Abstract
Objectives
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder with symptoms of abnormal defecation and abdominal discomfort. Psychological factors are well known to be involved in onset and exacerbation of IBS. A few studies have reported effectiveness of traditional herbal (Kampo) medicines in IBS treatment. Yokukansan (YKS) has been shown to have anti-stress and anxiolytic effects. We investigated the effect of YKS on defecation induced by stress and involvement of oxytocin (OT), a peptide hormone produced by the hypothalamus, in order to elucidate the mechanism of YKS action.
Methods and results
Male Wistar rats were divided into four groups; control, YKS (300 mg/kg PO)-treated non-stress (YKS), acute stress (Stress), and YKS (300 mg/kg PO)-treated acute stress (Stress+YKS) groups. Rats in the Stress and Stress+YKS groups were exposed to a 15-min psychological stress procedure involving novel environmental stress. Levels of plasma OT in the YKS group were significantly higher compared with those in the Control group (P < 0.05), and OT levels in the Stress+YKS group were remarkably higher than those in the other groups (P < 0.01). Next, rats were divided into four groups; Stress, Stress+YKS, Atosiban (OT receptor antagonist; 1 mg/kg IP)-treated Stress+YKS (Stress+YKS+B), and OT (0.04 mg/kg IP)-treated acute stress (Stress+OT) groups. Rats were exposed to acute stress as in the previous experiment, and defecation during the stress load was measured. Administration of YKS or OT significantly inhibited defecation; however, administration of Atosiban partially abolished the inhibitory effect of YKS. Finally, direct action of YKS on motility of isolated colon was assessed. YKS (1 mg/mL, 5 mg/mL) did not inhibit spontaneous contraction.
Conclusion
These results suggested that YKS influences stress-induced defecation and that increased OT secretion may be a mechanism underlying this phenomenon.
Introduction
Irritable Bowel Syndrome (IBS) is a disorder with an increasing number of cases in recent years. The prevalence rate in Japan is 10%–15% and the number of patients is estimated to be 12 million.Citation1,Citation2 IBS is a functional gastrointestinal disorder characterized by the presence of recurrent abdominal pain with abnormalities in stool frequency and form. No physical or biochemical abnormalities underlying the symptoms have been found. IBS is thought to be caused by diverse etiologies, and psychological factors are often related to its onset and deterioration.Citation3,Citation4 Psychological abnormalities affect intestinal motility through the central nervous system, for example, colon movement is accelerated by negative emotions such as anxiety, fear, and stress.Citation5,Citation6 Intestinal function and central nervous system are known to be closely related, referred to as “brain-gut interaction”. This interaction is suggested to be influenced by the hypothalamic–pituitary–adrenal (HPA) axis, the autonomous nervous system, and the immune system.Citation6,Citation7
IBS patients are categorized into subgroups including IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), IBS with mixed diarrhea and constipation (IBS-M), and unclassified IBS. Drug therapy used for IBS-D primarily includes serotonin-3 (5-HT)3 receptor antagonists, anticholinergic drugs, antidiarrheal drugs, and selective serotonin reuptake inhibitors.Citation2,Citation8 Recently, effective examples of traditional herbal medicines (Kampo medicines) such as Keishikashakuyakuto and Rikkunshito have been reported.Citation9
Yokukansan (YKS) is a Kampo medicinal formula composed of seven medicinal herbs ().Citation10 YKS has been used for the treatment of emotional irritability, neurosis, insomnia, and night terrors and temper tantrums in children.Citation10 In clinical practice, YKS has been used in behavioral and psychological symptoms of dementia,Citation11,Citation12 pain disorders,Citation13 and anxiety disorders.Citation14,Citation15 The anti-stressCitation16,Citation17 and anxiolytic effectsCitation18,Citation19 of YKS have been reported in basic research studies. In a clinical report, SempukuCitation20 reported a case wherein Yokukansankachinpihange, which includes the nine ingredients found in YKS, was effective in treating IBS. Therefore, we expected that YKS may also be effective in treating IBS patients affected psychologically.
