Abstract
Objective
The aim of the study was to investigate changes of brain neural homogeneity in retinal detachment (RD) patients using the regional homogeneity (ReHo) method to understand their relationships with clinical features.
Materials and methods
A total of 30 patients with RD (16 men and 14 women), and 30 healthy controls (HCs) (16 men and 14 women) closely matched in age and sex were recruited. Resting-state functional magnetic resonance imaging scans were performed for all subjects. The ReHo method was used to investigate the brain regional neural homogeneity. Patients with RD were distinguished from HCs by receiver operating characteristic curve. The relationships between the mean ReHo signal values in many brain regions and clinical features in RD patients were calculated by Pearson correlation analysis.
Results
Compared with HCs, RD patients had significantly decreased ReHo values in the right occipital lobe, right superior temporal gyrus, bilateral cuneus and left middle frontal gyrus. Moreover, we found that the mean ReHo signal of the bilateral cuneus showed positive relationships with the duration of the RD (r=0.392, P=0.032).
Conclusion
The RD patients showed brain neural homogeneity dysfunction in the visual pathway, which may underline the pathological mechanism of RD patients with acute vision loss. Besides, the ReHo values can reflect the progress of the RD disease.
Introduction
Retinal detachment (RD) is a severe eye disease in which the retina separates from the layer underneath. RD is often characterized by floaters, flashes and visual field defects.Citation1 According to a survey in Scotland, the annual incidence of rhegmatogenous RD is 12.05 per 100,000 in Scotland.Citation2 The occurrence of RD is associated with various risks including high myopia,Citation3 ocular traumaCitation4 and vitreous detachment.Citation5 The RD is accompanied by a variety of complications such as proliferative vitreoretinopathy (PVR),Citation6 vitreous hemorrhageCitation7 and even blindness.Citation8 At present, surgery is the main treatment of RD.Citation9
Currently, ocular ultrasound and optical coherence tomography (OCT) are the noninvasive technologies used to detect RD. The ocular ultrasound was effective for diagnosis of RD.Citation10 However, ultrasound can only roughly judge for RD, as it cannot indicate the scope and degree of RD. The OCT can not only make effective diagnoses of RD,Citation11 but it can also provide useful information about the microstructure changes of the retinal layers in RD patients.Citation12 Besides, the OCT can help in surgical planning of RD.Citation13 Moreover, the spectral-domain OCT can reveal the integrity of the intermediate line and changes in the thickness of the outer nuclear layer, which is an important index for the recovery of visual outcomes after RD repair.Citation14 The abovementioned researches only focus on the abnormal ocular changes in RD patients. Visual pathways include the retina through the optic nerve to the visual cortex. However, whether RD leads to abnormalities of other visual systems, including the visual pathways and the visual cortex, remains unknown.
Resting-state functional magnetic resonance imaging (rs-fMRI) is an effective method, which has been applied successfully to evaluate the changes of brain activity. The regional homogeneity (ReHo) method, an rs-fMRI measurement method, is thought to be a reliable and sensitive measurement, which can be used to evaluate coherence of the blood oxygen level-dependent signal among neighboring voxels of the whole brain at rest.Citation15,Citation16 The ReHo method is widely used to investigate the local synchronization of spontaneous fMRI signals. In our previous studies, the ReHo method has been successfully used to assess the neurological conditions in some eye diseases such as optic neuritisCitation17 and comitant strabismus.Citation18
Here, our study is the first to evaluate regional spontaneous neural activity changes in RD patients using the ReHo method. We hypothesized that RD might lead to abnormal visual cortex activity.
Materials and methods
Subjects
A total of 30 patients with RD (16 men and 14 women) were recruited from the Ophthalmology Department of the First Affiliated Hospital of Nanchang University. The inclusion criteria of the study in RD patients were 1) idiopathic RD patients with one or two retinal tear(s); 2) the range of RD being no more than two quadrants; and 3) bilateral eye without any ocular diseases (cataracts, glaucoma, optic neuritis, maculopathy, and so on).
The exclusion criteria of RD in the study were 1) recurrent RD or recurrent after RD repairment operation; 2) RD due to ocular trauma; 3) RD with serious related complications (PVR, vitreous hemorrhage, macular degeneration, and so on); 4) RD patients with a history of laser treatment or surgery; 5) with cardiovascular system diseases such as heart disease and hypertension and 6) with psychiatric disorders and cerebral infarction diseases.
