Abstract
Classic antidepressant drugs are modestly effective across the population and most are associated with intolerable side effects. Recently, numerous lines of evidence suggest that resveratrol (RES), a natural polyphenol, possesses beneficial therapeutic activity for depression. The aim of the present study was to explore whether RES exhibits an antidepressant-like effect in a depression model and to explore the possible mechanism. A depression model was established via chronic unpredictable mild stress (CUMS), after which the model rats in the RES and fluoxetine groups received a daily injection of RES or fluoxetine, respectively. The sucrose preference test, open field test, and forced swimming test were used to explore the antidepressant-like effects of RES. The activity of the hypothalamic–pituitary–adrenal (HPA) axis was evaluated by detecting the plasma corticosterone concentration and hypothalamic mRNA expression of corticotrophin-releasing hormone. The plasma interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α) concentrations were measured by enzyme-linked immunosorbent assay. Hippocampal protein expression of brain-derived neurotrophic factor (BDNF) and the Wnt/β-catenin pathway were analyzed by western blot. The results showed that RES relieved depression-like behavior of CUMS rats, as indicated by the increased sucrose preference and the decreased immobile time. Rats that received RES treatment exhibited reduced plasma corticosterone levels and corticotrophin-releasing hormone mRNA expression in the hypothalamus, suggesting that the hyperactivity of the HPA axis in CUMS rats was reversed by RES. Moreover, after RES treatment, the rats exhibited increased plasma IL-6, CRP, and TNF-α concentrations. Furthermore, RES treatment upregulated the hippocampal protein levels of BDNF and the relative ratio of p-β-catenin/β-catenin while downregulating the relative ratio of p-GSK-3β/GSK-3β. Our findings suggest that RES improved depressive behavior in CUMS rats by downregulating HPA axis hyperactivity, increasing BDNF expression and plasma IL-6, CRP, and TNF-α concentrations, and regulating the hippocampal Wnt/β-catenin pathway.
Introduction
Depression is one of the leading causes of disability, affecting up to 120 million people worldwide.Citation1 According to the World Health Organization Global Burden of Disease, depression is anticipated to become the leading cause of long-term disability by the year 2030.Citation57 Based on the monoamine neurotransmitter hypothesis of depression,Citation2 raising monoamine levels in synaptic clefts has been the main strategy for treating depressive patients over the past 60 years. However, not all patients with depressive disorders respond to classic antidepressants, and months of treatment are usually required, indicating that monoamine depletion might not be the unique pathogenesis of depression.Citation3 In addition to this, there is also growing evidence regarding adverse reactions to the clinical use of antidepressants, including dizziness, tremor, cognitive impairments, urinary retention, and sexual dysfunction.Citation4 Therefore, there is an urgent need to explore the pathogenesis of depression and improve the efficacy and safety of depression treatment with better drugs.
The stress response and the resulting hypothalamic–pituitary–adrenal (HPA) axial hyperactivity are considered to be one of the most powerful triggers for depressive disorders.Citation5 Preclinical and clinical studies have indicated that the stress response can result in depression-like behavior accompanied by hyperactivity of the HPA axis,Citation6,Citation7 as indicated by increased serum glucocorticoid concentrations and corticotrophin-releasing hormone (CRH) expression in the hypothalamus.Citation8 Chronic mild unpredictable stress (CUMS) has been used in animal depression research because it can simulate the representative symptoms of depression, including anhedonia and despairing behavior.Citation9 Given the roles of the HPA axis in the pathophysiology of depression, plasma corticosterone concentrations and hypothalamic CRH expression were evaluated in CUMS rats in this study.
Increasing evidence supports the view that depression is accompanied by activation of the inflammatory response system, and overproduction of pro-inflammatory cytokines may play a role in the pathophysiology of depressive disorders.Citation10,Citation11 It has been reported that the most robust evidence-based inflammatory markers associated with depressive disorder include interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α).Citation12 Moreover, numerous studies have demonstrated that increased levels of plasma IL-6 and CRP are related to an increased risk of depressive episodesCitation13,Citation14 and can even be used to predict subsequent depressive symptoms.Citation15 In animals, it has been shown that depression-like behaviors are induced by cytokinesCitation16 and cytokine inducers, including lipopolysaccharide administrationCitation17 and chronic mild stress.Citation18 Therefore, IL-6, CRP, and TNF-α levels were evaluated in CUMS rats with and without antidepressant administration in this study.
