Abstract
Objectives
Mitochondrial disorders (MIDs) frequently present as mitochondrial multiorgan disorder syndrome (MIMODS) at onset or evolve into MIMODS during the course. This study aimed to find which organs and/or tissues are most frequently affected by MIMODS, which are the most frequent abnormalities within an affected organ, whether there are typical MIMODS patterns, and to generate an MIMODS score to assess the diagnostic probability for an MID.
Methods
This is a retrospective evaluation of clinical, biochemical, and genetic investigations of adult patients with definite MIDs. A total of 36 definite MID patients, 19 men and 17 women, aged 29–82 years were included in this study. The diagnosis was based on genetic testing (n=21), on biochemical investigations (n=17), or on both (n=2).
Results
The number of organs most frequently affected was 4 ranging from 1 to 9. MIMODS was diagnosed in 97% of patients. The organs most frequently affected were the muscle (97%), central nervous system (CNS; 72%), endocrine glands (69%), heart (58%), intestines (55%), and peripheral nerves (50%). The most frequent CNS abnormalities were leukoencephalopathy, prolonged visually evoked potentials, and atrophy. The most frequent endocrine abnormalities included thyroid dysfunction, short stature, and diabetes. The most frequent cardiac abnormalities included arrhythmias, systolic dysfunction, and hypertrophic cardiomyopathy. The most frequent MIMODS patterns were encephalomyopathy, encephalo-myo-endocrinopathy, and encepalo-myo-endocrino-cardiopathy. The mean ± 2SD MIMODS score was 35.97±27.6 (range =11–71). An MIMODS score >10 was regarded as indicative of an MID.
Conclusion
Adult MIDs manifest as MIMODS in the vast majority of the cases. The organs most frequently affected in MIMODS are muscles, CNS, endocrine glands, and heart. An MIMODS score >10 suggests an MID.
Introduction
Mitochondrial disorders (MIDs) frequently present as multiorgan disorder syndrome (MODS) already at the onset of the disease or evolve into a mitochondrial multiorgan disorder syndrome (MIMODS) during the disease course.Citation1,Citation2 The term “multi-organ disorder syndrome” should not be mixed up with multiorgan dysfunction syndrome, which is used for acute multiorgan failure during severe system disease (eg, cardiogenic shock).Citation3 MODS may occur not only in MIDs but also in disorders other than MIDs, such as myotonic dystrophy, Fabry disease, and glycogen storage disease. In general, all tissues may be affected in an MID, but those with the highest demand of oxygen or with the highest energy requirements are most frequently involved. These organs include the skeletal muscle, the peripheral nerves, the central nervous system (CNS), endocrine organs, the eyes, the ears, the heart, the arteries, the gastrointestinal tract, the kidneys, the bone marrow, the cartilage,Citation4,Citation5 and the skin.Citation1 This retrospective study was carried out to assess which organs/tissues are the most frequently affected in MIMODS, which are the most frequent manifestations within an organ, and whether there are typical patterns of organ involvement suggesting an MID and to generate a score, for estimating the probability of a phenotype to represent an MID.
Methods
All patients with a definite MID diagnosed between 1992 and 2014 were retrospectively investigated. A “definite MID” was diagnosed if biochemical investigations (the determination of the respiratory chain complex activity in the muscle homogenate) revealed a single or multiple respiratory chain complex defect(s) or if a mutation causative for the phenotype could be identified (by Southern analysis, PCR, restriction fragment length polymorphism, polyacrylamide gel electrophoresis, or long PCR in lymphocytes or muscle). From each of the included patients, all available records were assessed for diseases in any of the frequently affected organs in MIDs. As a generally accepted definition of MIMODS is not available, MIMODS was diagnosed in case more than one tissue was affected. All previous and current information about history, clinical examination, and instrumental investigations available was relevant for assessing how many organs were affected in a patient. Within an organ, it was counted how many abnormalities per organ were present. Patients who are currently alive as well as those already deceased were considered.
Patients with equivocal results on biochemical investigations or in case of uncertainty about the pathogenicity of a mutation such as mtDNA polymorphisms were excluded. Also patients with a probable and possible MID were excluded. Probable MIDs were defined as those without a biochemical or genetic abnormality but with abnormal histochemical findings on muscle biopsy (cytochrome oxidase–negative fibers, ragged red fibers, nicotinamide adenine dinucleotide–negative fibers, and succinate dehydrogenase hyperreactive fibers), increased lipid droplets or glycogen storage, abnormally shaped or structured mitochondria, paracrystalline inclusions, abnormal cycle lactate stress test, elevated resting lactate, or the presence of a lactate peak on cerebral magnetic resonance spectroscopy (MRS). Although MID patients frequently present with dyslipidemia or hyperuricemia, they were not regarded as MID manifestations because of the potential nutritional influence. Hyperhidrosis was regarded as a manifestation of the autonomic nervous system (ANS), since this etiology is the most frequent among MIDs, but hyperhidrosis may be due to other causes as well. Abnormal visually evoked potentials (VEPs) were regarded as indicative of a CNS lesion after the exclusion of ophthalmologic abnormalities. Although arterial hypertension can be frequently found in MIDs, it was not assessed as a manifestation of an MID since arterial hypertension is frequently multicausal. Atherosclerosis was regarded as MID manifestation only if classical cardiovascular risk factors (ie, diabetes, arterial hypertension, hyperlipidemia, and smoking) were absent. Dysphagia was regarded as a gastrointestinal MID manifestation only if there were no depression and no cerebral abnormality. The present study did not assess differences of organ involvement between the onset of the MID and the last follow-up, and it was conducted in accordance with the Declaration of Helsinki. Verbal informed consent was obtained from all included patients. The research was approved by the local institutional review board of the NKH Rosenhügel.
