Abstract
Aims
Paliperidone palmitate 3-month (PP3M) represents a new long-acting injectable antipsychotic therapeutic option. This review aims: 1) to summarize available data relating to efficacy, safety, tolerability and costs of PP3M; 2) to describe hospitalization rate, occupational status, treatment preference, satisfaction, adherence and caregiver burden of patients with schizophrenia who participate in PP3M clinical trials; 3) to examine ethical implications, pros and cons of PP3M use and 4) to propose study designs to further assess PP3M.
Methods
On August 21, 2017, a search on PubMed about PPM3, without any filter restriction, was conducted and all available records were analyzed. Records written in a language other than English were excluded.
Results
Twenty-two records were included in this review: 6 reviews, 1 report, 4 pharmacokinetic studies, 2 cost-effectiveness analyses, 1 open-label clinical trial, 2 randomized controlled trials (RCTs), 5 studies based on these 2 RCTs and 1 observational study.
Discussion
According to these last 9 studies, when compared with placebo, PP3M showed a longer time to relapse and good safety and tolerability profiles. Furthermore, when compared with paliperidone palmitate 1 month (PP1M), PP3M treatment showed: 1) non-inferiority in terms of efficacy, safety, tolerability, rate of hospitalization, symptomatic and functional remission, treatment preference and variations of the occupational status; 2) a longer time to relapse after treatment discontinuation and 3) a similar reduction of the caregiver burden.
Conclusion
PP3M is the only 3-monthly long-acting injectable antipsychotic available on the market. This makes it a unique option of treatment, which could be chosen both in early and advanced phases of illness. Nonetheless, longer naturalistic follow-up studies, two-arm head-to-head superiority trials and mirror studies, based on real-world samples of patients, are needed to further assess long-term safety and advantages of this new option of treatment and to define patients’ sub-populations that would most beneficiate from it.
Introduction
Schizophrenia is a pervasive psychotic chronic condition, present in all cultures and historical periods.Citation1 It is one of the top 20 causes of disability worldwide:Citation2 World Health Organization reported that schizophrenia is responsible for 1.1% of the total disability-adjusted life years and 2.8% of the years lived with disability.Citation3 It has an important impact on patients’ quality of life and mortality, on patients’ families and on social and financial costs:Citation1,Citation4,Citation5 in developed countries the disorder justifies 1.5%–3.0% of health care expenses.Citation6
Schizophrenia has a prevalence estimated at 0.6%–0.8% and its lifetime prevalence is about 1% worldwide.Citation1 Typically, it is preceded by prodromal symptoms leading to a first psychotic episode starting in young adulthood.Citation5,Citation7 Individuals with schizophrenia have a shorter life expectancy than the general population, an increased risk of physical illness, especially cardiovascular disease, as well as higher rates of suicide and accidental injury.Citation5,Citation7,Citation8 Episodes of partial or full remission broken by relapses characterize the long-term course of schizophrenia and difficulties in global functioning:Citation5,Citation9 most patients are unable to reach at least one milestone, such as being in a stable relationship, having full-time competitive employment or having self-supported independent living.Citation10 Relapse in schizophrenia can be destructive often resulting in hospitalization.Citation11–Citation13 In addition, relapse can be strictly connected with a biological risk: it has been hypothesized that active psychosis reflects a period of disease progression to the extent that patients may not come back to their previous level of functioning and can become resistant to treatment.Citation14,Citation15 The early phase of schizophrenia, including the first 2 years up to 5 years after the onset, is thought to be essential in determining long-term prognosis.Citation16 Therefore, a continued treatment from the early phases of disease may preserve from structural brain changes and progression toward functional deterioration.Citation17–Citation20
Poor adherence to treatment and long-acting injectable (LAI) atypical antipsychotics
Antipsychotic therapy is the mainstay of schizophrenia treatment, and severity and frequency of disease symptoms can be adequately managed by adhering to the prescribed antipsychotic medication.Citation5,Citation21,Citation22 The most frequent cause of relapse in schizophrenia is poor adherence to antipsychotic treatment.Citation23,Citation24 When patients discontinue their medications, even after the first episode, the risk of symptomatic relapse increases dramatically.Citation25 In clinical settings, poor compliance is common, especially in the early stages of the disease,Citation26–Citation29 with between 40% and 60% of patients with schizophrenia partially or totally noncompliant with oral antipsychotics.Citation30,Citation31 It has been highlighted that up to 74% of people affected by schizophrenia started to discontinue their medications after 18 months and that up to 42% of patients stopped their therapy within 1 year after the first psychotic episode.Citation32,Citation33 Studies using more strict measurement methodology, such as pill count, electronic monitoring and blood drug level, often indicate higher levels of non-adherence.Citation34–Citation37 Moreover, the duration of follow-up certainly has a clear influence on the observed frequencies of non-adherence. Therefore, it is reasonable to consider that adherence is much lower in routine care than in clinical trials.Citation38 Poor adherence has serious repercussions on the course of the disease in terms of relapses.Citation39,Citation40 Once illness recurrence occurs, the severity of symptoms rapidly returns to levels similar to the initial psychotic episode.Citation25 This leads to important consequences: inability to work and hospitalization in about 70% of the cases, attempt to suicide in about 20% of the patients, worsening of caregivers’ quality of life and higher health care-related and indirect costs.Citation39–Citation41 Furthermore, relapses can result in poorer long-term outcomes,Citation42 such as disease progression and emergent treatment refractoriness.Citation43,Citation44