Recent evidence has indicated that decreased levels of brain-derived neurotrophic factor (BDNF) in the hippocampus can cause reduced proliferation of hippocampal neurons and result in depressive disorders.Citation19 Moreover, depression-like effects in rats induced by chronic stress or glucocorticoid administration have been reported to be related to reduced hippocampal BDNF levels.Citation20,Citation21 Furthermore, some antidepressants are reported to achieve their efficacy by increasing hippocampal BDNF expression.Citation22,Citation23 Although the underlying mechanisms remain unknown, it has been reported that BDNF appears to be a direct target of Wnt signaling.Citation24 Together with the fact that the canonical Wnt signaling pathway has been reported to be implicated in mood disorders such as bipolar disorder and major depression,Citation25,Citation26 the protein expression levels of BDNF and the Wnt/β-catenin pathway were further investigated in CUMS rats in the present study.
Resveratrol (trans-3,5,4′-trihydroxy-trans-stilbene, RES), a phytoalexin with antioxidant properties, is found in a wide range of foods, especially in grapes and red wine. In the last decade, RES has been shown to possess multiple biological and pharmacological activities such as antiinflammatory, antioxidant, anticarcinogenic, antiaging, neuroprotective, and cardioprotective effects.Citation27 Recently, RES was reported to exert antidepressant-like effects, alleviating depression-like symptoms/behaviors in rodent animal models.Citation28,Citation29 Additionally, it has been shown that RES treatment can alter the monoaminergic system and the molecular markers related to depression.Citation30,Citation31 However, it remains unknown whether RES can improve depression-like symptoms in CUMS rats, which involve a complicated balance of the HPA axis, the inflammatory reaction, BDNF, and the Wnt/β-catenin pathway.
In the present study, a rat depression model was established using CUMS. Behavior tests, including the open field test (OFT), sucrose preference test (SPT), and forced-swimming test (FST), were used to probe the effects of RES.
Experimental procedures
Drugs
RES was provided by Sigma Chemical Co. (St Louis, MO, USA). Fluoxetine hydrochloride (Prozac) was provided by Eli Lilly Pharmaceuticals. RES and fluoxetine were dissolved to become a mixed suspension in an aqueous solution of 0.5% sodium carboxymethyl cellulose.
Animals
After 7 days of adaptive breeding, 40 male adult Sprague Dawley (SD) rats were randomly allocated to four groups, including a control group (control +0.5% sodium carboxymethyl cellulose), a chronic unpredictable mild stress group (CUMS +0.5% sodium carboxymethyl cellulose), RES treatment group (15 mg/kg/day RES + CUMS), and a fluoxetine-treated CUMS group (2 mg/kg/day fluoxetine hydrochloride + CUMS). Rats were maintained at 21°C–25°C with 50%–60% relative humidity, under a 12/12 h light–dark cycle (lights on at 07:30 h). Rats in the control group were housed five per cage (43 cm length ×31 cm width ×19 cm height) with free access to water and food. Rats in the other three groups were housed five per cage and stressed according to the CUMS procedure described below. The experiments were performed after approval of the protocol by the Animal Care and Use Committee at Anhui Medical University and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No 85-23, revised 1985).
Chronic unpredictable mild stress procedure
Stressors were administered once daily between 08:40 and 10:40 for 3 weeks, with the exception of the 24 h duration stressors. The stressors were used to induce a depressive state, including 1) social crowding (10 rats per cage, 24 h); 2) swimming at 30°C (20 min); 3) in a cage tilted at 30° from the horizontal (24 h); 4) swimming at 8°C–10°C (5 min); 5) in a wet cage (24 h); 6) tail pinch (2 min); and 7) food and water deprivation (24 h). The different stressors were randomly distributed, with an interval of 7 days between repetitions. All stressors were administered three times within 21 days. The schedule of the experimental design is shown in .
Figure 1 Schedule of the experimental design.
Behavioral tests
Behavioral tests were executed in a noise-proof room. All behavioral tests were performed between 08:00 and 14:00, with matching between the groups.
Sucrose preference test
All the rats were singly housed in a cage (28 cm length ×17 cm width ×14.5 cm height) after a 12-h period of food and water deprivation. Then, all rats were given two bottles containing water or 2% sucrose solution. Sucrose and water bottles were placed in randomly assigned sides of the cage. Six hours later, the weights of water and sucrose consumed were measured. The rats’ sensitivity to reward was calculated as the percentage of total liquid intake attributed to the 2% sucrose solution, according to the following equation: Sucrose solution(g)/(Sucrose solution [g] + water [g]) ×100%.