Thirty healthy controls (HCs) (16 men and 14 women) who were age-, sex- and status-matched to subjects in the RD group were also enrolled for this study. All HCs met the following criteria: 1) no ocular disease with uncorrected visual acuity (VA) >1.0; 2) no psychiatric disorders (depression, bipolar disorder); and 3) ability to be scanned with an MRI (eg, no cardiac pacemaker or implanted metal devices).
The protocol of this research was approved by the First Affiliated Hospital of Nanchang University Medical Ethics Committee, and followed the tenets of the Declaration of Helsinki. All subjects provided written informed consent to participate.
MRI parameters
MRI scanning was performed on a 3-Tesla MR scanner (Trio, Siemens, Munich, Germany). The whole-brain T1-weighted images were obtained with spoiled gradient-recalled echo sequence with the parameters: (repetition time =1,900 ms, echo time =2.26 ms, thickness =1.0 mm, gap =0.5 mm, acquisition matrix =256×256, field of view =250×250 mm, flip angle =9°). Functional images with the parameters (repetition time =2,000 ms, echo time =30 ms, thickness =4.0 mm, gap =1.2 mm, acquisition matrix =64×64, flip angle =90°, field of view =220×220 mm, 29 axial) were corrected.
fMRI data processing
The functional images were analyzed as described previously.Citation18 Briefly, the data were filtered by software and preprocessed using Statistical Parametric Mapping SPM8 (The MathWorks, Inc., Natick, MA, USA) and Data Processing Assistant for rs-fMRI DPARSFA (Institute of Psychology, CAS., Beijing, People’s Republic of China) software.Citation19 The first 10 volumes of each subject were discarded because of the signal reaching equilibrium. After head motion was corrected, spatial smoothing was done. The fMRI images were detrended and band-pass-filtered (0.01–0.08 Hz) to reduce the effects of low-frequency drift and physiological high-frequency respiratory and cardiac noise.Citation20 Based on Kendall’s coefficient of concordance (KCC), each voxel in the brain was calculated voxelwise by applying a cluster size of 26 voxels. We did not regress the global signal out according to a previous study.Citation21 Finally, the remaining data were smoothed with a Gaussian kernel of 6×6×6 mm 3 full-width at half-maximum.
ReHo statistical analysis
ReHo computation was performed with REST software (Institute of Psychology, CAS.).Citation16 ReHo analysis was performed to assess the consistency and similarity for each individual by calculating the KCC of the time series of one given voxel with those of its adjacent voxels in a voxelwise analysis based on the assumption that a voxel was temporally similar to those of its neighbors.
Statistical analysis
The cumulative clinical measurements, including the duration of the onset of RD, were analyzed in the study with an independent-sample t-test using SPSS version 16.0 (SPSS Inc, Chicago, IL, USA) (P<0.05 significant differences).
Statistical analysis was performed with a general linear model analysis using the SPM8 toolkit. The two-sample t-tests were used to examine the differences in the ReHo maps between the RD groups and the health controls (P<0.01) for multiple comparisons using Gaussian random field theory (z>2.3, P<0.01, cluster >40 voxels, AlphaSim corrected).
The mean ReHo values in the different brain regions between the two groups were analyzed by the receiver operating characteristic (ROC) curves method. Pearson correlation was used to evaluate the relationship between the mean ReHo values in different brain regions in the RD group and behavioral performances (P<0.05 significant differences).
Results
Demographics and visual measurements
We did not find any significant differences in age (P=0.903) and weight (P=0.816), and there were significant differences in best-corrected VA-right (P<0.001) and best-corrected VA-left (P=0.001) between the two groups. Meanwhile, the mean values of the duration of RD were 24.05±19.61 days ().
Table 1 Demographics and clinical measurements by group
ReHo differences
Compared with HCs, RD patients had significantly decreased ReHo values in the right occipital lobe, right superior temporal gyrus (STG), bilateral cuneus and left middle frontal gyrus (MFG) ( [blue]; ) (z>2.3, P<0.01, cluster >40 voxels, AlphaSim corrected). The mean values of altered ReHo between the two groups are shown in . In the RD group, the mean ReHo signal of the bilateral cuneus showed the positive relationships with the duration of the RD (r=0.392, P=0.032) ().