Results
In total, 36 adult, Caucasian patients with a definite MID, 19 men and 17 women, aged 29–82 years, were included (). Meanwhile, 7 patients had died. In 21 of the included patients, the diagnosis was based on genetic testing, in 17 patients on biochemical investigations of the muscle homogenate, and in 2 patients on both (). Three patients carried a single mtDNA deletion, 15 patients carried a single mtDNA point mutation, and 2 patients carried a point mutation in more than one mtDNA gene. In a single patient, a depletion syndrome was diagnosed. Among those with a biochemical defect, 9 patients had a single complex defect and 8 had combined complex defects (). The most frequent single-complex defect concerned C1 (n=6) followed by C4 (n=3). The most frequent combined complex defect concerned C1-4 (n=4). In 2 patients, biochemical investigations and genetic investigations had been carried out.
Table 1 Characterization of the 36 adult MID patients included in this study
In one patient, only a single organ (muscle) was affected at the time of the last available investigation. In one patient, 2 organs were affected, in 4 patients 3 organs, in 10 patients 4 organs, in 4 patients 5 organs, in 3 patients 6 organs, in 8 patients 7 organs, in 3 patients 8 organs, and in 2 patients 9 organs (). None of the patients had involvement of >9 organs. The number of organs most frequently affected in MIMODS was 4. In total, 35 patients with MIMODS (97%) were identified. The organs most frequently affected in patients with MIMODS were the skeletal muscle (n=35), the CNS (n=27), the endocrine glands (n=26), the heart (n=21), the intestines (n=20), and the peripheral nerves (n=19; ). The ears (n=11), the bone marrow (n=11), the eyes (n=8), the kidneys (n=7), the skin (n=6), and the bones (n=4) were rarely affected organs. The only organ not affected in this cohort was the vasculature and the lungs. MIMODS was as frequent in women as in men ().
Figure 1 Frequency of organ involvement and gender difference among 36 patients with definite MID.
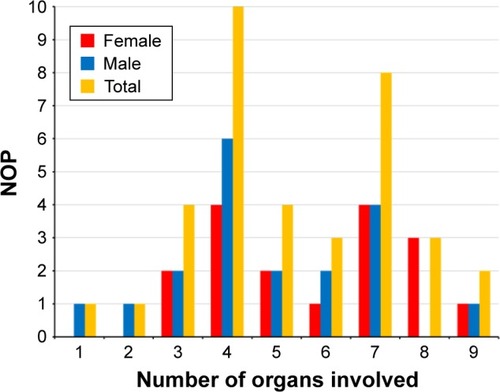
Table 2 Abnormalities (n=63) in organs frequently affected by an MID in the present investigation and the literature
Among the 35 patients with myopathy, 20 patients had weakness of the limb muscles, 19 had muscle wasting, 14 had myalgias, 13 had exercise intolerance, 10 had muscle cramps, 9 had ptosis, 8 had double vision, 6 had chronic progressive external ophthalmoplegia (CPEO), 6 had sore muscles, 5 had fasciculations, 2 had muscle stiffness, and 2 patients had a history of a muscle rupture. In one patient, respiratory muscles were involved. In 34 patients, creatine kinase (CK) was determined and elevated in 20 patients. In a single patient, myoglobinuria was detected. The lactate stress test on a cycle ergometer was carried out in 26 patients and was abnormal (indicative of an MID) in 15 patients. The electromyography (EMG) was carried out in 35 patients and myogenic in 13, neurogenic in 7, and normal in 15 patients. Among the 27 patients with CNS involvement, 12 had leukoencephalopathy, 7 had abnormal VEPs, 7 had focal or diffuse atrophy, 6 had tremor, 6 had cognitive impairment, 5 had spasticity, 4 had epilepsy, 4 had ataxia or dysmetria, 2 had migraine, 2 had dysarthria, 1 had basal ganglia calcification, 1 had an arachnoid cyst, and 1 had restless leg syndrome (RLS; ). None of the patients underwent MRS or reported a stroke-like episode. Among the 26 patients with endocrine abnormalities, 20 had thyroid dysfunction, 12 had short stature, 9 had diabetes, 7 had osteoporosis, 5 had hypogonadism, 2 had parathyroid dysfunction, 2 had prostate hypertrophy, and 1 each had ovarian cysts, endometriosis, and gynecomastia, respectively. Among the 21 patients with cardiac involvement, 14 had arrhythmias, 8 had systolic dysfunction or heart failure, 5 had hypertrophic cardiomyopathy, 2 had dilative cardiomyopathy, 4 had valve abnormalities, 4 had noncompaction (left ventricular hypertrabeculation [LVHT]), and 1 each had pericarditis and abnormal myocardial texture, respectively (). Five were supplied with a pacemaker, but none with an implantable cardioverter defibrillator. Among the 20 patients with gastrointestinal abnormalities, 9 had hepatopathy with only elevated liver enzymes, 5 had diarrhea, 3 had diverticulosis, 3 had steatosis hepatis, 2 had pancreatitis, and 1 each had hyperemesis, hepatomegaly, nonspecific colitis, and liver cysts, respectively (). Among the 19 patients with peripheral nervous system involvement (ie, myopathy, neuropathy, or neuronopathy), 18 had neuropathy with motor, sensory, or ANS involvement and 1 had neuronopathy. The ANS was affected in 4 patients manifesting as hyperhidrosis.