Open field test
The open field arena, 100×100 cm in size, was divided into 16 square areas. Rats were placed randomly at one corner of the apparatus. During a 5-min observation period, all rat movements were recorded by a camera interfaced to a computer running the ANY maze video imaging software (Stoelting Co, Wood Dale, IL, USA). After testing each individual rat, the open field arena surface was cleaned with 75% ethyl alcohol. The total distance was recorded to measure locomotion. The distance, frequency, and duration in the center, and frequencies of grooming and defecation were recorded to measure anxiety. The frequency of rearing was recorded to measure exploratory behavior.
Forced swimming test
The FST was conducted by placing the rat in a cylinder (60 cm in height ×25 cm in diameter) containing 30 cm of water at 24°C–25°C. The FST paradigm includes two sections: an initial 15-min pretest followed by a 5-min test 24 h later. A rat was considered to be immobile when it remained floating in the water in an upright position, making only very small movements to keep its head above the water. A rat was considered to be struggling when it made active movements with its forepaws in and out of the water along the side of the swim chamber. A rat was considered to be swimming when it made active swimming or circular movements.
Measurement of plasma corticosterone, IL-6, CRP, and TNF-α
Twenty-four hours after the FST, the rats were anesthetized by an intraperitoneal (i.p.) injection of chloral hydrate (300 mg/kg, Sinopharm Chemical Reagent Co., Ltd, Shanghai Shi, China). Then, blood was collected from the abdominal aorta in evacuated tubes with EDTA-K2. The plasma concentrations of corticosterone, IL-6, CRP, and TNF-α were measured using an enzyme-linked immunosorbent assay (ELISA; corticosterone: Enzo Life Sciences, Inc., Farmingdale, NY, USA; IL-6, CRP, and TNF-α: Yuanye Biotech. Co., LTD, Shanghai, China) according to the manufacturer’s instructions.
RNA isolation and real-time PCR
After blood collection, rats were sacrificed by decapitation. Then, the hypothalamus was rapidly dissected on ice, frozen quickly in liquid nitrogen, and stored at −80°C. Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Identical amounts of RNA (2 μg) were reverse transcribed into cDNA using a commercial real-time polymerase chain reaction (RT-PCR) kit (Promega, Fitchburg, WI, USA) according to the manufacturer’s instructions. qPCR was performed using the SYBR Green PCR Kit (Applied Biosystems, Waltham, MA, USA) and an ABI Prism 7000 Sequence Detector system in a 25 μL volume for 40 cycles (15 s at 95°C; 60 s at 62°C) for rat β-actin and CRH. The primers used were β-actin Forward (5′-TTGCTGACAGGATGCAGAA-3′), Reverse (5′-ACCAATCCACACAGAGTACTT-3′); CRH Forward (5′-CAGAACAACAGTGCGGGCTCA-3′), Reverse (5′-AAGGCAGACAGGGCGACAGAG-3′). The relative amount of the target gene was calculated using the 2−ΔΔCt method.
Western blot assays
Frozen tissue from the entire hippocampus was homogenized in ice-cold radioimmunoprecipitation assay (RIPA) buffer plus Complete Protease Inhibitor (Roche, Indianapolis, IN, USA). The dissolved proteins were collected after centrifugation at 10,000 g for 15 min at 4°C, and then, the supernatant was collected. Protein quantitation was conducted using a Lowry Protein Assay Kit (Meiji Biotech. Co., LTD., Shanghai, China). The same quantity (~40–50 μg) of protein from each rat was separated by 12% SDS-PAGE and then transferred onto a polyvinylidene difluoride membrane (Amersham Biosciences, Buckinghamshire, UK). The membrane was blocked with 5% skim milk for 3 h, probed with the primary antibodies targeting β-actin (1:800; Bioworld Technology, Inc., St Louis Park, MN, USA), BDNF (1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), GSK-3β, p-GSK-3β (Ser9), β-catenin, or p-β-catenin (1:1,000; Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight, and then incubated with a horseradish peroxidase-conjugated secondary antibody (1:10,000) at 37°C for 2 h. The membranes were developed using an enhanced chemiluminescence detection system (Pierce Biotechnology, Rockford, IL, USA).
Statistical analysis
All tests were performed using the SPSS Graduate Pack 17.0 statistical software package (SPSS, Chicago, IL, USA). Data were expressed as the mean ± SEM. The between-group effects on body weight were analyzed by a repeated measures one-way analysis of variance (ANOVA) with treatment group and weeks as the factors. Statistical analyses of behavioral performance, plasma corticosterone, IL-6, CRP, and TNF-α concentration, and expression of CRH, BDNF, p-GSK-3β/GSK-3β, or p-β-catenin/β-catenin were carried out by ANOVA followed by least significant difference (LSD) post hoc tests. A value of P<0.05 was regarded as significant.