Figure 1 Spontaneous brain activity in the RDs and HCs. Significant activity differences were observed in the right occipital lobe, right superior temporal gyrus, bilateral cuneus and left middle frontal gyrus for multiple comparisons using Gaussian random field theory (z>2.3, P<0.01, cluster >40 voxels, AlphaSim corrected). (A) and (B) The mean values of altered ReHo values between the RD and HC groups (C).
Figure 2 Correlations between the mean ReHo signal values and behavioral performance.
Abbreviations: ReHo, regional homogeneity; RD, retinal detachment.
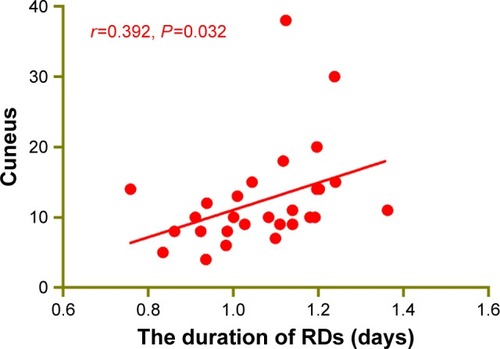
Table 2 Brain areas with significantly different ReHo values between two groups
Receiver operating characteristic curve
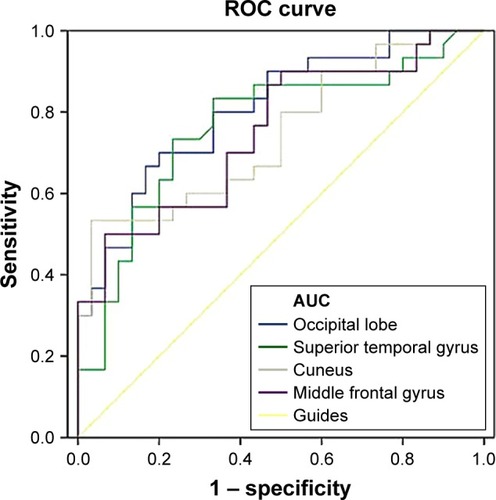
We speculated that the ReHo differences between the two groups might be useful diagnostic markers. Thus, the mean ReHo values in the different brain regions were analyzed by the ROC curves method. The areas under the ROC curve were as follows: the right occipital lobe (0.807); right STG (0.764); bilateral cuneus (0.744) and left MFG (0.750) (RDs < HCs) ().
Figure 3 ROC curve analysis of the mean ReHo values for altered brain regions.
Abbreviations: HCs, healthy controls; RD, retinal detachment; ReHo, regional homogeneity; ROC, receiver operating characteristic.
Discussion
To our knowledge, the study is the first to investigate the regional spontaneous neural activity changes in RD patients using the ReHo method. In our study, RD patients had significantly decreased ReHo values in the right occipital lobe, right STG, bilateral cuneus and left MFG. Moreover, we found that the mean ReHo signal of the bilateral cuneus showed positive relationships with the duration of the RD (r=0.392, P=0.032).
The occipital lobe is the anatomical region of the visual cortex, which plays a critical role in visual processing. The primary visual cortex is an important part of the occipital lobe, which receives the visual signals from the lateral geniculate body through visual radiation.Citation22 The normal function of the retinal ganglion cells plays a critical role in visual stimulation. The pathological mechanism of RD is when the retinal neuroepithelium separates from the pigment epithelium.Citation23 The RD may lead to impaired visual evoked potentials.Citation24 Besides, the RD patients were associated with visual loss.Citation25 In our study, we found that the RD patients had significantly decreased ReHo values in the right occipital lobe, which reflected the dysfunction of the local synchronization of brain activities in the brain region. Therefore, we speculated that RD not only leads to abnormal function of the retina, but also affects the function of the visual cortex.