Among the 11 patients with otologic involvement, 11 had hypoacusis or anacusis and 4 had tinnitus. Among the 10 patients with hematological abnormalities, 10 had anemia, 2 had thrombocytopenia, and 1 had leucopenia. Affection of the bones/cartilage resulted in polyarthralgia (n=2), arthrosis (n=2), facial dysmorphism (n=1), and hypertelorism (n=1). Among the 9 patients with ophthalmologic involvement, 2 had cataract, 3 had glaucoma, 2 had pigmentary retinopathy, and 2 had optic atrophy. Among the 7 patients with renal involvement, 5 had renal insufficiency, 2 had nephrolithiasis, and 1 had renal cysts. Seven patients had dermal involvement manifesting as lipomatosis (n=2), psoriasis (n=1), basal cell carcinoma (n=1), seborrheic eczema (n=1), and atopic dermatitis (n=1). The number of abnormalities in all the affected organs per patient ranged from 1 to 21.
Myopathy manifested with 15 of 15 different abnormalities, CNS involvement with 11 of 18 abnormalities, endocrine involvement with 7 of 10 abnormalities, cardiac involvement with 4 of 9 abnormalities, gastrointestinal involvement with 7 of 8 abnormalities, ophthalmologic involvement with 4 of 4 abnormalities, otologic involvement with 2 of 2 abnormalities, renal involvement with 3 of 4 abnormalities, hematologic involvement with 3 of 3 abnormalities, bone/cartilage involvement with 4 of 4, and dermal involvement with 4 of 10 abnormalities. In none of the patients could mitochondrial vasculopathy be unequivocally attributed to MID. Among the 10 patients with a neoplasm (28%), 6 had developed a benign neoplasm and 4 had a malignant neoplasm.
No typical pattern of organ involvement could be extracted from this study, but most of the patients presented with myopathy, CNS disease, endocrine abnormalities, and cardiac dysfunction. As 35 patients had myopathy and 27 patients had CNS involvement, encephalo-myopathy was the most frequent pattern of organ involvement. However, more frequently than 2 organs, 3 or 4 organs were simultaneously involved resulting encephalo-myo-endocrinopathy or encephalo-myo-endocrino-cardiomyopathy.
The MIMODS score was calculated as the sum of 3 figures: 1) the number of organs affected (maximal number =13); 2) the number of abnormalities within an organ (maximal number =94 [2–18 per organ]) (); and 3) the number of hits a PubMed search for each single-organ involvement by means of the search terms “specific abnormality,” “mitochondrial,” “human,” and “mutation” generated. If the number of hits exceeded 100, 3 points were additionally assigned for an abnormality, 2 points in case of 51–100 hits, and 1 point in case of 1–50 hits. The mean ± SD of the MIMODS score calculated from 35 patients (one patient did not have MIMODS) was 35.97±27.6 ranging from 11 to 71. An MIMODS score >10 was thus regarded as indicative of an MID. If MIMODS was defined as >2 organ involvement, a score >14 was indicative of MID.
Discussion
This study shows that MIMODS is frequent among non-selected patients with a definite MID. The most frequently involved organs in MIMODS are the muscle, CNS, endocrine glands, heart, and the gastrointestinal tract. Limb weakness, encephalopathy, thyroid dysfunction, and arrhythmias were the most frequent abnormalities of myopathic, CNS, endocrine, and cardiac involvement. The most frequent pattern of organ involvement in MIMODS was encephalo-myopathy, encephalo-myo-endocrinopathy, and encephalo-myo-endocrino-cardiomyopathy. An MIMODS score >10 suggests an MID.
The multiorgan nature of most MIDs is well established in the literature and supported by several studies ( and ).Citation6 Whether MIMODS is more frequent in specific MIDs than in nonspecific MIDs is unknown. Among the specific MIDs, MIMODS has been particularly reported in Pearson syndrome,Citation7–Citation10 Kearns–Sayre syndrome (KSS), CPEO, sensory ataxic neuropathy with dysarthria and ophthalmoparesis, and mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS).Citation11–Citation14 However, MIMODS is also a frequent manifestation of nonspecific MIDs.Citation15–Citation21 The number of organs affected in MIMODS is assumed to depend on the disease duration, as MIDs are progressive disorders in the majority of cases with deterioration and affection of an increasing number of organs over time. Nearly all patients with single-organ involvement at the onset of the disease develop MIMODS over years. Often, this may occur not earlier than after several years of clinical stability.