LAI antipsychotics were developed in order to limit both hidden and overt non-adherence to antipsychotic drugs. LAIs has some known disadvantages such as pain on the injection site, lack of flexibility in dose adjustments and patients’ perception of stigma and coercion.Citation40 However, they have potential advantages such as complete tracking of the drug consumption and full adherence in early stages of the disease.Citation45,Citation46 In addition, LAIs do not need daily administration, which may be perceived by patients as a practical advantage, and minimize the risk of harmful drug use.Citation47,Citation48 It has also been suggested that pharmacokinetic (PK) differences associated with the route of administration may be a possible advantage for LAI over oral formulations. The higher bioavailability of LAI formulations may help identify the lower effective dose, reducing unnecessary toxic serum levels of the drug.Citation49 Furthermore, a reduced fluctuation of serum drug levels, and therefore a more stable receptor occupancy,Citation50 may reduce adverse events.Citation49,Citation51 Moreover, in case of sudden treatment interruption, plasmatic drug levels would decrease abruptly. On the contrary, after the discontinuance of the treatment, LAI antipsychotics assure a progressive decrease of the plasma drug levels. This difference might lower the risk of the so-called “super-sensitivity psychosis”, a severe disease relapse triggered by sudden antipsychotic withdrawal.Citation52 Furthermore, LAIs facilitate the regular contact between patients and physician and allow physicians to rule out non-adherence as a cause of relapse. At last, should a patient miss an injection, there remains some time to act to avert a crisis.Citation53
Among LAIs, second-generation long-acting antipsychotics (SGA-LAIs) combine the advantages of SGAs with a long-acting formulation.Citation10 The SGA-LAIs available on the market are risperidone LAI (RLAI), olanzapine LAI (OLAI), aripiprazole long-acting one month (AOM) and paliperidone palmitate (PP) long-acting 1 month (PP1M) and 3 month (PP3M). Scientific evidence about superiority of SGA-LAIs to SGA oral formulations is controversial. On one hand, randomized controlled trials (RCTs), considered to be the “gold standard” for clinical trial design, do not support the clinical viewpoint that LAI antipsychotics are generally superior to oral formulations in terms of effectiveness, safety and tolerability. On the other hand, observational studies (eg, cohort and mirror studies), that encompass the concept of effectiveness in a naturalistic-pragmatic setting (more representative of real-life clinical practice), show a greater benefit of LAIs over oral antipsychotics.Citation54–Citation57 RCTs have high internal validity and allow specific signal detection in carefully selected patient population. Moreover, randomization and blinding of the RCTs can adequately control confounding effects. However, RCTs are frequently affected by selection bias, so they have limited external validity and generalizability to a wider clinical patient population.Citation56 Observational naturalistic studies analyze real-world patients, limiting selection bias and improving generalizability and external validity. Also, they include pragmatic outcomes such as hospitalizations and all-cause treatment discontinuation. Nevertheless, this kind of study design can be confounded by factors that vary over time and, without a separate control group, this type of confounding cannot be controlled for.Citation56 Therefore, in order to answer questions of clinical efficacy and effectiveness of LAIs in the range of patients generally seen during routine clinical practice, both RCTs and naturalistic studies are required.Citation57 About this topic, a recent meta-analysis of head-to-head RCTs comparing the principal SGA-LAIs and their oral counterparts highlighted that high-quality evidence suggests that AOM may provide some small advantages compared with its oral preparation; moderate quality evidence showed that there is no clinical benefit for RLAI; evidence for OLAI was imprecise and therefore not able to rule out neither clinically meaningful superiority nor inferiority vs oral olanzapine; no evidence for PP was available in July 2016.Citation54 To achieve stronger evidence and recommendations about the use of SGA-LAIs, more head-to-head comparisons between drugs are expected,Citation57 such as the European Long-Acting Antipsychotics in Schizophrenia Trial (EULAST), which compares oral and LAI formulations of aripiprazole and paliperidone (NCT02146547). These investigations will provide data for further meta-analytic approaches regarding the relative usefulness of the different drugs and formulations available. Future research and experiences will also help to identify which clinical subpopulation may obtain greater benefit from these new formulations, not only in controlling symptoms but also in terms of cognitive performance, functioning, and quality of life.Citation58
Methods
On August 21, 2017, an electronic search on PubMed about PP3M, without any filter or MESH restriction, was performed, using the following search string: (“3 months” OR “three months” OR “3 monthly” OR “three monthly” OR “3-month” OR “three-month” OR “3-monthly” OR “three-monthly”) AND “paliperidone”. The query translation of PubMed search engine was as follows: (“3 months” [All Fields] OR “three months” [All Fields] OR “3 monthly” [All Fields] OR “three monthly” [All Fields] OR “3-month” [All Fields] OR “three-month” [All Fields] OR “3-monthly” [All Fields] OR “three-monthly” [All Fields]) AND “paliperidone” [All Fields].
This string was developed because, in scientific literature, PP3M is called in different ways. In particular, the words “three months” (3M) of PP3M have many different spellings listed in the proposed string. These spellings were connected by “OR” logical operator. This string guaranteed a high-sensitive search, limiting spelling selection of published works indexed in PubMed. Furthermore, an “AND” logical operator was used to connect these equivalent spellings of 3M with the word “paliperidone” of PP3M. This logical connective led to a high specific search that selected mostly works on topic.
All kinds of publications (ie, original contributions and reviews) were included. Publications must concern PP3M as principal issue. Publications written in a language other than English were excluded.
Results
The search described in the previous section provided 38 records. Among them, 16 were excluded: 13 because they did not concern PP3M and 3 because they were not written in English. Thus, this review included 22 records indexed on PubMed: 6 reviews, 1 report, 4 PK studies, 2 cost-effectiveness analyses, 1 open-label clinical trial (OCT), 2 double-blind (DB) RCTs, 5 studies based on these 2 RCTs and 1 observational study. The selection process and a schematic representation of the results are represented in the literature search flowchart ().
Figure 1 Literature search flowchart.