Results
RES administration improved depression-like behavior caused by CUMS in rats
shows the effect of RES on the changes in body weight and behavior caused by CUMS. The repeated measures ANOVA (four treatments ×3 weeks with repeated measures on weeks) showed that both the treatment and the time affected body weight (treatment effect: (F[3,36] =4.162, P=0.047); time effect: (F[3,108] =634.376, P<0.001); interaction: (F[9,108] =3.880, P=0.004)). The LSD test showed that the CUMS rats, with or without RES treatment, gained less body weight during the 3-week stress period compared with controls (F[3,36] =13.177, P=0.002). However, no significant differences between CUMS rats and CUMS rats treated with RES or fluoxetine were found ().
Figure 2 Effect of RES on the body weight and behavior of the CUMS rats.
Abbreviations: CUMS, chronic unpredictable mild stress; FST, forced swimming test; OFT, open field test; RES, resveratrol.
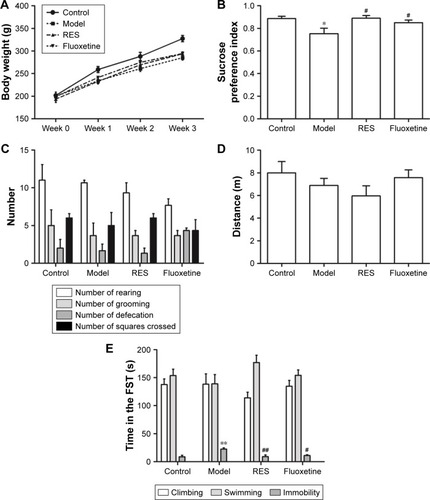
Compared to the control group, the sucrose preference of CUMS rats was significantly lower, which could be reversed by RES or fluoxetine treatment (F[3,36] =4.289, P=0.044; ).
In the OFT, no differences in performance, including the number of rearings (F[3,36] =1.312, P=0.336; ), groomings (F[3,36] =0.222, P=0.878; ), defecations (F[3,36] =2.778, P=0.110; ), squares crossed (F[3,36] =0.462, P=0.717; ), and distance (F[3,36] =1.178, P=0.377; ), were observed among the four groups.
In the FST, no differences in the climbing (F[3,36] =0.820, P=0.519; ) or swimming (F[3,36] =1.487, P=0.290; ) time were observed among the four groups. However, the CUMS rats were immobile for a longer time (F[3,36] =7.272, P=0.011) than controls, which was reversed by RES and fluoxetine treatment ().
RES administration decreased hyperactivity of the HPA axis caused by CUMS
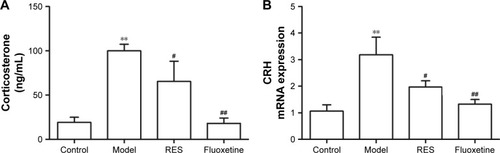
After three continuous weeks of CUMS, the plasma corticosterone concentrations of the CUMS rats were remarkably higher than that of the control rats (F[3,36] =9.747, P=0.002; ). Treatment with RES or fluoxetine decreased the elevated corticosterone levels (). Consistently, hypothalamic CRH mRNA expression was inhibited by treatment with RES or fluoxetine (F[3,36] =6.223, P=0.017; ).
Figure 3 Effects of RES on the plasma corticosterone and the expression of CRH mRNA in the hypothalamus in CUMS rats.
Abbreviations: CRH, corticotrophin-releasing hormone; CUMS, chronic unpredictable mild stress; RES, resveratrol.
RES administration decreased the elevated IL-6, CRP, and TNF-α concentrations induced by CUMS in rats
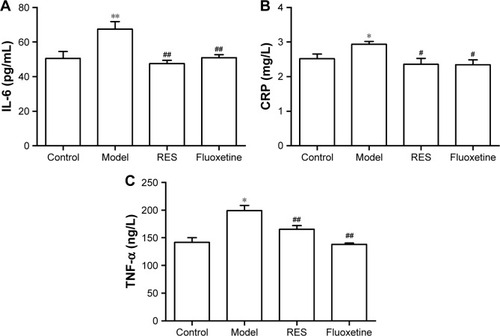
As shown in , compared with the control group, the plasma IL-6 (F[3,36] =7.600, P=0.01; ), CRP (F[3,36] =4.0554, P=0.049; ), and TNF-α (F[3,36] =14.998, P=0.001; ) concentrations of model rats were significantly higher than those of the controls, which was reversed by the RES or fluoxetine treatment.