Table 3 Abnormalities (n=31) in organs more rarely affected by an MID in the present investigation and the literature
According to the literature, any organ may be affected in an MID.Citation6 However, the most frequently affected organs are the muscle, the CNS, endocrine glands, and the heart, which is in line with the findings of the present study.Citation6 Frequently affected are also the eyes and ears, which was not confirmed by the presently investigated cohort. This is most likely due to the development of ophthalmologic and otologic abnormalities only later in the disease course. More rarely involved are arteries, intestines, kidneys, the hematological system, lungs, bones, cartilage, and skin. and list the organ abnormalities described in MIDs. Among the various abnormalities found in organs of MID patients, some are more frequent than others ( and ). In MID patients with myopathy, the most frequently described abnormalities include CPEO, ptosis, and exercise intolerance (>100 citations). More rarely described are limb weakness, muscle wasting, and hyper-CK-emia (51–100 citations; ). Among CNS abnormalities, the most frequent are epilepsy, cerebral atrophy, ataxia, Parkinson syndrome, and dementia (>100 citations). Stroke-like episodes and migraine occur more rarely (51–100 citations). Among the abnormalities of the endocrine glands, the most frequent is by far diabetes (>100 citations). The second most frequent is short stature (51–100 citations; ). Among the cardiac abnormalities, the most frequent is cardiomyopathy (>100 citations). Heart failure/systolic dysfunction, arrhythmias, and arterial hypertension occur more rarely (51–100 citations). Among the gastrointestinal abnormalities, the most frequent is hepatopathy (elevated liver enzymes; >100 citations). Affection of the peripheral nerves manifests almost exclusively as neuropathy (). The most frequent ophthalmologic abnormalities include optic atrophy and pigmentary retinopathy (). Among the otologic abnormalities, hypoacusis is by far the most frequent. Renal insufficiency and anemia are the most frequent abnormalities among the nephrological and hematological abnormalities. Thus the combination of the key features ptosis, ophthalmoparesis, easy fatigability, epilepsy, cerebral atrophy, ataxia, Parkinson syndrome, dementia, diabetes, cardiomyopathy, hepatopathy, neuropathy, optic atrophy, retinopathy, and hypoacusis (all >100 citations) is highly suggestive of an MID.
In addition to clinical features, abnormalities detectable on routine instrumental investigations were included. For example, myopathy may manifest not only with classical clinical manifestations but also with instrumental abnormalities, particularly hyper-CK-emia and abnormal cycle lactate stress test. More rarely than in muscular dystrophies, the EMG may be myogenic. Myopathy may also manifest exclusively with reduced tendon reflexes. Hyponatremia can be occasionally observed in MIDs without being attributed to drugs, diet, emesis, sweating, or renal insufficiency. In these cases, Addison’s disease or Fanconi syndrome (low molecular weight proteins, glucose, amino acid, uric acid, phosphate, and bicarbonate not reabsorbed in proximal tubules resulting in proteinuria, glucosuria, aminoaciduria, hypophosphatemia, and metabolic acidosis)Citation22 should be considered. Renal tubular acidosis with or without Fanconi syndrome may be a rare manifestation of an MID.Citation23,Citation24 If the ANS is additionally affected in neuropathy, it may manifest as dry skin, hypohidrosis, or hyperhidrosis. In addition, there may be oversensitivity to light, sicca syndrome (xerostomia), decreased heart rate variability, orthostasis or syncope, gustatory sweating, obstipation, overactive bladder (detrusor overactivity), or impotence.
Diseases of the arteries such as atherosclerosis, dissection, ectasia, aneurysm formation, or spontaneous rupture are not well appreciated as a manifestation of an MID.Citation25–Citation29 The number of patients with such vascular abnormalities is limited to a few cases (). Nevertheless, there is an increasing evidence that these abnormalities can be clearly related to MID in the few available reports. Although hematological disease is increasingly recognized as a manifestation of MIDs, it is still underdiagnosed and underreported. Bone marrow involvement includes anemia, thrombocytopenia, and leukopenia. Among the sepcific MIDs, such hematological abnormalities occur in Pearson syndrome, KSS, Barth syndrome, mitochondrial myopathy, lactic acidosis and sideroblastic anemia, X-linked sideroblastic anemia, Leigh syndrome, Leber’s hereditary optic neuropathy, MELAS, and thiamine-responsive megaloblastic anemia.Citation30 However, hematological abnormalities occur more frequently in non-specific MIDs than in specific MIDs.Citation30 Whether eosinophilia, as found in one of the patients of the present study, should be attributed to MIDs remains speculative and requires further investigations. Dermatological involvement is also hardly recognized as mitochondrial, but there is growing evidence that MIDs also manifest in the skin or its appendages.Citation31 Dermatological involvement in MIDs includes psoriasis, vitiligo, baldness, alopecia, madarosis, hypertrichosis, skin rashes, seborrheic eczema, atopic dermatitis, or edema. Not all these aspects were found in the present study but reported in the literature (). Congenital heart disease as a manifestation of MIDs is also rare but has been described in single patients ().Citation32 Diverticulosis is also hardly recognized as a manifestation of MIDs but is prevalent particular among nonspecific MIDs, without being frequently reported. Ischemic stroke should not be regarded as a primary manifestation of MIDs but rather as secondary in case classical risk factors for atherosclerosis, such as mitochondrial diabetes, hyperlipidemia, arterial hypertension, severe heart failure, and LVHT, are present. Altogether, only 71% of the 94 reported abnormalities described in the literature () were also found in the present study.