Table 1 Component galenicals of YKS
It has been recently reported that oxytocin (OT) is involved in the control of intestinal motility.Citation21–Citation23 OT is a neuropeptide produced in the hypothalamus, acting centrally via nerve axons and systemically via blood circulation. Studies in animals have demonstrated that intracerebroventricular administration of OT inhibited production and biological activity of corticotropin releasing factor (CRF) in the paraventricular nucleus of the hypothalamus following acute or chronic stress, and controlled gastrointestinal motility induced by stress exposure.Citation24–Citation26 Moreover, OT and OT-receptor mRNA are expressed throughout the gastrointestinal tract,Citation27 and OT has been shown to inhibit colonCitation28,Citation29 and stomachCitation30 motility in vitro. It has also been reported that intraperitoneal administration (IP) of OT inhibited stress-induced colonic contraction in rats.Citation25 In the current study, the effects of YKS on increased intestinal motility induced by stress, and involvement of OT in YKS action were evaluated.
Materials and methods
Animals
Male Wistar rats (7–8 weeks old), purchased from Nippon Bio-Supp. Center (Tokyo, Japan), were used. During the experimental period, the animals were housed in standard plastic cages in our animal facilities at 25°C ± 2°C with 55% ± 5% humidity under a light/dark cycle of 12 h/12 h. Food (CLEA Japan, CE-2, Tokyo, Japan) and water were provided ad libitum. Experiments were performed as per the guidelines of the Committee of Animal Care and Welfare of Showa University. All experimental procedures were approved by the Committee of Animal Care and Welfare of Showa University (certificate number: 07061).
Drugs
Dry powdered extracts of YKS (Lot No 2110054010) used in the present study were supplied by Tsumura & Co. (Tokyo, Japan). The seven herbs comprising YKS () were mixed and extracted with purified water at 95.1°C for 1 h, a soluble extract was separated from insoluble waste and concentrated by removing water under reduced pressure. YKS was dissolved in distilled water and orally administered. OT (Peptide Institute, Osaka, Japan) and Atosiban, an OT-receptor antagonist (Sigma-Aldrich, St Louis, MO, USA), were dissolved in saline and intraperitoneally administered.
Influence of YKS and acute stress on OT secretion
To evaluate effects of YKS and acute stress on OT secretion, 28 rats were randomly divided into four groups (seven per group) as follows: (1) Control group; (2) YKS-treated non-stress (YKS) group; (3) acute stress (Stress) group, and (4) YKS-treated acute stress (Stress+YKS) group. YKS (300 mg/kg/day) was administered daily for 3 days to the YKS and Stress+YKS groups, and water was administered orally to the Control and Stress groups. In preliminary experiments, single administration of YKS did not show inhibitory effects on increased defecation (data not shown), and YKS was therefore pre-administered for 3 days. YKS dose was chosen based on the results of published studies.Citation17,Citation31 It has been reported that OT is involved in stress responses.Citation32,Citation33 In our study, we investigated the influence of acute psychological stress on OT secretion. On day 4, 1 h after administration of YKS or water, rats in the Stress and the Stress+YKS groups were exposed to 15 min of an acute stress procedure, involving novel environmental stress.Citation34,Citation35 Each rat was transferred from group-housed cages and placed in an opaque box (60 × 60 × 60 cm3) individually for 15 min. Next, all rats were anesthetized with intraperitoneal pentobarbital sodium (50 mg/kg; Kyoritsu Seiyaku, Somnopentyl, Tokyo, Japan), and blood samples were obtained from the inferior vena cava. To avoid influences of fluctuation in routine, all blood sampling was performed between 13:00 h and 15:00 h. Blood samples were centrifuged at 4°C and 3,000 rpm for 10 min, and supernatants collected. The plasma was stored at −80°C until measurements. Acetonitrile was used for the precipitation of plasma proteins. The thawed plasma was mixed with acetonitrile (Wako Pure Chemical Industries, Osaka, Japan) and centrifuged. Subsequently, the supernatant was evaporated and reconstituted with the assay buffer. Plasma OT level was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Enzo Life Sciences, Farmingdale, NY, USA), according to the manufacturer’s instructions.
Intraperitoneal administration of OT
To determine the dose of OT to be used in subsequent experiments, OT was intraperitoneally administered to rats and plasma OT concentrations were measured. Doses of OT were 0.02 mg/kg (OT0.02; n = 6), 0.04 mg/kg (OT0.04; n = 6), and 0.20 mg/kg (OT0.20; n = 6). These doses were determined by referring to a previous study.Citation24 It was reported that intra-peritoneal administration of OT (500 pmol per rat) inhibited water-avoidance stress-induced colonic contraction in rats. Control rats (n = 6) were intraperitoneally administered with saline. One hour after administration, plasma OT concentrations were measured using the method described above.
