenbergerALessons learned from open-label deep brain stimulation for Tourette syndrome: eight cases over 7 YearsTremor Other Hyperkinet Mov (N Y)20133
- NairGEvansABearREVelakoulisDBittarRGThe anteromedial GPi as a new target for deep brain stimulation in obsessive compulsive disorderJ Clin Neurosci20142181582110.1016/j.jocn.2013.10.00324524950
- CannonESilburnPCoyneTO’MaleyKCrawfordJDSachdevPSDeep brain stimulation of anteromedial globus pallidus interna for severe Tourette’s syndromeAm J Psychiatry201216986086610.1176/appi.ajp.2012.1110158322772329
- Martínez-FernándezRZrinzoLAviles-OlmosIDeep brain stimulation for Gilles de la Tourette syndrome: a case series targeting subregions of the globus pallidus internusMov Disord2011261922193010.1002/mds.2373421538528
- WelterM-LHouetoJ-LThoboisSAnterior pallidal deep brain stimulation for Tourette’s syndrome: a randomised, double-blind, controlled trialLancet Neurol20171661061910.1016/S1474-4422(17)30122-928645853
- WelterM-LMalletLHouetoJ-LInternal pallidal and thalamic stimulation in patients with Tourette syndromeArch Neurol20086595295710.1001/archneur.65.7.95218625864
- AckermansLTemelYCathDDeep brain stimulation in Tourette’s syndrome: two targets?Mov Disord20062170971310.1002/mds.2081616463374
- Martinez-TorresIHarizMIZrinzoLFoltynieTLimousinPImprovement of tics after subthalamic nucleus deep brain stimulationNeurology2009721787178910.1212/WNL.0b013e3181a9fad119451536
- ZabekMSobstylMKoziaraHDzierzeckiSDeep brain stimulation of the right nucleus accumbens in a patient with Tourette syndrome. Case reportNeurol Neurochir Pol20084255455919235110
- KuhnJLenartzDMaiJKDeep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndromeJ Neurol200725496396510.1007/s00415-007-0648-y17410328
- FlahertyAWWilliamsZMAmirnovinRDeep brain stimulation of the anterior internal capsule for the treatment of Tourette syndrome: technical case reportNeurosurgery200557E403 discussion E40316234657
- NeunerIPodollKJanouschekHMichelTMSheldrickAJSchneiderFFrom psychosurgery to neuromodulation: deep brain stimulation for intractable Tourette syndromeWorld J Biol Psychiatry20091036637610.1080/1562297080251331719005877
- BurdickAFooteKDGoodmanWLack of benefit of accumbens/capsular deep brain stimulation in a patient with both tics and obsessive-compulsive disorderNeurocase20101632133010.1080/1355479090356042220178034
- Vilela FilhoORagazzoPSilvaDRibeiroTOliveiraPBilateral globus pallidus externus deep brain stimulation for the treatment of Tourette syndrome: an on-going prospective controlled studyStereotact Funct Neurosurg2007854243
- PiedimonteFAndreaniJCMPiedimonteLBehavioral and motor improvement after deep brain stimulation of the globus pallidus externus in a case of Tourette’s syndromeNeuromodulation2013165558 discussion 5810.1111/j.1525-1403.2012.00526.x23240689
- ServelloDSassiMBrambillaADe novo and rescue DBS leads for refractory Tourette syndrome patients with severe comorbid OCD: a multiple case reportJ Neurol20092561533153910.1007/s00415-009-5123-519437063
- HauseuxP-ACyprienFCifLLong-term follow-up of pallidal deep brain stimulation in teenagers with refractory Tourette syndrome and comorbid psychiatric disorders: about three casesEur J Paediatr Neurol20172121421710.1016/j.ejpn.2016.06.00527436698
- Huisman-van DijkHMvan de SchootRRijkeboerMMMathewsCACathDCThe relationship between tics, OC, ADHD and autism symptoms: A cross-disorder symptom analysis in Gilles de la Tourette syndrome patients and family-membersPsychiatry Res201623713814610.1016/j.psychres.2016.01.05126826899
- IdrisZGhaniARIMarWIntracerebral haematomas after deep brain stimulation surgery in a patient with Tourette syndrome and low factor XIIIA activityJ Clin Neurosci2010171343134410.1016/j.jocn.2010.01.05420620064
- AckermansLTemelYBauerNJCVisser-VandewalleVDutch-Flemish Tourette Surgery Study GroupVertical gaze palsy after thalamic stimulation for Tourette syndrome: case reportNeurosurgery200761E1100 discussion E110018091260
- ServelloDSassiMGaetaMRicciCPortaMTourette syndrome (TS) bears a higher rate of inflammatory complications at the implanted hardware in deep brain stimulation (DBS)Acta Neurochir (Wien)201115362963210.1007/s00701-010-0851-y21052744
- DuitsAAckermansLCathDVisser-VandewalleVUnfavourable outcome of deep brain stimulation in a Tourette patient with severe comorbidityEur Child Adolesc Psychiatry20122152953110.1007/s00787-012-0285-622622600