Abnormalities that have not been previously described in MID patients but have been found in the present investigation include liver cysts, RLS, eosinophilia, arachnoidal cysts, prostate hypertrophy, and endometriosis. Whether these abnormalities can truly be regarded as manifestations of the mitochondrial defect and whether valve abnormalities, pericarditis, and abnormal myocardial texture should be regarded as an MID manifestation remain speculative. It is also not established that nephrolithiasis is an authentic manifestation of MIDs. According to our own experience, however, nephrolithiasis particularly occurs as an initial manifestation at the onset of adult MIDs. Syncope is multi-causal but if due to a seizure or a cardiac arrhythmia an MID should be considered if no other explanations impose oneself. Syncope may be also due to arterial hypotension, which in turn could be a manifestation of adrenal insufficiency or bradyarrhythmia.
Limitations of the study were that it was retrospective, thus not all patients went through the same clinical screening, that the records could not be completely pursued in each case, that the genetic defect was not identified in each case (biochemical defects could have been secondary), that the number of included patients was low, that no control group or disease control group (eg, autoimmune disorders and myotonic dystrophy) was investigated, that the score was not validated, that the score does not predict the severity of the disease, that the number of matching PubMed citations for each MID manifestation was only approximately determined, that the number of PubMed hits may not adequately reflect the frequency of abnormalities, and that not all organs potentially affected in MIDs were also investigated in each patient. A further limitation is that biochemical and genetic studies were carried out in only 2 patients. A single biochemical defect usually reflects a mutation in one of the genes encoding the subunits of the corresponding respiratory chain complex. Multiple biochemical defects usually reflect tRNA mutations or mtDNA depletion.
Conclusion
This study shows that adult MIDs manifest as MIMODS in the vast majority of the cases; that organs most frequently affected in MIMODS are the muscle, CNS, endocrine glands, and heart; and that an MIMODS score >10 suggests an MID and thus could be a helpful parameter to assess, by nonsophisticated, clinical means, whether an MID is present or not.
Disclosure
The authors report no conflicts of interest in this work.
References
- FinstererJJariusCEichbergerHPhenotype variability in 130 adult patients with respiratory chain disordersJ Inherit Metab Dis20012456057611757584
- PfefferGSirrsSWadeNKMezeiMMMultisystem disorder in late-onset chronic progressive external ophthalmoplegiaCan J Neurol Sci20113811912321156440
- SchmidtHLotzeUGhanemARelation of impaired inter-organ communication and parasympathetic activity in chronic heart failure and multiple-organ dysfunction syndromeJ Crit Care20142936737324529299
- LüHBZhouYHuJZLeiGHZhuMLiKHMitochondrial DNA deletion mutations in articular chondrocytes of cartilage affected by osteoarthritisZhong Nan Da Xue Xue Bao Yi Xue Ban20063164064417062921
- ChangMCHungSCChenWYAccumulation of mitochondrial DNA with 4977-bp deletion in knee cartilage – an association with idiopathic osteoarthritisOsteoarthritis Cartilage2005131004101116165375
- SchapiraAHMitochondrial diseasesLancet20123791825183422482939
- ParkJRyuHJangWNovel 5.712 kb mitochondrial DNA deletion in a patient with Pearson syndrome: a case reportMol Med Rep20151153741374525543536
- WilliamsTBDanielsMPuthenveetilGChangRWangRYAbdenurJEPearson syndrome: unique endocrine manifestations including neonatal diabetes and adrenal insufficiencyMol Genet Metab201210610410722424738
- ManeaEMLevergerGBellmannFPearson syndrome in the neonatal period: two case reports and review of the literatureJ Pediatr Hematol Oncol20093194795119881395
- LohiOKuuselaALArolaMA novel deletion in a Pearson syndrome infant with hypospadias and cleft lip and palateJ Inherit Metab Dis2005281165116616435219
- DemarestSTWhiteheadMTTurnaciogluSPearlPLGropmanALPhenotypic analysis of epilepsy in the mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes-associated mitochondrial DNA A3243G mutationJ Child Neurol2014291249125625038129
- GurrieriCKivelaJEBojanićKAnesthetic considerations in mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes syndrome: a case seriesCan J Anaesth20115875176321656321
- PronickiMSykut-CegielskaJMierzewskaHDiversity of clinical symptoms in A3243G mitochondrial DNA mutation (MELAS syndrome mutation)Med Sci Monit20028CR767CR77312444382
- Bandettini di PoggioMNestiCBrunoCMeschiniMCSchenoneASantorelliFMDopamine-agonist responsive Parkinsonism in a patient with the SANDO syndrome caused by POLG mutationBMC Med Genet20131410524099403