Acute stress-induced defecation
It is known that defecation in rats is increased by an acute stress load, and this model is used for studies on IBS-D.Citation36 Thirty-six rats were randomly divided into four groups (nine per group) as follows: (1) acute stress (Stress); (2) YKS-treated acute stress (Stress+YKS); (3) Atosiban-treated Stress+YKS (Stress+YKS+B); and (4) OT-treated acute stress (Stress+OT). YKS (300 mg/kg/day) or water was orally administered daily for 3 days. On day 4, rats in the Stress+OT group were administered OT (0.04 mg/kg, IP) 1 h before the stress load, and those in the Stress+YKS+B group were administered Atosiban (1 mg/kg, IP) 10 min before YKS administration. In the Stress and Stress+YKS groups, saline was administered instead of Atosiban or OT (). One hour after administration of YKS or water, rats were exposed to acute stress, and defecation during the stress load was measured using a balance. Atosiban dose was determined based on a previous study.Citation37
Table 2 Drugs administered in the study of stress-induced defecation
Influence of YKS and acute stress on corticosterone secretion
Levels of plasma corticosterone, which reflects activation of the HPA axis, were measured.Citation38 Twenty-four rats were randomly divided into four groups (six per group) as follows: (1) Control; (2) acute stress (Stress); (3) YKS-treated acute stress (Stress+YKS); and (4) OT-treated acute stress (Stress+OT). Drug administration, stress load, and blood sampling were conducted as described above. Corticosterone levels were measured using an ELISA kit (Enzo Life Sciences).
Recording of colon contraction
We investigated whether the inhibitory effect of YKS on increased defecation was mediated via direct action on the bowel. Segments of the distal colon were isolated from five intact rats and were longitudinally cut into 15 × 5 mm2 strips, and then transferred to a 20-mL organ bath. The organ baths contained aerated (5% CO2, 95% O2) Kreb’s solution (composed of NaCl 6.9 g/L, KCl 0.36 g/L, KH2PO4 0.16 g/L, MgSO4 0.14 g/L, CaCl2 0.28 g/L, NaHCO3 2.1 g/L, and glucose 1.8 g/L) maintained at 37°C. The colon segments were suspended in Kreb’s solution with an initial tension of 2.0 g for recording. Then, YKS dissolved in Kreb’s solution was perfused into the bath at 5 mL/min after stable colonic contractions were established. The YKS concentrations used were 1 mg/mL and 5 mg/mL based on previous reports.Citation39 Colon contractions were recorded with a force displacement transducer (FD Pickup TB-611T; Nihon Koden, Tokyo, Japan) and a PowerLab data acquisition system (ADInstruments, Dunedin, New Zealand).
Statistical analysis
All experimental data are presented as mean ± standard error of mean (SEM). Statistical significance of differences between groups was evaluated using paired t-test for comparisons between two groups, and one-way analysis of variance (ANOVA) for comparisons among >2 groups. Post hoc comparisons between the four groups were performed using the Tukey’s post hoc test. All P-values < 0.05 were considered to be statistically significant.
Results
Influence of YKS and acute stress on OT secretion
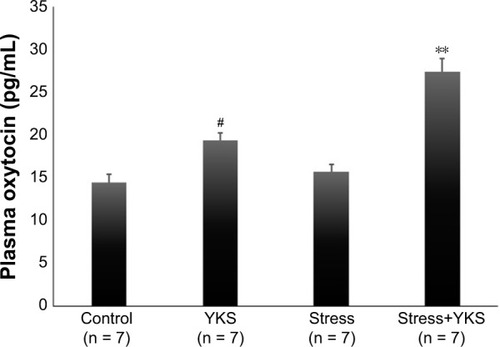
The influence of YKS and acute psychological stress on OT secretion was investigated. Administration of 300 mg/kg/day YKS significantly increased plasma OT levels compared with those in the control group (Control, 14.47 ± 0.96 pg/mL; YKS, 19.43 ± 0.86 pg/mL; P < 0.05). No significant difference was observed between the Control and Stress groups (Stress, 15.74 ± 0.83 pg/mL); however, OT level in the Stress+YKS group was markedly increased compared with the other groups (Stress+YKS, 27.47 ± 1.47 pg/mL; P < 0.01) ().