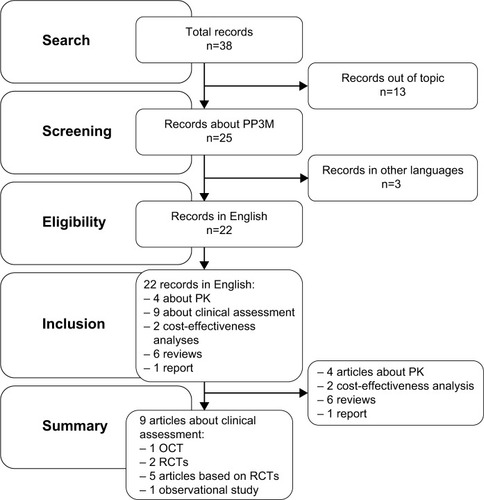
This review will describe the main findings relating to the formulation, pharmacodynamics, PK, safety, tolerability and efficacy of PP3M, followed by a brief presentation about practical issues encountered with this new formulation. In addition, an overview of possible advantages and disadvantages of PP3M compared with PP1M will be reported. In the final part of this review, PP3M place in therapy and proposals of study designs to further assess this LAI formulation will be discussed. The OCT, the 2 RCTs, the 5 articles based on the RCTs and the observational study are summarized in .
Table 1 Clinical studies about PP3M
The OCT was a Phase I, single-dose, randomized, open label study, conducted to investigate the PK, the safety and the tolerability of PP3M in patients with schizophrenia.Citation59 The first RCT compared PP3M with placebo in order to assess the efficacy and safety of PP3M.Citation60 The second RCT aimed to demonstrate non-inferiority of PP3M compared with PP1M in terms of relapse rates, changes in Positive and Negative Syndrome Scale (PANSS) scores, Clinical Global Impression-Severity (CGI-S) score and Personal and Social Performance (PSP) score.Citation61 Savitz et al,Citation62 Katz et al,Citation63 Chirila et al,Citation64 and Gopal et alCitation65 studied outcome measures within the samples or sub-samples of the 2 previously described RCTs. In details, Savitz et alCitation62 analyzed data derived from the previously mentioned non-inferiority RCT comparing PP3M with PP1M treatment; to investigate symptomatic and functional remission in the DB phase of this RCT, Katz et alCitation63 investigated native English-speaking trial participants’ and English-speaking investigators’ judgments about paliperidone formulations and adherence; Chirila et alCitation64 compared occupational status and health care resource use between treatment groups (PP3M vs placebo and PP3M vs PP1M), using data from the whole samples of the 2 RCTs and Gopal et alCitation65 evaluated caregiver burden in the 2 RCTs. Weiden et alCitation66 made a post-hoc analysis comparing median time to relapse across the treatment withdrawal arms of 3 different RCTs (Kramer et al,Citation67 Hough et al,Citation68 and Berwaerts et alCitation60), which compares the 3 formulations of paliperidone with placebo. Joshi et alCitation69 performed an observational retrospective cohort study, using pharmacy and medical claims data of the Symphony Health Solutions database from May 2014 to September 2017. This study described baseline characteristics and treatment patterns of patients with schizophrenia initiated on PP3M in a real-world setting in the USA.
Discussion
Overview of pharmacology, PKs of paliperidone palmitate 3-monthly injection
Formulation properties
PP3M contains a racemic mixture of the active ingredient paliperidone (9-OH risperidone), an atypical antipsychotic belonging to the chemical class of benzisoxazole derivatives, derived from risperidone.Citation70,Citation71 PP is the palmitate salt ester of paliperidone. It is very slightly soluble in polar solvent.Citation70,Citation71 Tiny drug crystals are created and dispersed in an aqueous suspension (NanoCrystal technology). These crystals are nanoparticles, usually defined as having a size between 1 and 1,000 nm. These tiny drug crystals are dispersed in an aqueous suspension (nanosuspensions), which is the LAI formulation of PP.Citation72 The PP3M formulation utilizes Nano-Crystal technology similar to the PP1M but with increased particle size, allowing an extended sustained release.Citation59 These nanoparticles dissolve slowly after intramuscular (IM) injection before being hydrolyzed to paliperidone by the esterases present in muscle tissue. Then paliperidone diffuses into the systemic circulation.Citation72,Citation73 PP3M is available in dose strengths of 273, 410, 546 and 819 mg paliperidone palmitate that undergo hydrolysis, resulting in dose strengths of 175, 263, 350 and 525 mg of paliperidone, respectively.Citation72
Pharmacodynamics
Therapeutic efficacy of paliperidone likely occurs through its antagonism of both central dopamine D2 and serotonin 5-HT2A receptors. Plasma concentrations of 10–17 ng/mL were estimated to correspond to 70%–80% occupancy of D2 receptors.Citation74 A D2 receptor occupancy of 65%–80% is generally considered optimal, with levels exceeding this conferring an increased risk of extrapyramidal symptoms.Citation75 Paliperidone is also an antagonist at α1- and α2-adrenergic receptors and H1 histaminergic receptors.Citation75,Citation76 This activity profile of paliperidone may explain some of the potential adverse effects of the drug, such as orthostatic hypotension and weight gain.Citation76 Unlike risperidone, paliperidone does not block beta adrenoceptors, muscarinic cholinoceptors or peptidergic receptors. This receptor profile, more selctive than the one of risperidone, could avoid further side effects.Citation70
Pharmacokinetics
Nanoparticles of PP3M dissolve slowly after IM injection; release starts as early as day 1 and lasts for up to 18 months.Citation72 The paliperidone plasma concentration time profiles for the corresponding PP1M and PP3M doses are similar. The principal differences concern the pre-dose plasma concentrations of PP3M: they are 21% lower than the concentrations observed following PP1M administration. Mean peak-to-trough ratios are higher following PP3M administration (range: 1.86–2.54) than PP1M administration (range: 1.30–1.63).Citation59 With regard to metabolism and elimination, information from the studies of oral paliperidone are reported: ~59% of a single dose of the drug is excreted unchanged in the urine; ~80% of the radioactivity related to a single oral dose of 14C paliperidone is recovered in the urine and 11% in the feces, indicating a lack of extensive hepatic metabolization.Citation70 Results obtained in in vitro studies suggest that CYP2D6 and CYP3A4 may be implicated in the metabolism of paliperidone, although no evidence of their role derive from in vivo studies: inhibitors or inducers of CYP2D6 and CYP3A4 do not significantly influence paliperidone plasma levels and no significant induction or inhibitory properties of paliperidone on the cytochrome system have been demonstrated.Citation59
Dosing and switching
PP3M injections should be started at a dose 3.5-fold multiple of the preceding dose of PP1M, and administered in either the deltoid or the gluteal muscle at the time scheduled for the next PP1M dose.Citation72 Available marketed doses are listed in .