The cuneus is a part of the occipital lobe, which plays an important role in visual processing. The anteromedial cuneus is needed to interact with the primary visual cortex V1 to encode visual information to the extrastriate cortices.Citation26 Meanwhile, the cuneus is part of the visual pathway, which is involved in spatial location.Citation27 Moreover, the dysfunction of the cuneus is seen in many diseases, such as trigeminal neuralgiaCitation28 and panic disorder.Citation29 In our study, we found that the RD patients showed significantly decreased ReHo values in the bilateral cuneus, which indicated the dysfunction of the local synchronization of brain activities. Moreover, the ReHo signal of the bilateral cuneus showed positive relationships with the duration of the RD (r=0.392, P=0.032). Thus, we speculated that the RD patients were associated with impaired brain activities in the cuneus. Besides, the degree of impaired cuneus may reflect the progress of the RD disease.
The STG is located in the temporal lobe, which plays an important role in auditory processingCitation30 and auditory memory.Citation31 The STG is also involved in visual search insightsCitation32 and visual information processing.Citation33 In our study, we found that the RD patients showed decreased ReHo values in the right STG, which reflects the impaired synchronization of brain activities in the STG. We speculated that RD might lead to the dysfunction of auditory and visual information processing.
The MFG is a part of frontal gyrus, which is involved in the contingency awarenessCitation34 and cognition.Citation35 Besides, the MFG is associated with executive attention.Citation36 In our study, we found that the RD patients had significantly decreased ReHo values in the left MFG, which might indicate that RD leads to the dysfunction of cognitive activities.
Conclusion
In summary, our results showed that the RD patients had brain neural homogeneity dysfunction in the visual pathway, which may underline pathological mechanisms of the RD patients with acute vision loss. Besides, the ReHo values can reflect the progress of the RD disease.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No: 81660158, 81160118, 81460092, 81400372); Jiangxi Province Voyage Project (No: 2014022); Natural Science Key Project of Jiangxi Province (No: 20161ACB21,017); Youth Science Foundation of Jiangxi Province (No: 20151BAB215016); Technology and Science Foundation of Jiangxi Province (No: 20151BBG70223); Health Development Planning Commission Science Foundation of Jiangxi Province (No: 20175115, 20175116).
Disclosure
The authors report no conflicts of interest in this work.
References
- LumiXHawlinaMGlavačDAging of the vitreous: from acute onset floaters and flashes to retinal detachmentAging Res Rev2015217177
- MitryDCharterisDGYorstonDThe epidemiology and socioeconomic associations of retinal detachment in Scotland: a two-year prospective population-based studyInvest Ophthalmol Vis Sci201051104963496820554615
- BabaTOhno-MatsuiKFutagamiSPrevalence and characteristics of foveal retinal detachment without macular hole in high myopiaAm J Ophthalmol2003135333834212614751
- LeeTHChenYHKuoHKRetinal detachment associated with basketball-related eye traumaAm J Ophthalmol20171809710128600149
- WilliamsKMWattLWilliamsonTHAcute symptomatic posterior vitreous detachment and delayed retinal breaksActa Ophthalmol2011891e100e10120662799
- JoeresSKirchhofBJoussenAMPVR as a complication of rhegmatogeneous retinal detachment: a solved problem?Br J Ophthalmol200690679679716714271
- SarrafizadehRHassanTSRubyAJIncidence of retinal detachment and visual outcome in eyes presenting with posterior vitreous separation and dense fundus-obscuring vitreous hemorrhageOphthalmology2001108122273227811733270
- ChenKHChenLRBilateral retinal detachment with subsequent blindness in a pregnant woman with severe pre-eclampsiaTaiwan J Obstet Gynecol201352114214423548240
- MannersSNgJQKemp-CaseyAChowKKangCYPreenDBRetinal detachment surgery in Western Australia (2000–2013): a whole-population studyBr J Ophthalmol Epub201748
- YoonessiRHussainAJangTBBedside ocular ultrasound for the detection of retinal detachment in the emergency departmentAcad Emerg Med201017991391720836770
- AkibaJKonnoSSatoEYoshidaARetinal detachment and retinoschisis detected by optical coherence tomography in a myopic eye with a macular holeOphthalmic Surg Lasers200031324024210847504