- HansroteSCroulSSelakMKalmanBSchwartzmanRJExternal ophthalmoplegia with severe progressive multiorgan involvement associated with the mtDNA A3243G mutationJ Neurol Sci2002197636711997068
- de KeyzerYValayannopoulosVBenoistJFMultiple OXPHOS deficiency in the liver, kidney, heart, and skeletal muscle of patients with methylmalonic aciduria and propionic aciduriaPediatr Res200966919519342984
- LowECrushellEBHartySBRyanSPTreacyEPReversible multiorgan system involvement in a neonate with complex IV deficiencyPediatr Neurol20083936837018940565
- MeulemansASenecaSLagaeLA novel mitochondrial transfer RNA(Asn) mutation causing multiorgan failureArch Neurol2006631194119816908752
- LehmannDSchubertKJoshiPRA novel m.7539C>T point mutation in the mt-tRNA(Asp) gene associated with multisystemic mitochondrial diseaseNeuromuscul Disord201525818425447692
- PitceathlyRDTaanmanJWRahmanSCOX10 mutations resulting in complex multisystem mitochondrial disease that remains stable into adulthoodJAMA Neurol2013701556156124100867
- OláhováMHaackTBAlstonCLA truncating PET100 variant causing fatal infantile lactic acidosis and isolated cytochrome c oxidase deficiencyEur J Hum Genet201523793593925293719
- EzguFSenacaSGunduzMSevere renal tubulopathy in a newborn due to BCS1L gene mutation: effects of different treatment modalities on the clinical courseGene201352836436623892085
- YangGZouLWangJMitochondrial disorders manifested as renal tubular acidosis and recurrent seizuresChin Med J (Engl)2014127198924824270
- GaiXGhezziDJohnsonMAMutations in FBXL4, encoding a mitochondrial protein, cause early-onset mitochondrial encephalomyopathyAm J Hum Genet20139348249523993194
- Brunetti-PierriNPignatelliRFouladiNDilation of the aortic root in mitochondrial disease patientsMol Genet Metab201110316717021406331
- SakharovaAVKalashnikovaLAChaĭkovskaiaRPMorphological signs of mitochondrial cytopathy in skeletal muscles and micro-vessel walls in a patient with cerebral artery dissection associated with MELAS syndromeArkh Patol2012745156 Russian
- RytherRCCho-ParkYALeeJWCarotid dissection in mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodesJ Neurol201125891291421076841
- KalashnikovaLASakharovaAVDobryninaLAMitochondrial arteriopathy as a cause of spontaneous dissection of cerebral arteriesZh Nevrol Psikhiatr Im S S Korsakova2010110Suppl 2311 Russian
- TaySHNordliDRJrBonillaEAortic rupture in mitochondrial encephalopathy, lactic acidosis, and stroke-like episodesArch Neurol20066328128316476819
- FinstererJFrankMHematological abnormalities in mitochondrial disordersSingapore Med J20155641241926243978
- MancusoMOrsucciDAngeliniCPhenotypic heterogeneity of the 8344A>G mtDNA “MERRF” mutationNeurology2013802049205423635963
- KleefstraTWortmannSBRodenburgRJMitochondrial dysfunction and organic aciduria in five patients carrying mutations in the Ras-MAPK pathwayEur J Hum Genet20111913814421063443
- CaballeroPECandelaMSAlvarezCITejerinaAAChronic progressive external ophthalmoplegia: a report of 6 cases and a review of the literatureNeurologist200713333617215725
- SpyropoulosAManfordMHorvathRNear-identical segregation of mtDNA heteroplasmy in blood, muscle, urinary epithelium, and hair follicles in twins with optic atrophy, ptosis, and intractable epilepsyJAMA Neurol2013701552155524126373
- VissingCRDunoMOlesenJHRecurrent myoglobinuria and deranged acylcarnitines due to a mutation in the mtDNA MT-CO2 geneNeurology2013801908191023616164
- BlakelyELAlstonCLLeckyBDistal weakness with respiratory insufficiency caused by the m.8344A>G “MERRF” mutationNeuromuscul Disord20142453353624792523
- RubegniACardaioliEChiniEA case of 3243A>G mutation in mtDNA presenting as apparently idiopathic hyperCKemiaJ Neurol Sci201433823223424468540
- GdyniaHJSperfeldADKnirschUBenign symmetric lipomatosis with axonal neuropathy and abnormalities in specific mitochondrial tRNA regionsEur J Med Res20061154554617182368
- HanischFMüllerTMuserADeschauerMZierzSLactate increase and oxygen desaturation in mitochondrial disorders–evaluation of two diagnostic screening protocolsJ Neurol200625341742316619117
- McKelviePMarottaRThorburnDRChinJPunchihewaSCollinsSA case of myelopathy, myopathy, peripheral neuropathy and subcortical grey matter degeneration associated with recessive compound heterozygous POLG1 mutationsNeuromuscul Disord20122240140522357363
- DeschauerMWieserTNeudeckerSLindnerAZierzSMitochondrial 3243A–>G mutation (MELAS mutation) associated with painful muscle stiffnessNeuromuscul Disord1999930530710407850
- BarrientosACasademontJGrauJMOftalmoplejía externa progresiva y síndrome de Kearns-Sayre: estudio clínico y molecular de 6 casos [Progressive external ophthalmoplegia and the Kearns-Sayre syndrome: a clinical and molecular study of 6 cases]Med Clin (Barc)1995105180184 Spanish7630231