Table 2 Conversion between PP1M and PP3M doses
Treatment with PP3M should only be initiated in patients who had adequately responded to and tolerated treatment with PP1M for at least 4 months.Citation72 Due to the slow release profile, treatment with PP3M is not indicated for use in acutely symptomatic patients or in patients who are transitioning from oral or other, non-PP1M, LAI antipsychotic therapy.Citation72 It is recommended that the last 2 PP1M doses prior to switching to PP3M are the same and patients are supposed to be clinically stable at the end of the PP1M dosing before transitioning to PP3M.Citation72 According to PK simulations, during maintenance therapy of PP3M, changes in plasma concentrations allow a flexible dosing window of 2 weeks for regularly scheduled 3-monthly injections ().Citation72
Table 3 Management of missed PP3M injections
PP3M injection procedure
One significant difference between the preparation of PP1M and PP3M is the force of shaking required to re-suspend the syringe contents. The PP3M syringe must be shaken vigorously for at least 15 seconds with a loose wrist before injecting the formulation to ensure a homogenous suspension. Improper shaking could result in clumping and inability to express the entire medication content from the syringe barrel.Citation72 Furthermore, a suitable syringe must be used: in the RCT comparing PP3M and PP1M, the use of a shorter syringe and the consequent lower dose administration of PP3M caused the exclusion of some patients from the study.Citation61,Citation62
Clinical efficacy, safety and tolerability of PP for schizophrenia
Efficacy and hospitalization rate
Efficacy of PP3M was evaluated against placeboCitation60 and in terms of non-inferiority against PP1MCitation61 in 2 different DB RCTs summarized in . The first study was stopped by an independent data monitoring committee for greater efficacy of PP3M compared to placebo: during the DB phase, 29% of patients in the placebo group experienced a relapse event against 9% in the group receiving PP3M.Citation60 The final data analysis included 305 patients (PP3M: n=160; placebo: n=145) and showed superiority of PP3M over placebo in delaying time to relapse of schizophrenia symptoms (P<0.001; hazard ratio=3.81; 95% CI, 2.08–6.99), a result confirmed by Cox proportional hazards models.Citation60 Furthermore, Weiden et al post-hoc studyCitation66 showed longer time to relapse after PP3M treatment discontinuation compared to that of oral paliperidone and PP1M: in particular, after suspension of the treatment, 50% of patients treated with PP3M remained relapse free for ~13 months, instead of 6 months with PP1M treatment withdrawal. Also, according to this study, the relapse risk (hazard ratio) was 2.08 higher for patients discontinuing PP1M than for those discontinuing PP3M.Citation66 However, as these data come from a descriptive post-hoc analysis and not a meta-analysis of the datasets of the three different RCTs (Kramer et al,Citation67 Hough et alCitation68 and Berwaerts et alCitation60), caution must be used when interpreting this study. Furthermore, this was not a head-to-head discontinuation RCT comparing oral paliperidone, PP1M and PP3M in the same trial; therefore, the evidence of this post-hoc analysis has strong study design and generalizability limitations.
The non-inferiority Phase III RCTCitation61 demonstrated that PP3M-treated group had relapse rates similar to PPM1-treated group, based on Kaplan–Meier estimates. Variations from DB baseline in Positive and Negative Symptom Scale (PANSS) total score and subscale scores, in Clinical CGI-S and in PSP scores were similar in the two experimental groups.Citation61 Furthermore, PP3M and PP1M treatments showed comparable symptomatic (defined according to Andreasen’s criteria on PANSS) and functional remissions (PSP >70) during the last 6 months of DB phase. In addition, most patients who achieved remission at DB baseline maintained their remission status throughout the DB phase.Citation62
Moreover, health care resource use was studied in the 2 described RCTsCitation60,Citation61 in terms of hospitalization odds.Citation64 The placebo group showed a higher rate of hospitalization for either psychiatric and social reasons or social reason alone compared to PP3M.Citation64 No difference in terms of hospitalizations was observed between PP3M and PP1M groups.Citation64
Safety and tolerability
Safety and tolerability were examined in the 2 previously described RCTs and in the Phase I OCT.Citation59 The latter one showed that headache and nasopharyngitis were the most common (>7%) treatment-emergent adverse events (TEAEs) and described a safety and tolerability profile similar to those of PP1M.Citation59 Compared with placebo, PP3M treatment demonstrated a similar proportion on TEAEs developed during the DB phase of the study: 62% of the subjects who were treated with PP3M injections and 58% of the subjects who received placebo injections had at least one TEAE. TEAEs noted more frequently in the group receiving PP3M than in the placebo group were headache (9% vs 4%), weight increase (9% vs 3%), nasopharyngitis (6% vs 1%) and akathisia (4% vs 1%).Citation60 In the non-inferiority Phase III RCT, safety and tolerability profiles of PP3M and PP1M were comparable over the whole DB phase of the study. Withdrawal rates due to TEAEs were low and comparable for both treatments.Citation61 Serious TEAEs were mostly of a psychiatric nature and similar between both groups.Citation61 Weight gain, nasopharyngitis and anxiety were the most common TEAEs in both groups.Citation61 Other TEAEs such as extrapyramidal symptoms, suicidality, agitation and aggression, somnolence and sedation, tachycardia, orthostatic hypotension, QTc interval prolongation, potentially prolactin-related and weight gain-related TEAEs had a similar frequency in both experimental groups.Citation61
Pregnancy
With its release during 18 months from the injection, PP3M makes more likely fetal exposition to paliperidone. However, few data about paliperidone effects in pregnancy are available in scientific literature. Two case reports of women in treatment with PP1M during pregnancy reported no congenital malformation and no perinatal complications of the newborns.Citation77,Citation78
Other features of PP3M and ethical implications
Quality of life, satisfaction and adherence
To our knowledge, no studies about quality of life, satisfaction and adherence of PP3M-treated patients with schizophrenia have been performed, yet. Longer follow-up studies should be carried out in order to compare switching from PP1M to PP3M treatment.