Figure 4 Effects of RES on the plasma concentrations of IL-6, CRP, and TNF-α in CUMS rats.
Abbreviations: CRP, C-reactive protein; CUMS, chronic unpredictable mild stress; IL-6, interleukin-6; RES, resveratrol; TNF-α, tumor necrosis factor-α.
RES administration increased BDNF protein expression and decreased activation of the canonical Wnt pathway in the hippocampus of CUMS rats
shows the protein expression levels of GSK-3β, p-GSK-3β (Ser9), β-catenin, p-β-catenin, and BDNF in the hippocampus. Compared to the control group, the hippocampal protein levels of BDNF were lower in the CUMS rats. However, these differences were ameliorated by RES or fluoxetine (F[3,36] =11.948, P=0.003; ).
Figure 5 Effects of RES on the protein expressions of BDNF and the Wnt/β-catenin pathway in the hippocampi of CUMS rats.
Abbreviations: BDNF, brain-derived neurotrophic factor; CUMS, chronic unpredictable mild stress; RES, resveratrol.
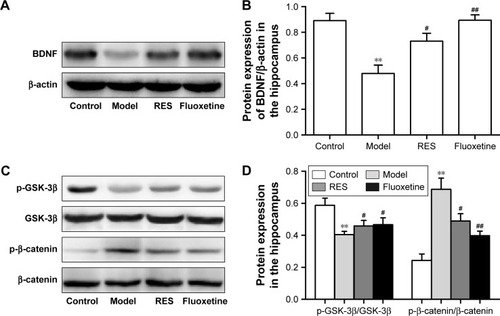
A lower relative ratio of p-GSK-3β/GSK-3β (F[3,36] =4.524, P=0.039; ) and a higher relative ratio of p-β-catenin/β-catenin (F[3,36] =14.943, P=0.001; ) were observed in the hippocampus of the model rats compared with the control group, which were reversed by the RES or fluoxetine treatments.
Discussion
In the present study, we investigated the antidepressant effects of RES using the CUMS rat depression model. The results showed that RES can improve the depression-like behavior exhibited by rats subjected to CUMS, as indicated by an increased sucrose preference index in the SPT and a decreased immobile time in the FST. Rats that received RES treatment had lower plasma corticosterone concentrations and hypothalamic CRH mRNA expression levels, indicating that RES treatment reversed the hyperactivity of the HPA axis observed in CUMS rats. RES treatment also increased the protein expression of BDNF and decreased the activation of the canonical Wnt pathway in the hippocampus of CUMS rats.
CUMS is often utilized in laboratory animals to mimic unpredictable life stressors that may contribute to the development of major depressive disorders in humans.Citation32 In the present study, rats displayed typical anhedonia behavior and despairing behavior after 3 weeks of chronic stress, as indicated by their decreased sucrose preference in the SPT and their increased immobile time in the FST, which showed that the rat model of depression was established successfully. The OFT provides simultaneous analysis of locomotion, exploration, and anxiety.Citation33 In the present study, no differences in performance in the OFT, including the amount of rearing, grooming, and defecation, the number of squares crossed and distance were observed among the four groups, indicating that RES and CUMS had no significant effect on the locomotor activity, exploration, and anxiety level of the rats. The SPT is generally used to evaluate anhedonia, defined as impairments in the ability to pursue, experience, and/or learn about pleasure, which is a typical symptom of depression in rodents.Citation34 Immobile time in the FST is taken as an index of despair behavior, which is another prominent symptom of depression.Citation35 In the present study, the CUMS rats showed a reduced sucrose preference index in the SPT and an increased immobile time in the FST, indicating depression-like symptoms in CUMS rats, which was consistent with the previous results.Citation36
It has been shown in clinical studies that RES is well tolerated with the dosages ranging from 200 to 1,000 mgCitation37,Citation38 daily. In terms of the formula for dose translation based on body surface area (BSA), the corresponding dose should range from 18 to 90 mg/kg in rats. With respect to preclinical studies, it has been reported that RES (15 mg/kg ×16 days) can improve the depression-like behavior in subclinical hypothyroidism rats.Citation39 Consistently, another study also demonstrated that RES (10 and 40 mg/kg) exerts an antidepressant-like effect in an animal model of depression.Citation28 Therefore, a dose of 15 mg/kg/day was selected in the present study.