- Müller-VahlKRCathDCCavannaAEEuropean clinical guidelines for Tourette syndrome and other tic disorders. Part IV: deep brain stimulationEur Child Adolesc Psychiatry20112020921710.1007/s00787-011-0166-421445726
- SmeetsAYJMDuitsAALeentjensAFGThalamic deep brain stimulation for refractory Tourette syndrome: clinical evidence for increasing disbalance of therapeutic effects and side effects at long-term follow-upNeuromodulation20182119720210.1111/ner.1255628102636
- AlhoEJLAlhoATDLGrinbergLHigh thickness histological sections as alternative to study the three-dimensional microscopic human sub-cortical neuroanatomyBrain Struct Funct201822331121113210.1007/s00429-017-1548-229094303
- KaidoTOtsukiTKanekoYTakahashiAOmoriMOkamotoTDeep brain stimulation for Tourette syndrome: a prospective pilot study in JapanNeuromodulation2011142123128 discussion 12910.1111/j.1525-1403.2010.00324.x21992198
- ServelloDZekajESalehCLangeNPortaMDeep Brain Stimulation in Gilles de la Tourette Syndrome: What Does the Future Hold? A Cohort of 48 PatientsNeurosurgery20167819110010.1227/NEU.000000000000100426348012
- HouetoJLKarachiCMalletLTourette’s syndrome and deep brain stimulationJ Neurol Neurosurg Psychiatry200576799299515965209
- GallagherCLGarellPCMontgomeryEBJrHemi tics and deep brain stimulationNeurology2006663E1216476922
- ShieldsDCChengMLFlahertyAWGaleJTEskandarENMicroelectrode-guided deep brain stimulation for Tourette syndrome: within-subject comparison of different stimulation sitesStereotact Funct Neurosurg2008862879118073521
- KuhnJLenartzDHuffWTransient Manic-like Episode Following Bilateral Deep Brain Stimulation of the Nucleus Accumbens and the Internal Capsule in a Patient With Tourette SyndromeNeuromodulation200811212813122151046
- KuhnJGaebelWKlosterkoetterJWoopenCDeep brain stimulation as a new therapeutic approach in therapy-resistant mental disorders: ethical aspects of investigational treatmentEur Arch Psychiatry Clin Neurosci2009259Suppl 2S135S14110.1007/s00406-009-0055-8 Review19876671
- DueckAWoltersAWunschKDeep brain stimulation of globus pallidus internus in a 16-year-old boy with severe tourette syndrome and mental retardationNeuropediatrics200940523924210.1055/s-0030-124751920221961
- FoltynieTMartinez-TorresIZrinzoLImprovement in vocal & motor tics following dbs motor Gpi for Tourette sindrome, not accompanied by subjective improvement in quality of life – Case ReportMov Disord200924S497S498
- MarcegliaSServelloDFoffaniGThalamic single-unit and local field potential activity in Tourette syndromeMov Disord201025330030820108375
- LeeMWYAu-YeungMMHungKNWongCKDeep brain stimulation in a Chinese Tourette’s syndrome patientHong Kong Med J201117214715021471596
- KuhnJBartschCLenartzDHuysDDaumannJWoopenCClinical effectiveness of unilateral deep brain stimulation in Tourette syndromeTransl Psychiatry20111e5222833207
- HwynnNTagliatiMAltermanRLImprovement of both dystonia and tics with 60 Hz pallidal deep brain stimulationInt J Neurosci2012122951952222494180
- HuasenBMcCrearyREvansJPotterGSilverdaleMCervical myelopathy secondary to Tourette’s syndrome managed by urgent deep brain stimulationMov Disord201429445245324395181
- PatelNJimenez-ShahedJSimultaneous improvement of tics and parkinsonism after pallidal DBSParkinsonism Relat Disord20142091022102324957594
- ZekajESalehCPortaMServelloDTemporary deep brain stimulation in Gilles de la Tourette syndrome: A feasible approach?Surg Neurol Int2015612226290773
- TestiniPZhaoCZSteadMDuffyPSKlassenBTLeeKHCentromedian-Parafascicular Complex Deep Brain Stimulation for Tourette Syndrome: A Retrospective StudyMayo Clin Proc201691221822526848003
- DwarakanathSHegdeAKetanJ“I swear, I can’t stop it!” – A case of severe Tourette’s syndrome treated with deep brain stimulation of anteromedial globus pallidus internaNeurol India20176519910228084249
- SmeetsAYJMDuitsAAPlantingaBRDeep Brain Stimulation of the internal globus pallidus in refractory Tourette SyndromeClin Neurol Neurosurg2016142545926811866
- OkunMSFooteKDWuSSA trial of scheduled deep brain stimulation for Tourette syndrome: moving away from continuous deep brain stimulation paradigmsJAMA Neurol2013701859423044532
- VandewalleVvan der LindenCGroenewegenHJCaemaertJStereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamusLancet1999353724