Caregiver burden
A study by Gopal et alCitation65 showed that switching from an oral antipsychotic to either PP1M or PP3M can significantly reduce caregiver burden. This mirror image study was performed on data derived from the non-inferiority Phase III RCT comparing PP1M and PP3M.Citation61 For this reason, both treatment groups received a monthly injection and caregiver burden reduction because diminished number of administrations was not detectable.Citation65 To our knowledge, no studies on this topic in the transition from PP1M to PP3M in a real-world setting are available.
Antipsychotic preference according to formulation
Katz et alCitation63 analyzed preferences between oral antipsychotic treatments, PP1M and PP3M with a survey performed by English native speaking and English-speaking physicians who participated in the 2 RCTsCitation60,Citation61 as previously described.
According to this study, patients and physicians preferred LAIs over oral antipsychotics and were willing to accept reduced efficacy in exchange for switching from an oral formulation to an LAI. Physicians showed a greater preference for 3-month over 1-month LAI.Citation63
Occupational status variation
Chirila et alCitation64 described the occupational status of patients who participated in the 2 RCTsCitation60,Citation61 as previously described. According to that study, no significant differences in terms of variation of the occupational status were observed between PP3M and placebo and PP3M and PP1M treatments, probably because of the short time of follow-up and consequently the small number of patients who changed their occupational status during the 2 trials.Citation64 Although not statistically significant, at the end of the follow-up period (from week 41 until week 53), improvement from baseline in occupational status was slightly higher in the PP3M than in the PP1M group.Citation64
Ethical implications
Both the RCT comparing PP3M with placeboCitation60 and the RCT comparing PP3M with PP1MCitation61 were conducted in compliance with the Declaration of Helsinki, consistent with Good Clinical Practices and applicable regulatory requirements and with the approval of independent ethical committees of the participant center. Nonetheless, some ethical critics were made against these studies. First of all, comparing a new drug with placebo might be considered ethically debatable and clinically irrelevant when effective agents are available.Citation79 In this particular case, the European Medicines Agency (EMA) pointed out the relevance of placebo-controlled studies when assessing the efficacy of new LAIs, arguing that recent schizophrenia trials showed only minimal differences between active treatments and placebo, and therefore an assessment of the absolute effect is required to establish efficacy.Citation80 With reference to the study comparing PP3M and PP1M, it has been pointed out that, as there is no validated and shared method of choice of the non-inferiority margin, the demonstration of non-inferiority leaves uncertainty on whether the 2 drugs are really equivalent and does not establish whether the new drug tested is associated with additional benefits over the control.Citation79
Advantages and disadvantages of PP3M compared with PP1M
Advantages and disadvantages of PP3M, compared with PP1M treatment, are summarized in .
Figure 2 Comparison between PP3M and PP1M pros and cons.
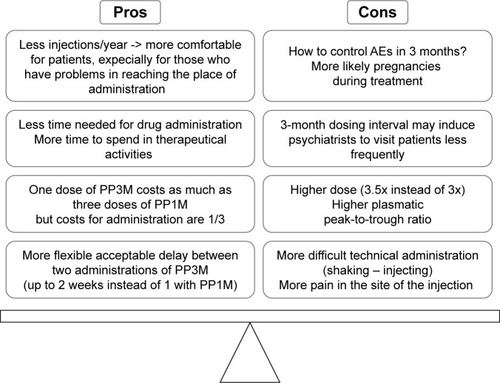
One of the most positive features of PP3M is notable that patients need to receive an injection just 4 times per year. The lower number of injections is more comfortable for patients, especially for those patients who cannot easily reach the place of administration. Furthermore, less time is needed for drug administration so that saved time and resources can be available for other therapeutic activities, for example, rehabilitation. At the same time, psychiatric services with poor human resources should control the risk to visit the patient exclusively 4 times a year on the day of PP3M administration and not according to the health needs of the patient. In terms of costs, three doses of PP1M cost as much as the equivalent dose of 1 PP3M injection, but the administration costs are reduced to one-third. Moreover, 2 economic studies evaluated cost-utility and cost-effectiveness simulations on the use of PP3M in Spain and in the Netherlands.Citation81,Citation82 According to these studies, PP3M resulted to be cost-effective for treating chronic schizophrenia and dominated PP1M in all analyses.Citation81,Citation82
Finally, PP3M administration protocol admits more flexible delays in PP3M injections interval compared with PP1M. In fact, according to PP3M administration regimen, 2 weeks of delays after 3 planned months of inter-injections interval can be tolerated instead of 1 week with PP1M.