Hyperactivity of the HPA axis is one of the most powerful factors that trigger depressive disorders.Citation5 It has been reported that RES treatment significantly reduces the increased serum corticosterone level in a stressed animal model induced by repeated corticosterone treatment, which has been performed widely in adult rodents in various depression models.Citation40 Pang et al studied the effect of RES isomers on the HPA axis.Citation41 The result showed that trans-RES may exert its antidepressant-like effect against post-stroke depression by modulating the HPA axis. Consistent with these studies, rats that received RES treatment had decreased plasma corticosterone concentrations and CRH mRNA expression in the hypothalamus in the present study, suggesting that the RES has a regulatory effect on the hyperactivity of the HPA axis in CUMS rats.
Given that inflammation may play a role in depression, anti-inflammatory agents may be a useful antidepressant therapy or adjuvant to traditional therapies. Research involving RES treatment in rodent models of inflammatory diseases have demonstrated downregulation of inflammation-induced biomarkers, including proinflammatory mediators (IL-1β, IL-6, TNF-α, and TNF-β),Citation42 and upregulation of inflammation-reduced biomarkers, including antiinflammatory protein (IL-10).Citation43 Consistently, the plasma IL-6, CRP, and TNF-α concentrations of model rats increased significantly, which was reversed by the RES or fluoxetine treatment in the present study. Thus, as one of the initial pharmacological activities, the antiinflammatory activity of RES might be involved in its antidepressant-like effects.
GSK-3β regulates phosphorylation and subsequent degradation of β-catenin, thereby preventing aberrant activation of the canonical Wnt pathway.Citation44 Clinical studies have assessed the levels or activity of total GSK-3β protein in the prefrontal cortex in patients with mood disorders, including depression.Citation45 Evidence also suggests that inhibition of GSK-3β might contribute to an antidepressant effect.Citation46 Consistent with the result that the enzymatic activity of GSK-3β was increased in depressed suicide victims,Citation45 lower ratios of p-GSK-3β/GSK-3β in the hippocampus were found in the CUMS rats, which could underlie depression-like behavior. The decreased ratio of p-GSK-3β/GSK-3β was ameliorated by RES treatment. L803-mts, a known GSK-3β inhibitor,Citation47 was reported to induce an antiimmobility effect in the FST. Moreover, a lower GSK-3β expression level in heterozygotic GSK-3β+/− mice is associated with a reduced immobile time in the FST.Citation48 These studies, together with the result of this study, suggest a role for GSK-3β in the antidepressant effects of RES and highlight GSK-3β as a potential target in the treatment of human depression.
β-catenin, a substrate of GSK-3β, is involved in cerebral development, cognitive function, and dendritic growth.Citation49,Citation50 Increasing evidence has indicated that β-catenin levels in the hippocampus can serve as a marker for antidepressant effects.Citation51 Moreover, β-catenin protein levels in the postmortem prefrontal cortices of depressed patients were lower compared to healthy controls.Citation52 Consistently, the ratio of p-β-catenin/β-catenin was increased in the hippocampus of the model rats, which was reversed by the RES treatment. The findings from this study, together with the reports mentioned above, reinforce the observation that RES might involve regulation of the canonical Wnt pathway in its antidepressant-like effect.
BDNF, a neurotrophin, is involved in neuronal proliferation, development, differentiation, and maturation.Citation53 There are several reports on the functional interaction between the BDNF and Wnt signaling cascades. Recent studies have demonstrated that BDNF expression is regulated by the Wnt signaling pathway.Citation54 Another study reported that BDNF and Wnt signaling cooperatively regulate dendritic spine formation.Citation55 Moreover, BDNF also mobilizes synaptic vesicles, enhances synapse formation, regulates axonal morphogenesis, and promotes neurite growth from neonatal cochlear ganglion explants by disrupting β-catenin interactions.Citation56 In the present study, our results showed that RES treatment could upregulate the reduced hippocampal protein levels of BDNF in CUMS rats. Given the relationship between BDNF and the canonical Wnt pathway, it is logical to hypothesize that the upregulation of BDNF expression caused by RES might be due to its effect on the canonical Wnt pathway.
Conclusion
In conclusion, our results demonstrate that RES ameliorated depression-like behavior in CUMS rats. This effect may be due, at least in part, to regulation of the HPA axis, inflammatory markers, BDNF, and the Wnt/β-catenin pathway in the hippocampus.
Disclosure
The authors report no conflicts of interest in this work.