Figure 1 Plasma oxytocin levels (pg/mL) following administration of YKS and acute stress load.
Abbreviations: ANOVA, analysis of variance; SEM, standard error of mean; YKS, Yokukansan.
Intraperitoneal administration of OT
Plasma OT concentrations were measured 1 h after intraperitoneal administration of OT (0, 0.02, 0.04, and 0.20 mg/kg). The results are shown in . The plasma OT level in the OT (0.04) group was almost identical to that in the Stress+YKS group (27.47 ± 1.47 pg/mL; ); therefore, OT dose in subsequent experiments was set to 0.04 mg/kg.
Table 3 Plasma OT concentrations
Acute stress-induced defecation
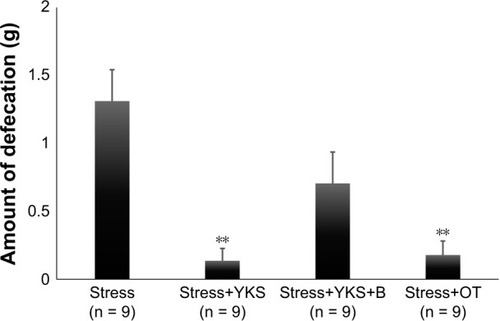
Defecation during the 15-min stress load was measured by weighing. Acute stress load-induced defecation (1.31 ± 0.23 g), and defecation in the absence of stress were significantly suppressed by pre-administrations of YKS (0.14 ± 0.09 g) and OT (0.18 ± 0.11 g) (P < 0.01). However, administration of Atosiban decreased the effect of YKS (0.71 ± 0.23 g) ().
Figure 2 Amount of defecation (g) following the acute stress load.
Abbreviations: ANOVA, analysis of variance; B, blocker of oxytocin receptor; OT, oxytocin; SEM, standard error of mean; YKS, Yokukansan.
Influence of YKS and acute stress on corticosterone secretion
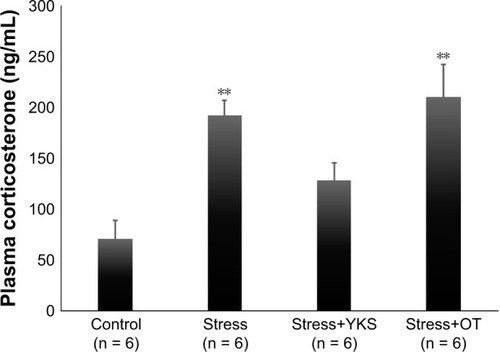
Influence of YKS and acute psychological stress on plasma corticosterone levels were also investigated. Plasma corticosterone level was significantly increased in the Stress and Stress-OT groups compared with that in the Control group (Control, 71.08 ± 17.43 ng/mL; Stress, 192.61 ± 14.72 ng/mL; Stress+OT, 210.33 ± 32.40 ng/mL; P < 0.01); however, there was no significant difference between the Control and Stress+YKS groups (128.51 ± 17.06 ng/mL) (P = 0.266) ().
Figure 3 Plasma corticosterone levels (ng/mL) following administration of YKS and the acute stress procedure.
Abbreviations: ANOVA, analysis of variance; OT, oxytocin; SEM, standard error of mean; YKS, Yokukansan.
Influence of YKS on isolated colon contraction
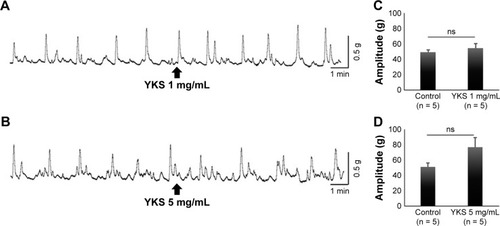
Spontaneous muscle contractions vary in their tension and frequency among muscle preparations. Thus, baseline recordings were obtained as controls during 10 min prior to administration of YKS and compared with a 10-min period after YKS administration to assess response. Representative contraction waves are shown in (YKS 1 mg/mL) and 4B (YKS 5 mg/mL). Contractile amplitude (g) was obtained by integrating area under the contractile wave above baseline. The amplitudes before and after administration of 1 mg/kg YKS did not show a significant difference (before, 49.15 ± 3.23 g; after, 54.63 ± 6.18 g) (). The value after administration of 5 mg/kg YKS was higher compared with that before administration (before, 50.85 ± 5.04 g; after, 76.57 ± 12.32 g) (P = 0.052) (). Overall, YKS did not appear to directly inhibit colonic motility.