- DesguerreIHullyMRioMNabboutRMitochondrial disorders and epilepsyRev Neurol (Paris)201417037538024810279
- FinstererJZarrouk MahjoubSMitochondrial epilepsy in pediatric and adult patientsActa Neurol Scand201312814115223480231
- Devaux-BricoutMGréventDLebreASAspect en IRM cérébrale des maladies mitochondriales. Algorithme décisionnel des maladies mitochondriales les plus fréquentes [Aspect of brain MRI in mitochondrial respiratory chain deficiency. A diagnostic algorithm of the most common mitochondrial genetic mutations]Rev Neurol (Paris)2014170381389 French24768439
- DallabonaCDiodatoDKevelamSHNovel (ovario) leukodystrophy related to AARS2 mutationsNeurology2014822063207124808023
- Unal GulsunerHGulsunerSMercanFNMitochondrial serine protease HTRA2 p.G399S in a kindred with essential tremor and Parkinson diseaseProc Natl Acad Sci U S A2014111182851829025422467
- Inczedy-FarkasGTrampushJWPerczel ForintosDMitochondrial DNA mutations and cognition: a case-series reportArch Clin Neuropsychol20142931532124777554
- DossSLohmannKSeiblerPRecessive dystonia-ataxia syndrome in a Turkish family caused by a COX20 (FAM36A) mutationJ Neurol201426120721224202787
- ZsurkaGBeckerFHeinenMMutation in the mitochondrial tRNA(Ile) gene causes progressive myoclonus epilepsySeizure20132248348623601850
- LovanAul HaqIBalakrishnanNDiagnostic challenges in movement disorders: Sensory Ataxia Neuropathy Dysarthria and Ophthalmoplegia (SANDO) syndromeBMJ Case Rep201310.1136/bcr-2013-010343
- AnglinREGarsideSLTarnopolskyMAMazurekMFRosebushPIThe psychiatric manifestations of mitochondrial disorders: a case and review of the literatureJ Clin Psychiatry20127350651222579150
- CoomansHBarrosoBBertandeauEMutisme et troubles du comportement aigus révélant un syndrome MELAS [Mutism and acute behavioral disorders revealing MELAS syndrome]Rev Neurol (Paris)2011167847851 French21514610
- RonchiDGaroneCBordoniANext-generation sequencing reveals DGUOK mutations in adult patients with mitochondrial DNA multiple deletionsBrain20121353404341523043144
- FinstererJBastovanskyADilative arteriopathy and leucencephalopathy as manifestations of a neurometabolic diseaseOpen Neurol J20159283126191091
- NaghizadehFVargaETMolnárMJHollóGRetinal ganglion cell layer and visual function in patients with progressive external ophthalmoplegia caused by common mtDNA deletionIdeggyogy Sz201467335341 Hungarian25518262
- HaasRHZolkipliZMitochondrial disorders affecting the nervous systemSemin Neurol20143432134025192510
- van BergeLHamiltonEMLinnankiviTLeukoencephalopathy with brainstem and spinal cord involvement and lactate elevation: clinical and genetic characterization and target for therapyBrain20141371019102924566671
- HopkinsSESomozaAGilbertDLRare autosomal dominant POLG1 mutation in a family with metabolic strokes, posterior column spinal degeneration, and multi-endocrine diseaseJ Child Neurol20102575275619815814
- CassandriniDSavastaSBozzolaMMitochondrial DNA deletion in a child with mitochondrial encephalomyopathy, growth hormone deficiency, and hypoparathyroidismJ Child Neurol20062198398517092469
- D’AcoKEMannoMClarkeCGaneshJMeyersKESondheimerNMitochondrial tRNA(Phe) mutation as a cause of end-stage renal disease in childhoodPediatr Nephrol20132851551923135609
- VaranasiSSFrancisRMBergerCEPapihaSSDattaHKMitochondrial DNA deletion associated oxidative stress and severe male osteoporosisOsteoporos Int19991014314910501795
- MulliezEBlanckaertMBlanckaertJAcute manifestation of LHON and coincidental finding of a pituitary adenoma: a case reportBull Soc Belge Ophtalmol2000277354211126672
- ZhuoGFengGLengJYuLJiangYA 9-bp deletion homoplasmy in women with polycystic ovary syndrome revealed by mitochondrial genome-mutation screenBiochem Genet20104815716320094848
- FinstererJBittnerRBodingbauerMEichbergerHStöllbergerCBlazekGComplex mitochondriopathy associated with 4 mtDNA transitionsEur Neurol200044374110894993
- LimongelliGTome-EstebanMDejthevapornCRahmanSHannaMGElliottPMPrevalence and natural history of heart disease in adults with primary mitochondrial respiratory chain diseaseEur J Heart Fail20101211412120083621
- CardenaMMMansurAJPereira AdaCFridmanCA new duplication in the mitochondrially encoded tRNA proline gene in a patient with dilated cardiomyopathyMitochondrial DNA201324464922954281
- BatesMGBourkeJPGiordanoCd’AmatiGTurnbullDMTaylorRWCardiac involvement in mitochondrial DNA disease: clinical spectrum, diagnosis, and managementEur Heart J2012333023303322936362
- GuoHZhuangXYZhangAMPresence of mutation m.14484T>C in a Chinese family with maternally inherited essential hypertension but no expression of LHONBiochim Biophys Acta201218221535154322749828
- Zarrouk MahjoubSMehriSOurdaFTransition m.3308T>C in the ND1 gene is associated with left ventricular hypertrabeculation/noncompactionCardiology201111815315821625124