References
- KimHDHestermanJCallTSIRT1 mediates depression-like behaviors in the nucleus accumbensJ Neurosci201636328441845227511015
- BaldessariniRJThe basis for amine hypotheses in affective disorders. A critical evaluationArch Gen Psychiatry197532910871093241308
- FordACIn irritable bowel syndrome, antispasmodics and antidepressants improve abdominal pain and global assessment and symptom scores, but there is no evidence for the effectiveness of bulking agentsEvid Based Med201217411411522440557
- GivensCJAdverse drug reactions associated with antipsychotics, antidepressants, mood stabilizers, and stimulantsNurs Clin North Am201651230932127229284
- SwaabDFBaoAMLucassenPJThe stress system in the human brain in depression and neurodegenerationAging Res Rev200542141194
- EvansJSunYMcGregorAConnorBAllopregnanolone regulates neurogenesis and depressive/anxiety-like behavior in a social isolation rodent model of chronic stressNeuropharmacology20126381315132622939998
- GarzaJCGuoMZhangWLuXYLeptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3beta/beta-catenin signalingMol Psychiatry201217879080822182938
- WangSSYanXBHofmanMASwaabDFZhouJNIncreased expression level of corticotropin-releasing hormone in the amygdala and in the hypothalamus in rats exposed to chronic unpredictable mild stressNeurosci Bull201026429730320651811
- KoprdovaRBogiEBelovicovaKChronic unpredictable mild stress paradigm in male Wistar rats: effect on anxiety- and depressive-like behaviorNeuro Endocrinol Lett201637Suppl 110311028263537
- LangUEBorgwardtSMolecular mechanisms of depression: perspectives on new treatment strategiesCell Physiol Biochem201331676177723735822
- MaesMAndersonGKuberaMBerkMTargeting classical IL-6 signalling or IL-6 trans-signalling in depression?Expert Opin Ther Targets201418549551224548241
- DowlatiYHerrmannNSwardfagerWA meta-analysis of cytokines in major depressionBiol Psychiatry201067544645720015486
- LiuYAl-SayeghHJabrahRWangWYanFZhangJAssociation between C-reactive protein and depression: modulated by gender and mediated by body weightPsychiatry Res2014219110310824909972
- Wium-AndersenMKOrstedDDNordestgaardBGElevated C-reactive protein, depression, somatic diseases, and all-cause mortality: a Mendelian randomization studyBiol Psychiatry201476324925724246360
- ValkanovaVEbmeierKPAllanCLCRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studiesJ Affect Disord2013150373674423870425
- Sukoff RizzoSJNealSJHughesZAEvidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypesTransl Psychiatry20122e199
- ZhangFFuYZhouXDepression-like behaviors and heme oxygenase-1 are regulated by Lycopene in lipopolysaccharide-induced neuroinflammationJ Neuroimmunol20162981827609268
- YouZLuoCZhangWPro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: involvement in depressionBehav Brain Res2011225113514121767575
- BaiMZhuXZhangYAbnormal hippocampal BDNF and miR-16 expression is associated with depression-like behaviors induced by stress during early lifePLoS One2012710e4692123056528
- AdachiNNumakawaTNakajimaSGlucocorticoid affects dendritic transport of BDNF-containing vesiclesSci Rep201551268426239075
- ZuXZhangMLiWAntidepressant-like Effect of Bacopaside I in Mice Exposed to Chronic Unpredictable Mild Stress by Modulating the Hypothalamic-Pituitary-Adrenal Axis Function and Activating BDNF Signaling PathwayNeurochem Res Epub2017731
- WangHZhaoYWangYJAntidepressant-like effects of tetrahydroxystilbene glucoside in mice: Involvement of BDNF signaling cascade in the hippocampusCNS Neurosci Ther201723762763628547794
- JiangHChenSLiCThe serum protein levels of the tPA-BDNF pathway are implicated in depression and antidepressant treatmentTransl Psychiatry201774e107928375203
- YangJWMaWLuoTBDNF promotes human neural stem cell growth via GSK-3beta-mediated crosstalk with the Wnt/beta-catenin signaling pathwayGrowth Factors2016341–2193227144323
- MuneerAWnt and GSK3 Signaling Pathways in Bipolar Disorder: Clinical and Therapeutic ImplicationsClin Psychopharmacol Neurosci201715210011428449557
- InksterBNicholsTESaemannPGPathway-based approaches to imaging genetics association studies: Wnt signaling, GSK3beta substrates and major depressionNeuroImage201053390891720219685
- ParkEJPezzutoJMThe pharmacology of resveratrol in animals and humansBiochim Biophys Acta2015185261071111325652123
- HurleyLLAkinfiresoyeLKalejaiyeOTizabiYAntidepressant effects of resveratrol in an animal model of depressionBehav Brain Res20142681724717328