Figure 4 Effect of YKS on isolated colonic motility. Representative contraction wave acquired when administering YKS (1 mg/mL) (A) and (5 mg/mL) (B). Contractile amplitudes (g) obtained by integrating area under the contractile wave above baseline (1 mg/mL) (C) and (5 mg/mL) (D).
Abbreviations: ns, not significant; SEM, standard error of mean; YKS, Yokukansan.
Discussion
This study addressed the use of Kampo medicines as potential treatment for patients with IBS-D, by evaluating effectiveness and mechanism of YKS in a rat model with increased defecation induced by stress.Citation25
It has been reported that YKS shows 5-HT1A receptor agonist action,Citation18 5-HT2A receptor downregulation,Citation40 inhibition of glutamate secretion,Citation41 enhancement of glutamate clearance from extracellular fluid by astrocytes,Citation42 and N-methyl-d-aspartate (NMDA) receptor antagonism.Citation43 However, no reports concerning its action on OT secretion are present. Therefore, in the present study, we examined the influence of YKS on OT secretion and whether peripheral OT is involved in the control of defecation induced by acute stress.
First, influences of pre-administration of YKS and acute psychological stress on OT secretion in rats were investigated. Administration of YKS promoted peripheral secretion of OT, which was further increased under the 15-min novel environmental stress conditions, although OT level did not change under the stress alone (). Previous studies showed that some acute stressors,Citation44,Citation45 such as immobilization, ether exposure, and forced swimming, increase the plasma OT levels but not after novel stress load.Citation44,Citation46
The level was unchanged in the present study as well. The trends in the secretion of OT seem to be different depending on the types of stressors. These results suggested that administration of YKS might enhance sensitivity of OT neurons to stress. Zheng et alCitation24 reported that adaptation to stress may involve upregulation of OT expression in the hypothalamus, which in turn attenuates CRF expression. YKS is thus expected to enhance resistance against stress.
Next, the inhibitory effect of YKS on defecation induced by stress was examined, and the amount of feces in the Stress+YKS group significantly decreased compared with that in the Stress group (). Mizoguchi et alCitation47 also reported that pre-administration of YKS in aged rats for 3 months inhibited the anxiety-related defecation under a novel environment and that the increases of serotonin and dopamine in the prefrontal cortex were also involved. Therefore, we investigated whether this action is a result of OT secretion. Atosiban, an OT receptor antagonist, was intraperitoneally administered before stress load. The result showed that the inhibitory effect of YKS was partially obstructed by Atosiban. Moreover, OT was intraperitoneally injected to result in a plasma OT level similar to that due to YKS administration (, ), and defecation was significantly decreased similar to that caused by YKS. Intraperitoneal injection of OT and Atosiban is not expected to result in brain entry due to the blood–brain barrier.Citation48,Citation49 Plasma corticosterone concentration, which reflects activation of the HPA axis, was also measured in this study. The level was significantly increased by the stress load; however, this significant increase was prevented by pre-administration of YKS (ns, comparing Stress+YKS and Control groups), but not OT. Central administration of OT inhibits the release of CRF, adrenocorticotropic hormone (ACTH), and corticosterone,Citation24,Citation25 also suggesting that OT was not delivered into the brain. Finally, the lack of a direct inhibitory effect of YKS on colonic motility was confirmed with studies on isolated colons (). As described above, OT has a regulatory effect on bowel movement, acting on the OT receptor in the gastrointestinal tract.Citation25,Citation27–Citation30 Based on the above results, YKS may control stress-induced defecation at least partially via peripheral OT secretion.
Paraventricular nuclei in the hypothalamus also deliver OT to several forebrain nuclei. YKS administration has a high probability of increasing OT secretion in the brain because the peripheral OT level increased. Intraventricular administration of OT has been shown to inhibit accelerated colonic motility induced by acute stress,Citation25 highlighting the need for more detailed studies to clarify the central mechanism of YKS.