- KimJHParkDYHaHSKangSWTopographic changes of retinal layers after resolution of acute retinal detachmentInvest Ophthalmol Vis Sci201253117316732123033395
- AugerGWinderSSpectral domain OCT: an aid to diagnosis and surgical planning of retinal detachmentsJ Ophthalmol2011201172536222254129
- GharbiyaMGrandinettiFScavellaVCorrelation between spectral-domain optical coherence tomography findings and visual outcome after primary rhegmatogenous retinal detachment repairRetina2012321435321778929
- ZangYJiangTLuYHeYTianLRegional homogeneity approach to fMRI data analysisNeuroimage200422139440015110032
- TononiGMcIntoshARRussellDPEdelmanGMFunctional clustering: identifying strongly interactive brain regions in neuroimaging dataNeuroimage1998721331499558645
- ShaoYCaiFQZhongYLAltered intrinsic regional spontaneous brain activity in patients with optic neuritis: a resting-state functional magnetic resonance imaging studyNeuropsychiatr Dis Treat2015113065307326715848
- HuangXLiSHZhouFQAltered intrinsic regional brain spontaneous activity in patients with comitant strabismus: a resting-state functional MRI studyNeuropsychiatr Dis Treat2016121303130827350747
- Chao-GanYYu-FengZDPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRIFront Syst Neurosci201041320577591
- LoweMJMockBJSorensonJAFunctional connectivity in single and multislice echoplanar imaging using resting-state fluctuationsNeuroimage1998721191329558644
- MurphyKBirnRMHandwerkerDAJonesTBBandettiniPAThe impact of global signal regression on resting state correlations: are anti-correlated networks introduced?Neuroimage200944389390518976716
- ChenWZhuXHCorrelation of activation sizes between lateral geniculate nucleus and primary visual cortex in humansMagn Reson Med200145220220511180426
- SchubertHDStructural organization of choroidal colobomas of young and adult patients and mechanism of retinal detachmentTrans Am Ophthalmol Soc200510345747217057813
- OzgürSEsginHMacular function of successfully repaired macula-off retinal detachmentsRetina200727335836417460592
- Di LauroSCastrejónMFernándezILoss of visual acuity after successful surgery for macula-on rhegmatogenous retinal detachment in a prospective multicentre studyJ Ophthalmol2015201582186426640704
- VanniSTanskanenTSeppäMUutelaKHariRCoinciding early activation of the human primary visual cortex and anteromedial cuneusProc Natl Acad Sci U S A20019852776278011226316
- RaoHZhouTZhuoYFanSChenLSpatiotemporal activation of the two visual pathways in form discrimination and spatial location: a brain mapping studyHum Brain Mapp2003182798912518288
- PariseMKuboTTDoringTMTukamotoGVincentMGasparettoELCuneus and fusiform cortices thickness is reduced in trigeminal neuralgiaJ Headache Pain2014151724661349
- LaiCHWuYTDecreased regional homogeneity in lingual gyrus, increased regional homogeneity in cuneus and correlations with panic symptom severity of first-episode, medication-naïve and late-onset panic disorder patientsPsychiatry Res2013211212713123352831
- HowardMAVolkovIOMirskyRAuditory cortex on the human posterior superior temporal gyrusJ Comp Neurol20004161799210578103
- Muñoz-LópezMInsaustiRMohedano-MorianoAMishkinMSaundersRCAnatomical pathways for auditory memory II: information from rostral superior temporal gyrus to dorsolateral temporal pole and medial temporal cortexFront Neurosci2015915826041980
- GharabaghiAFruhmann BergerMTatagibaMKarnathHOThe role of the right superior temporal gyrus in visual search-insights from intraoperative electrical stimulationNeuropsychologia200644122578258116750545
- BeauchampMSLeeKEArgallBDMartinAIntegration of auditory and visual information about objects in superior temporal sulcusNeuron200441580982315003179
- CarterRMO’DohertyJPSeymourBKochCDolanRJContingency awareness in human aversive conditioning involves the middle frontal gyrusNeuroimage20062931007101216246595
- AchironAChapmanJTalSBercovichEGilHAchironASuperior temporal gyrus thickness correlates with cognitive performance in multiple sclerosisBrain Struct Funct2013218494395022790785
- AnderssonMYstadMLundervoldALundervoldAJCorrelations between measures of executive attention and cortical thickness of left posterior middle frontal gyrus – a dichotic listening studyBehav Brain Funct200954119796388