- HungPCWangHSChungHTHwangMSRoLSPulmonary hypertension in a child with mitochondrial A3243G point mutationBrain Dev20123486686822455997
- JiaZWangXQinYCoronary heart disease is associated with a mutation in mitochondrial tRNAHum Mol Genet2013224064407323736300
- Blanco-GrauABonaventura-IbarsIColl-CantíJIdentification and biochemical characterization of the novel mutation m.8839G>C in the mitochondrial ATP6 gene associated with NARP syndromeGenes Brain Behav20131281282024118886
- UusimaaJEvansJSmithCClinical, biochemical, cellular and molecular characterization of mitochondrial DNA depletion syndrome due to novel mutations in the MPV17 geneEur J Hum Genet20142218419123714749
- CardaioliEDa PozzoPMalfattiEA second MNGIE patient without typical mitochondrial skeletal muscle involvementNeurol Sci20103149149420232099
- VernyCAmati-BonneauPLetournelFMitochondrial DNA A3243G mutation involved in familial diabetes, chronic intestinal pseudo-obstruction and recurrent pancreatitisDiabetes Metab20083462062618955007
- TakahashiYIidaKTakenoRHepatic failure and enhanced oxidative stress in mitochondrial diabetesEndocr J20085550951418445996
- Maniura-WeberKTaylorRWJohnsonMAA novel point mutation in the mitochondrial tRNA(Trp) gene produces a neurogastrointestinal syndromeEur J Hum Genet20041250951215054399
- Perez-AtaydeARFoxVTeitelbaumJEMitochondrial neurogastrointestinal encephalomyopathy: diagnosis by rectal biopsyAm J Surg Pathol199822114111479737248
- FinstererJMitochondrial neuropathyClin Neurol Neurosurg200510718118615823672
- ZhaoDWangZHongDZhangWYuanYChronic progressive external ophthalmoplegia coexistent with motor neuron disease in a patient with a novel large-scale mitochondrial DNA deletionClin Neurol Neurosurg20131151490149223266267
- La MorgiaCCaporaliLGandiniFAssociation of the mtDNA m.4171C>A/MT-ND1 mutation with both optic neuropathy and bilateral brainstem lesionsBMC Neurol20141411624884847
- DunoMWibrandFBaggesenKRosenbergTKjaerNFrederiksenALA novel mitochondrial mutation m.8989G>C associated with neuropathy, ataxia, retinitis pigmentosa – the NARP syndromeGene201351537237523266623
- NucciCMartucciAMancinoRCerulliLGlaucoma progression associated with Leber’s hereditary optic neuropathyInt Ophthalmol201333757722983441
- HirakiNUdakaTYamamotoHKadokawaYOhkuboJSuzukiHMitochondrial neurogastrointestinal encephalomyopathy associated with progressive hearing lossJ Laryngol Otol20101241007100920546644
- RiveraHMartín-HernándezEDelmiroAA new mutation in the gene encoding mitochondrial seryl-tRNA synthetase as a cause of HUPRA syndromeBMC Nephrol20131419524034276
- LiuHMTsaiLPChienYHA novel 3670-base pair mitochondrial DNA deletion resulting in multi-systemic manifestations in a childPediatr Neonatol20125326426822964285
- GürgeyAOzalpIRötigAA case of Pearson syndrome associated with multiple renal cystsPediatr Nephrol1996106376388897573
- DanpureCJLumbMJBirdseyGMZhangXAlanine:glyoxylate aminotransferase peroxisome-to-mitochondrion mistargeting in human hereditary kidney stone diseaseBiochim Biophys Acta20031647707512686111
- SobeninIASazonovaMAPostnovAYBobryshevYVOrekhovANMitochondrial mutations are associated with atherosclerotic lesions in the human aortaClin Dev Immunol2012201283246422997526
- BurrageLCTangSWangJMitochondrial myopathy, lactic acidosis, and sideroblastic anemia (MLASA) plus associated with a novel de novo mutation (m.8969G>A) in the mitochondrial encoded ATP6 geneMol Genet Metab201411320721225037980
- MurakiKNishimuraSGotoYNonakaISakuraNUedaKThe association between haematological manifestation and mtDNA deletions in Pearson syndromeJ Inherit Metab Dis1997206977039323565
- TanigawaJKanekoKHondaMTwo Japanese patients with Leigh syndrome caused by novel SURF1 mutationsBrain Dev20123486186522410471
- HartyLCBinieckaMO’SullivanJMitochondrial mutagenesis correlates with the local inflammatory environment in arthritisAnn Rheum Dis20127158258822121133
- RanjbarSHAmiriPAmoliMMSoltaniAA new mitochondrial mutation in a patient with diabetes mellitus, deafness, hydronephrosis and joint contracturesJ Pediatr Endocrinol Metab2008211185118919189693
- FinstererJKovacsGGPsoriasis, bulbar involvement, and diarrhea in late myoclonic epilepsy with ragged-red fibers-syndrome due to the m.8344A > G tRNA (Lys) mutationIran J Neurol2017161454928717435
- CarmiEDefossezCMorinGMELAS syndrome (mitochondrial encephalopathy with lactic acidosis and stroke-like episodesAnn Dermatol Venereol20011281031103511907964
- TuliniusMHOldforsAHolmeEAtypical presentation of multisystem disorders in two girls with mitochondrial DNA deletionsEur J Pediatr199515435427895754
- TrifunovicAWredenbergAFalkenbergMPremature ageing in mice expressing defective mitochondrial DNA polymeraseNature200442941742315164064
- FinstererJBrunnerSMadarosis from mitochondriopathyActa Ophthalmol Scand20058362863016188012