Almost all OT neurons express both 5-HT1A and 5-HT2A receptors,Citation50 and administration of 5-HT1A receptor agonists increases secretion of OT. However, administration of a 5-HT2A receptor agonist was shown to induce functional desensitization of 5-HT1A receptors, and pre-administration of a 5-HT2A receptor antagonist prevented desensitization of 5-HT1A receptors.Citation51 As mentioned above, YKS has been shown to have 5-HT1A receptor agonist actionCitation18 and a 5-HT2A receptor downregulation effect.Citation40 In this study, YKS was pre-administered for 3 days before the stress load because single administration was not effective in preliminary experiments. Antagonist action on 5-HT2A receptors for 3 days might enhance sensitivity of 5-HT1A receptors in OT neurons. In the neuropharmacological studies of YKS, geissoschizine methyl ether, an alkaloid synthesized by Uncariae cum Uncis ramulus, has been identified as an active compound and to have a partial 5-HT1A receptor agonistic actionCitation52 and 5-HT2A receptor antagonistic action.Citation53 Accordingly, the actions of Uncariae cum Uncis ramulus via the 5-HT1A and 5-HT2A receptors may represent one of the mechanisms via which YKS affects OT neurons. Therefore, future studies are required to identify crude medicinals that regulate OT secretion as well as to identify their mechanisms. This is the first report to show that YKS increases plasma OT level and the possibility of using YKS to treat increased defecation induced by stress, similar to symptoms of abnormal defecation in IBS-D. OT is also reported to downregulate mesenteric afferent sensitivity through the nNOS-NO-KATP pathway,Citation54 and therefore action of YKS via OT is also expected to play an analgesic role in modulating the decline of the pain threshold in IBS.
Conclusion
Our findings suggest that administration of YKS enhances sensitivity of OT neuron to stress, elevates secretion of OT, and controls stress-induced defecation. YKS is expected to be useful in treating abnormal defecation induced by stress.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
The authors are grateful to Tsumura & Co. for generously providing YKS and to Prof Guo Shi-yu for generously providing instructions for recording colonic motility.
Disclosure
The authors report no conflicts of interest in this work.
References
- MiwaHPrevalence of irritable bowel syndrome in Japan: Internet survey using Rome III criteriaPatient Prefer Adherence2008214314719920955
- The Japanese Society of GastroenterologyEvidence-based clinical practice guidelines for irritable bowel syndrome1st edTokyo, Nankodo2014
- ThompsonWGLongstrethGFDrossmanDAHeatonKWIrvineEJMüller-LissnerSAFunctional bowel disorders and functional abdominal painGut199945211431147
- DrossmanDAChangLCheyWDKellowJTackJWhiteheadWERome IV functional gastrointestinal disorders – disorders of gut-brain interaction14th edCarolinaRaleigh2016
- RaoSSHatfieldRASulsJMChamberlainMJPsychological and physical stress induce differential effects on human colonic motilityAm J Gastroenterol19989369859909647034
- FukudoSSaitoKSagamiYKanazawaMCan modulating corticotropin releasing hormone receptors alter visceral sensitivity?Gut200655214614816407379
- SaitoKKasaiTNaguraYItoHKanazawaMFukudoSCorticotropin-releasing hormone receptor 1 antagonist blocks brain-gut activation induced by colonic distention in ratsGastroenterology200512951533154316285953
- FukudoSKanekoHAkihoHEvidence-based clinical practice guidelines for irritable bowel syndromeGastroenterology20155011130
- OkaTOkumiHNishidaSEffects of Kampo on functional gastrointestinal disordersBiopsychosoc Med201481524447839
- de CairesSSteenkampVUse of Yokukansan (TJ-54) in the treatment of neurological disorders: a reviewPhytother Res20102491265127020812276
- MizukamiKAsadaTKinoshitaTA randomized cross-over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioural and psychological symptoms of dementiaInt J Neuropsychopharmacol200912219119919079814