The main disadvantages of PP3M can be summarized as follows. First of all, despite the tolerance demonstrated by patients during the PP1M phase of treatment, adverse events related to paliperidone therapy might appear during PP3M treatment. The control of these side effects, especially if starting soon after a PP3M administration, could be a clinical challenge for psychiatrists but, above all, a real health problem for patients. In addition, a 3-month dosing interval may induce psychiatrists to visit patient less frequently, for example only in concomitance with PP3M administration. Even if PK studies demonstrated a similar exposure to paliperidone with PP3M and PP1M, a 3.5-fold higher dose of PP3M is equivalent to a single dose of PP1M. In other words, a larger quantity of PP is injected in patients treated with PP3M. These data, in addition to the known higher plasmatic mean peak-to-trough ratio of PP3M, could lead to long-term adverse effects, somewhat different from those of PP1M.
PP3M injections need more technical carefulness to be correctly administered compared with PP1M procedure. In fact, a longer period of shaking and a longer injecting procedure are required. Finally, the larger volume administered with PP3M could be more painful in the injected muscle tissue.
Possible study designs to further assess PP3M
Further studies are needed to asses PP3M features more clearly. Some proposals of study design are described in .
Figure 3 Some possible study designs needed to further assess PP3M.
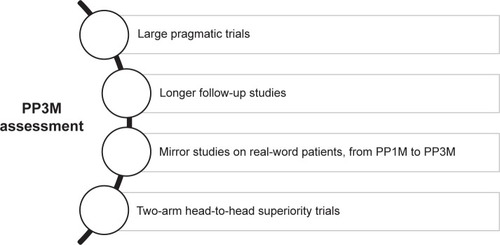
To our knowledge, the only observational study on PP3M is a retrospective cohort study that chose the date of the first approved claim for PP3M as the index date.Citation69 According to its experimental design, this study does not inform about the effect of PP3M treatment. In this scenery, large pragmatic trials and mirror studies on real-world patients switching from PP1M to PP3M would be useful in defining advantages and disadvantages of PP3M confronted with PP1M. Furthermore, 2-arm head-to-head superiority trials could directly compare PP3M with PP1M and with other SGA-LAIs. Regardless of the study design adopted, longer follow-ups are needed to study PP3M treatment safety and impact on patients’ adherence, functioning, quality of life and satisfaction. Finally, further studies are necessary to determine which subgroups of patients would most beneficiate from PP3M treatment.
Conclusion
In conclusion, PP3M is the only 3-month LAI antipsychotic available on the market. This makes it a unique option of treatment, which can be chosen both in early and advanced phases of illness. Nonetheless, further studies are needed to assess long-term safety, impact on quality of life and on functioning and to define patients’ sub-populations that would most beneficiate from this new option of treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
- McGrathJSahaSChantDWelhamJSchizophrenia: a concise overview of incidence, prevalence, and mortalityEpidemiol Rev2008301677618480098
- VosTFlaxmanADNaghaviMYears lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet2013380985921632196
- MurrayCJLopezADJamisonDTThe global burden of disease in 1990: summary results, sensitivity analysis and future directionsBull World Health Org19947234955098062404
- CarràGCazzulloCLClericiMThe association between expressed emotion, illness severity and subjective burden of care in relatives of patients with schizophrenia. Findings from an Italian populationBMC Psychiatry201212114022974195
- National Collaborating Centre for Mental Health UKPsychosis and Schizophrenia in Adults: Treatment and Management: Updated Edition 2014LondonNational Institute for Health and Care Excellence (UK) NICE guidelines updated in 2014
- KnappMMangaloreRSimonJThe global costs of schizophreniaSchizophr Bull200430227929315279046
- HowesODMurrayRMSchizophrenia: an integrated sociodevelopmental-cognitive modelLancet201438399291677168724315522
- SahaSChantDMcGrathJA systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time?Arch Gen Psychiatry200764101123113117909124
- WiersmaDWanderlingJDragomireckaESocial disability in schizophrenia: its development and prediction over 15 years in incidence cohorts in six European centresPsychol Med200030051155116712027051
- MontemagniCFrieriTRoccaPSecond-generation long-acting injectable antipsychotics in schizophrenia: patient functioning and quality of lifeNeuropsychiat Dis Treat201612917929
- AlmondSKnappMFrancoisCToumiMBrughaTRelapse in schizophrenia: costs, clinical outcomes and quality of lifeBr J Psychiatry2004184434635115056580
- Ascher-SvanumHZhuBFariesDEThe cost of relapse and the predictors of relapse in the treatment of schizophreniaBMC Psychiatry2010101220059765
- WeidenPJOlfsonMCost of relapse in schizophreniaSchizophr Bull19952134194297481573
- WyattRJResearch in schizophrenia and the discontinuation of antipsychotic medicationsSchizophr Bull1997231399050108
- LiebermanJAAlvirJMKoreenAPsychobiologic correlates of treatment response in schizophreniaNeuropsychopharmacology199614Suppl 313S21S8866739
- BirchwoodMToddPJacksonCEarly intervention in psychosis. The critical period hypothesisBr J Psychiatry Suppl19981723353599764127
- McEvoyJPThe importance of early treatment of schizophreniaBehav Healthc20072744043
- MurrayRMQuattroneDNatesanSShould psychiatrists be more cautious about the long-term prophylactic use of antipsychotics?Br J Psychiatry2016209536136527802977
- PantelisCYücelMWoodSJMcGorryPDVelakoulisDEarly and late neurodevelopmental disturbances in schizophrenia and their functional consequencesAust N Z J Psychiatry200337439940612873323