- LiuSLiTLiuHResveratrol exerts antidepressant properties in the chronic unpredictable mild stress model through the regulation of oxidative stress and mTOR pathway in the rat hippocampus and prefrontal cortexBehav Brain Res201630219119926801825
- XuYZhangCWuFPiperine potentiates the effects of trans-resveratrol on stress-induced depressive-like behavior: involvement of monoaminergic system and cAMP-dependent pathwayMetab Brain Dis201631483784826946512
- LiuDZhangQGuJResveratrol prevents impaired cognition induced by chronic unpredictable mild stress in ratsProg Neuropsychopharmacol Biol Psychiatry201449212924184538
- SunJWangFHongGAntidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stressNeurosci Lett201661815916626957230
- WalshRNCumminsRAThe open-field test: a critical reviewPsychol Bull197683348250417582919
- PappMWillnerPMuscatRAn animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stressPsychopharmacology199110422552591876670
- HanTKLeeJKLeemYHChronic exercise prevents repeated restraint stress-provoked enhancement of immobility in forced swimming test in ovariectomized miceMetab Brain Dis201530371171825344674
- YazirYUtkanTAriciogluFInhibition of neuronal nitric oxide synthase and soluble guanylate cyclase prevents depression-like behavior in rats exposed to chronic unpredictable mild stressBasic Clin Pharmacol Toxicol2012111315416022385503
- WitteAVKertiLMarguliesDSFloelAEffects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adultsJ Neurosci201434237862787024899709
- ChowHHGarlandLLHeckman-StoddardBMA pilot clinical study of resveratrol in postmenopausal women with high body mass index: effects on systemic sex steroid hormonesJ Transl Med20141222325115686
- GeJFXuYYQinGChengJQChenFHResveratrol Ameliorates the Anxiety- and Depression-Like Behavior of Subclinical Hypothyroidism Rat: Possible Involvement of the HPT Axis, HPA Axis, and Wnt/beta-Catenin PathwayFront Endocrinol (Lausanne)201674427252679
- AliSHMadhanaRMKVAResveratrol ameliorates depressive-like behavior in repeated corticosterone-induced depression in miceSteroids2015101374226048446
- PangCCaoLWuFThe effect of trans-resveratrol on post-stroke depression via regulation of hypothalamus-pituitary-adrenal axisNeuropharmacology20159744745625937213
- RahalKSchmiedlin-RenPAdlerJResveratrol has antiinflammatory and antifibrotic effects in the peptidoglycan-polysaccharide rat model of Crohn’s diseaseInflamm Bowel Dis201218461362322431488
- ImlerTJJrPetroTMDecreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17+ IL-10+T cells, CD4(−) IFN-gamma + cells, and decreased macrophage IL-6 expressionInt Immunopharmacol20099113414319022403
- VoletiBDumanRSThe roles of neurotrophic factor and Wnt signaling in depressionClin Pharmacol Ther201291233333822205198
- KaregeFPerroudNBurkhardtSAlteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victimsBiol Psychiatry200761224024516876135
- JopeRSRohMSGlycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventionsCurr Drug Targets20067111421143417100582
- Kaidanovich-BeilinOMilmanAWeizmanAPickCGEldar-FinkelmanHRapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampusBiol Psychiatry200455878178415050857
- O’BrienWTHarperADJoveFGlycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithiumJ Neurosci200424306791679815282284
- YuXMalenkaRCBeta-catenin is critical for dendritic morphogenesisNat Neurosci20036111169117714528308
- DaleTCSignal transduction by the Wnt family of ligandsBiochem J1998329Pt 22092239425102
- Pilar-CuellarFVidalRPazosASubchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, beta-catenin and antidepressant-like effectsBr J Pharmacol20121654b1046105721627639
- KaregeFPerroudNBurkhardtSProtein levels of beta-catenin and activation state of glycogen synthase kinase-3beta in major depression. A study with postmortem prefrontal cortexJ Affect Disord20121361–218518822036797
- HendersonCERole of neurotrophic factors in neuronal developmentCurr Opin Neurobiol19966164708794045
- YiHHuJQianJHackamASExpression of brain-derived neurotrophic factor is regulated by the Wnt signaling pathwayNeuroreport201223318919422198686
- HiesterBGGalatiDFSalinasPCJonesKRNeurotrophin and Wnt signaling cooperatively regulate dendritic spine formationMol Cell Neurosci20135611512723639831
- MullenLMPakKKChavezEKondoKBrandYRyanAFRas/p38 and PI3K/Akt but not Mek/Erk signaling mediate BDNF-induced neurite formation on neonatal cochlear spiral ganglion explantsBrain Res20121430253422119396
- WHODepression Available from: http://www.who.int/mediacentre/factsheets/fs369/en/Accessed April 5, 2017