- OkaharaKIshidaYHayashiYEffects of Yokukansan on behavioral and psychological symptoms of dementia in regular treatment for Alzheimer’s diseaseProg Neuropsychopharmacol Biol Psychiatry201034353253620170698
- NakamuraYTajimaKKawagoeIKanaiMMitsuhataHEfficacy of traditional herbal medicine, Yokukansan on patients with neuropathic painMasui2009581012481255 Japanese19860227
- LinehanMMTutekDAHeardHLArmstrongHEInterpersonal outcome of cognitive behavioral treatment for chronically suicidal borderline patientsAm J Psychiatry199415112177117767977884
- ShinnoHUtaniEOkazakiSSuccessful treatment with Yi-Gan San for psychosis and sleep disturbance in a patient with dementia with Lewy bodiesProg Neuropsychopharmacol Biol Psychiatry20073171543154517688986
- ShimizuSTanakaTTohyamaMMiyataSYokukansan normalizes glucocorticoid receptor protein expression in oligodendrocytes of the corpus callosum by regulating microRNA-124a expression after stress exposureBrain Res Bull2015114495525857947
- KatahiraHSunagawaMWatanabeDAntistress effects of Kampo medicine “Yokukansan” via regulation of orexin secretionNeuropsychiatr Dis Treat20171386387228360524
- YamaguchiTTsujimatsuAKumamotoHAnxiolytic effects of yokukansan, a traditional Japanese medicine, via serotonin 5-HT1A receptors on anxiety-related behaviors in rats experienced aversive stressJ Ethnopharmacol2012143253353922819689
- ShimizuSTanakaTTakedaTTohyamaMMiyataSThe Kampo medicine yokukansan decreases microRNA 18 expression and recovers glucocorticoid receptors protein expression in the hypothalamus of stressed miceBiomed Res Int2015201579728026106615
- SempukuSRetrospective clinical study of Yokukansankachinpihange done at Sempuku clinicJ Japan Assoc Oriental Psychosom Med2006211/21721 Japanese
- QinJFengMWangCYeYWangPSLiuCOxytocin receptor expressed on the smooth muscle mediates the excitatory effect of oxytocin on gastric motility in ratsNeurogastroenterol Motil200921443043819309416
- WangRGuoLYSuoMYRole of the nitrergic pathway in motor effects of oxytocin in rat proximal colonNeurogastroenterol Motil201628121815182327302181
- AmicoJAMiedlarJACaiHMVollmerRROxytocin knockout mice: a model for studying stress-related and ingestive behavioursProg Brain Res2008170536418655871
- ZhengJBabygirijaRBülbülMCerjakDLudwigKTakahashiTHypothalamic oxytocin mediates adaptation mechanism against chronic stress in ratsAm J Physiol20102994946953
- MatsunagaMKonagayaTNogimoriTInhibitory effect of oxytocin on accelerated colonic motility induced by water-avoidance stress in ratsNeurogastroenterol Motil2009218856e5919298230
- BabygirijaRZhengJLudwigKTakahashiTCentral oxytocin is involved in restoring impaired gastric motility following chronic repeated stress in miceAm J Physiol Regul Integr Comp Physiol20102981R157R16519889866
- MonsteinHJGrahnNTruedssonMOhlssonBOxytocin and oxytocin-receptor mRNA expression in the human gastrointestinal tract: a polymerase chain reaction studyRegul Pept20041191–2394415093695
- XieDPChenLBLiuCYLiuJZLiuKJEffect of oxytocin on contraction of rabbit proximal colon in vitroWorld J Gastroenterol20039116516812508375
- YangXXiTFLiYXOxytocin decreases colonic motility of cold water stressed rats via oxytocin receptorsWorld J Gastroenterol20142031108861089425152590
- DuridanovaDBNedelchevaMDGagovHSOxytocin-induced changes in single cell K+ currents and smooth muscle contraction of guinea-pig gastric antrumEur J Endocrinol199713655315389186274
- UchidaNEgashiraNIwasakiKFujiwaraMYokukansan inhibits social isolation-induced aggression and methamphetamine-induced hyperlocomotion in rodentsBiol Pharm Bull200932337237519252280
- NeumannIDWiggerATornerLHolsboerFLandgrafRBrain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleusJ Neuroendocrinol200012323524310718919
- NeumannIDInvolvement of the brain oxytocin system in stress: interactions with the hypothalamo-pituitary-adrenal axisProg Brain Res200213914716212436933