- PerkinsDOGuHBotevaKLiebermanJARelationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysisAm J Psychiatry2005162101785180416199825
- HasanAFalkaiPWobrockTWorld Federation of Societies of Biological Psychiatry (WFSBP) Task Force on Treatment Guidelines for SchizophreniaWorld federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistanceWorld J Biol Psychiatry201213531837822834451
- HasanAFalkaiPWobrockTWFSBP Task force on Treatment Guidelines for SchizophreniaWorld federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effectsWorld J Biol Psychiatry201314124423216388
- LindenmayerJPLiu-SeifertHKulkarniPMMedication nonadherence and treatment outcome in patients with schizophrenia or schizoaffective disorder with suboptimal prior responseJ Clin Psychiatry200970799099619497244
- SuzukiTUchidaHTakeuchiHTsuboiTHiranoJMimuraMA review on schizophrenia and relapse a quest for user-friendly psychopharmacotherapyHum Psychopharmacol201429541442625055792
- EmsleyRChilizaBAsmalLHarveyBHThe nature of relapse in schizophreniaBMC Psychiatry20131315023394123
- ColdhamELAddingtonJAddingtonDMedication adherence of individuals with a first episode of psychosisActa Psychiatr Scand2002106428629012225495
- TiihonenJHaukkaJTaylorMHaddadPMPatelMXKorhonenPA nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophreniaAm J Psychiatry2011168660360921362741
- MillerBJA review of second-generation antipsychotic discontinuation in first-episode psychosisJ Psychiatr Pract200814528930018832960
- MillerBJBodenheimerCCrittendenKSecond-generation antipsychotic discontinuation in first episode psychosis: an updated reviewClin Psychopharmacol Neurosci201192455323429653
- NovickDHaroJMSuarezDPerezVDittmannRWHaddadPMPredictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophreniaPsychiatry Res20101762–310911320185182
- VelliganDILamFEreshefskyLMillerALPsychopharmacology: perspectives on medication adherence and atypical antipsychotic medicationsPsychiatr Serv200354566566712719495
- LiebermanJAStroupTSMcEvoyJPClinical Antipsychotic Trials of Intervention Effectiveness (CATIE) InvestigatorsEffectiveness of antipsychotic drugs in patients with chronic schizophreniaN Engl J Med2005353121209122316172203
- KahnRSFleischhackerWWBoterHEUFEST study groupEffectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trialLancet200837196181085109718374841
- Di MatteoMRVariations in patients’ adherence to medical recommendations: a quantitative review of 50 years of researchMed Care200442320020915076819
- JónsdóttirHOpjordsmoenSBirkenaesABMedication adherence in outpatients with severe mental disorders: relation between self-reports and serum levelJ Clin Psychopharmacol201030216917520520290
- DassaDBoyerLBenoitMBourcetSRaymondetPBottaiTFactors associated with medication non-adherence in patients suffering from schizophrenia: a cross-sectional study in a universal coverage healthcare systemAust N Z J Psychiatry2010441092192820932206
- VelliganDIWangMDiamondPRelationships among subjective and objective measures of adherence to oral antipsychotic medicationsPsychiatr Serv20075891187119217766564
- KaneJMKishimotoTCorrellCUNon-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategiesWorld Psychiatry201312321622624096780
- HaddadPMBrainCScottJNonadherence with antipsychotic medication in schizophrenia: challenges and management strategiesPatient Relat Outcome Meas20145436225061342
- BrissosSVeguillaMRTaylorDBalanzá-MartinezVThe role of long-acting injectable antipsychotics in schizophrenia: a critical appraisalTher Adv Psychopharmacol20144519821925360245
- LauberCEichenbergerALuginbühlPKellerCRösslerWDeterminants of burden in caregivers of patients with exacerbating schizophreniaEur Psychiatry200318628528914611923
- LeuchtSTardyMKomossaKAntipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysisLancet201237998312063207122560607
- WiersmaDNienhuisFJSlooffCJGielRNatural course of schizophrenic disorders: a 15-year followup of a Dutch incidence cohortSchizophr Bull199824175859502547
- EmsleyROosthuizenPKoenLNiehausDMartinezLComparison of treatment response in second-episode versus first-episode schizophreniaJ Clin Psychopharmacol2013331808323277247
- LlorcaPMAbbarMCourtetPGuillaumeSLancrenonSSamalinLGuidelines for the use and management of long-acting injectable antipsychotics in serious mental illnessBMC Psychiatry20131334024359031
- TiihonenJHaukkaJTaylorMHaddadPMPatelMXKorhonenPA nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophreniaAm J Psychiatry2011168660360921362741
- PatelMXDavidASWhy aren’t depot antipsychotics prescribed more often and what can be done about it?Adv Psychiatr Treat2005113203211
- TaylorMNgKYShould long-acting (depot) antipsychotics be used in early schizophrenia? A systematic reviewAust N Z J Psychiatry201347762463023209308
- EreshefskyLMascarenasCAComparison of the effects of different routes of antipsychotic administration on pharmacokinetics and pharmacodynamicsJ Clin Psychiatry200364161823
- GefvertOErikssonBPerssonPPharmacokinetics and D2 receptor occupancy of long-acting injectable risperidone (Risperdal Consta™) in patients with schizophreniaInt J Neuropsychopharmacol200581273615710053
- WhitworthABFleischhackerWWAdverse effects of antipsychotic drugsCurr Opin Psychiatry1994717175
- MoncrieffJDoes antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapseActa Psychiatr Scand2006114131316774655
- KaneJMGarcia-RiberaCClinical guideline recommendations for antipsychotic long-acting injectionsBr J Psychiatry Suppl200952S63S6719880920
- OstuzziGBighelliISoRFurukawaTABarbuiCDoes formulation matter? A systematic review and meta-analysis of oral versus long-acting antipsychotic studiesSchizophr Res2016183102127866695