- SaegusaYTakedaHMutoSDecreased plasma ghrelin contributes to anorexia following novelty stressAm J Physiol Endocrinol Metab20113014E685E69621712530
- MatsunagaMKonagayaTNogimoriTInhibitory effect of oxytocin on accelerated colonic motility induced by water-avoidance stress in ratsNeurogastroenterol Motil200921856e5919298230
- WilliamsCLVillarRGPetersonJMBurksTFStress-induced changes in intestinal transit in the rat: a model for irritable bowel syndromeGastroenterology19889436116212828144
- CetinelSHancioğluSSenerEOxytocin treatment alleviates stress-aggravated colitis by a receptor-dependent mechanismRegul Pept20091601–314615219931575
- MiyashitaTYamaguchiTMotoyamaKUnnoKNakanoYShimoiKSocial stress increases biopyrrins, oxidative metabolites of bilirubin, in mouse urineBiochem Biophys Res Commun2006349277578016949032
- KitoYTeramotoNEffects of Hange-shashin-to (TJ-14) and Keishika-shakuyaku-to (TJ-60) on contractile activity of circular smooth muscle of the rat distal colonAm J Physiol Gastrointest Liver Physiol2012303910591066
- EgashiraNIwasakiKIshibashiARepeated administration of Yokukansan inhibits DOI-induced head-twitch response and decreases expression of 5-hydroxytryptamine (5-HT)2A receptors in the prefrontal cortexProg Neuropsychopharmacol Biol Psychiatry20083261516152018558456
- TakedaAItohHTamanoHYuzuriharaMOkuNSuppressive effect of Yokukansan on excessive release of glutamate and aspartate in the hippocampus of zinc-deficient ratsNutr Neurosci2008111414618510802
- KawakamiZIkarashiYKaseYGlycyrrhizin and its metabolite 18 beta-glycyrrhetinic acid in glycyrrhiza, a constituent herb of Yokukansan, ameliorate thiamine deficiency-induced dysfunction of glutamate transport in cultured rat cortical astrocytesEur J Pharmacol20106262–315415819818347
- KawakamiZIkarashiYKaseYIsoliquiritigenin is a novel NMDA receptor antagonist in kampo medicine YokukansanCell Mol Neurobiol20113181203121221691759
- IványiTWiegantVMde WiedDDifferential effects of emotional and physical stress on the central and peripheral secretion of neurohypophysial hormones in male ratsLife Sci19914813130913162002757
- ZelenaDLangnaeseKDomokosAVasopressin administration into the paraventricular nucleus normalizes plasma oxytocin and corticosterone levels in Brattleboro ratsEndocrinology200915062791279819246538
- OnakaTYagiKEffects of novelty stress on vasopressin and oxytocin secretion by the pituitary in the ratJ Neuroendocrinol1993543653698401560
- MizoguchiKTanakaYTabiraTAnxiolytic effect of a herbal medicine, yokukansan, in aged rats: involvement of serotonergic and dopaminergic transmissions in the prefrontal cortexJ Ethnopharmacol20091271707619799980
- ErmischABarthTRühleHJSkopkováJHrbasPLandgrafROn the blood-brain barrier to peptides: accumulation of labelled vasopressin, DesGlyNH2-vasopressin and oxytocin by brain regionsEndocrinol Exp198519129373872788
- McGoniglePPeptide therapeutics for CNS indicationsBiochem Pharmacol201283555956622051078
- ZhangYGrayTSD’SouzaDNDesensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons in vivoJ Pharmacol Exp Ther20043101596615064330
- ZhangYD’SouzaDRaapDKCharacterization of the functional heterologous desensitization of hypothalamic 5-HT1A receptors after 5-HT2A receptor activationJ Neurosci200121207919792711588165
- NishiAYamaguchiTSekiguchiKGeissoschizine methyl ether, an alkaloid in Uncaria hook, is a potent serotonin1A receptor agonist and candidate for amelioration of aggressiveness and sociality by yokukansanNeuroscience201220712413622314317
- UedaTUgawaSIshidaYShimadaSGeissoschizine methyl ether has third-generation antipsychotic-like actions at the dopamine and serotonin receptorsEur J Pharmacol20116711–3798621951966
- LiJXueBHanTOxytocin down-regulates mesenteric afferent sensitivity via the enteric OTR/nNOS/NO/KATP pathway in ratNeurogastroenterol Motil2015271516225346204