- BiagiECapuzziEColmegnaFLong-acting injectable antipsychotics in schizophrenia: literature review and practical perspective, with a focus on aripiprazole once-monthlyAdv Ther20173451036104828382557
- CorrellCUKishimotoTKaneJMRandomized controlled trials in schizophrenia: opportunities, limitations, and trial design alternativesDialogues Clin Neurosci201113215517221842613
- FagioliniARoccaPDe GiorgiSSpinaEAmodeoGAmoreMClinical trial methodology to assess the efficacy/effectiveness of long-acting antipsychotics: Randomized controlled trials vs naturalistic studiesPsychiatry Res201724725726427936437
- BernardoMBioqueMThree-month paliperidone palmitate-a new treatment option for schizophreniaExpert Rev Clin Pharmacol20169789990427206330
- RavenstijnPRemmerieBSavitzAPharmacokinetics, safety, and tolerability of paliperidone palmitate 3-month formulation in patients with schizophrenia: a phase-1, single-dose, randomized, open-label studyJ Clin Pharmacol201656333033926189570
- BerwaertsJLiuYGopalSEfficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trialJAMA Psychiatry201572883083925820612
- SavitzAJXuHGopalSEfficacy and safety of paliperidone palmitate 3-month formulation for patients with schizophrenia: a randomized, multicenter, double-blind, noninferiority studyInt J Neuropsychopharmacol2016197Pyw01826902950
- SavitzAJXuHGopalSNuamahIHoughDMathewsMPaliperidone palmitate 3-month treatment results in symptomatic remission in patients with schizophrenia: a randomized, multicenter, double-blind, and non-inferiority studyIntern Clin Psychopharmacol2017326329336
- KatzEGHauberBGopalSPhysician and patient benefit–risk preferences from two randomized long-acting injectable antipsychotic trialsPatient Prefer Adherence2016102127213927799749
- ChirilaCNuamahIWoodruffKHealth care resource use analysis of paliperidone palmitate 3 month injection from two phase 3 clinical trialsCurr Med Res Opin20173361083109028277864
- GopalSXuHMcQuarrieKCaregiver burden in schizophrenia following paliperidone palmitate long acting injectables treatment: pooled analysis of two double-blind randomized phase three studiesNPJ Schizophr2017312328751663
- WeidenPJKimEBermakJTurkozIGopalSBerwaertsJDoes half-life matter after antipsychotic discontinuation? A relapse comparison in schizophrenia with 3 different formulations of paliperidoneJ Clin Psychiatry2017787e813e82028640988
- KramerMSimpsonGMaciulisVPaliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomized, double-blind, placebo-controlled studyJ Clin Psychopharmacol200727161417224706
- HoughDGopalSVijapurkarULimPMorozovaMEerdekensMPaliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled studySchizophr Res20101162–310711719959339
- JoshiKLafeuilleMHBrownBBaseline characteristics and treatment patterns of patients with schizophrenia initiated on once-every-three-months paliperidone palmitate in a real-world settingCurr Med Res Opin201733101763177228741387
- European Medicines AgencyTrevicta (paliperidone palmitate) prolonged release suspension for injection: EU summary of product characteristics2016 Available from: http://www.ema.europa.eu/Accessed April 30, 2017
- Janssen Pharmaceuticals IncInvega Trinza (paliperidone palmitate) extended-release injectable suspension, for intramuscular use: US prescribing information2016 Available from: http://www.janssencns.com/Accessed April 30, 2017
- GopalSVermeulenANandyPPractical guidance for dosing and switching from paliperidone palmitate 1 monthly to 3 monthly formulation in schizophreniaCurr Med Res Opin201531112043205426306819
- GildayENasrallahHAClinical pharmacology of paliperidone palmitate a parenteral long-acting formulation for the treatment of schizophreniaRev Recent Clin Trials2012712922023179
- KarlssonPHargarterLDenckerEPharmacokinetics and dopamine d2 and serotonin 5-HT2A receptor occupancy of paliperidone in healthy subjects: two open-label, single-dose studies (PAL-115)Pharmacopsychiatry2007405A106
- RemingtonGMamoDLabelleAA PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidoneAm J Psychiatry2006163339640116513859
- NasrallahHAAtypical antipsychotic-induced metabolite side effects: insights from receptor-binding profilesMol Psychiatry2008131273517848919
- RodríguezFZVegaCBHernándezMSMacíasJGLealFVUse of paliperidone palmitate throughout a schizoaffective disorder patient’s gestation periodPharmacopsychiatry2017501384027414740
- ÖzdemirAKPakŞCCananFGeçiciÖKuloğluMGücerMKPaliperidone palmitate use in pregnancy in a woman with schizophreniaArch Womens Ment Health201518573974025599999
- OstuzziGPapolaDGastaldonCBarbuiCNew EMA report on paliperidone 3-month injections: taking clinical and policy decisions without an adequate evidence baseEpidemiol Psychiatr Sci201726323123328004623
- European Medicines Agency, Committee for Medicinal Products for Human Use (2012)Guideline on Clinical Investigation of Medicinal Products, Including Depot Preparations in the Treatment of SchizophreniaEMA/CHMP/40072/2010 Rev. 12012 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/10/WC500133437.pdfAccessed April 30, 2017
- EinarsonTRBerezaBGGarcia LlinaresIGonzález Martín MoroBTedouriFVan ImpeKCost-effectiveness of 3-month paliperidone treatment for chronic schizophrenia in SpainJ Med Econ201720101039104728678566
- EinarsonTRBerezaBGTedouriFVan ImpeKDeneeTRDriesPJCost-effectiveness of 3-month paliperidone therapy for chronic schizophrenia in the NetherlandsJ Med Econ201720111187119928762843
- European Medicine AgencySummary of product characteristics Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004066/WC500180640.pdfAccessed October 30, 2017
