Abstract
Antidepressant pharmacotherapy is to date the most often used treatment for depression, but the exact mechanism of action underlying its therapeutic effect is still unclear. Many theories have been put forward to account for depression, as well as antidepressant activity, but none of them is exhaustive. Neuroimmune endocrine impairment is found in depressed patients; high levels of circulating corticosteroids along with hyperactivation of the immune system, high levels of proinflammatory cytokines, low levels of melatonin in plasma and urine, and disentrainment of circadian rhythms have been demonstrated. Moreover, antidepressant treatment seems to correct or at least to interfere with these alterations. In this review, we summarize the complex neuroimmune endocrine and chronobiological alterations found in patients with depression and how these systems interact with each other. We also explain how antidepressant therapy can modify these systems, along with some possible mechanisms of action shown in animal and human models.
Introduction
Major depressive disorder is a widespread illness of great socioeconomic impact, and according to the World Hesalth Organization, will be the second leading cause of disability in terms of burden disease in the future. A US epidemiological study reports depression to have a lifetime prevalence of 16.2%.Citation1 Possible therapeutic strategies involve social, psychological, and pharmacological treatments. Current pharmacotherapy is associated with a 55%–70% lack of responsiveness in treated subjects, being also associated with a delayed onset of action of several weeks and important side effects. Therefore, there is a need for further investigation of possible treatments for depression. Immune endocrine disturbances have been shown to play a role in the pathophysiology of depression and to be restored by effective antidepressant treatment.Citation2–Citation7 Thus, agents which correct the neuroimmune endocrine imbalance have been proposed as potential novel therapeutics for depression. In this review, we will discuss the main mechanisms that support the hypothesis that antidepressants exert their therapeutic benefit by correcting immune and endocrine disturbances.
Neuroendocrine disturbances in depressed patients
Melatonin
Abnormalities in neuroendocrine regulation are widespread in depressive illness. One of the focuses of research in depressed patients has been melatonin, a naturally occurring “lights off “ hormone. Melatonin is released according to a daily rhythm, depending on the prevailing light/dark phase of the day.Citation8 Although many organs are now shown to produce it, the diurnal rhythm of melatonin in the blood is exclusively driven by its secretion from the pineal gland.Citation9 Temporal organization in humans presents a daily adjustment to the environmental light/dark cycle; during the night, the master circadian clock in the suprachiasmatic nuclei of the hypothalamus stimulates the pineal gland via a polysynaptic noradrenergic pathway.Citation10 This gland produces and releases the nocturnal hormone, melatonin, which circulates throughout the body and adjusts several bodily functions according to the existence and duration of darkness. During the day, environmental light detected by the retina adjusts the central clock in the suprachiasmatic nuclei, ie, melanopsin-containing ganglion cells send stimulatory glutamatergic signals to the suprachiasmatic nuclei that modulate the expression of specific clock genes suppressing the stimulation of the pineal gland.Citation11 This modulation will lead, in turn, to a reduction of circulating melatonin.Citation12,Citation13 Suprachiasmatic nuclei neurons also receive afferent serotonergic projections from the raphe nucleiCitation14,Citation15 which exert inhibitory control over the suprachiasmatic nuclei neuronal response to light.Citation16,Citation17 Melatonin synthesis is a multistage process that happens inside pinealocytes, which are the functional cells of the pineal gland. Melatonin is synthesized from tryptophan, which is hydroxylated by tryptophan hydroxylase, then decarboxylated into serotonin, transformed into N-acetylserotonin by arylalkylamine N-acetyltransferase (AANAT), which is the reported rate-limiting melatonin synthesis enzyme, and finally transformed into melatonin by acetyl serotonin-O-methyltransferase. Norepinephrine binds β1 adrenergic receptors from the membrane of the pinealocytes; these, through G protein adenylate cyclase, increase cytosolic cyclic adenosine monophosphate (cAMP), which stimulates the nuclear synthesis of AANAT and increases the rate of transformation of serotonin into melatonin.Citation18 Due to its regulation, it has been suggested that melatonin could be used as a readout of noradrenergic function after antidepressant administration.Citation19,Citation20 Moreover, to support the idea that melatonin synthesis is regulated by light and day cycles, expression of tryptophan-hydroxylase mRNA and the activity of the enzyme have been analyzed during the day; peak levels of both occur at night, which is when we can measure the highest level of circulating melatonin.Citation21
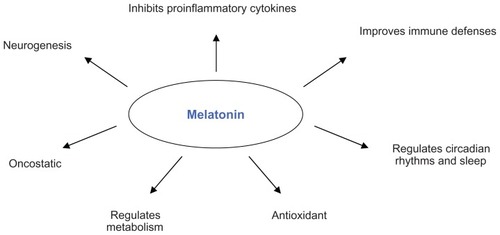
Melatonin is excreted from pinealocytes into the circulatory system where it exerts a wide range of activities (), ie, regulating circadian rhythms and sleep,Citation22 promoting neurogenesis,Citation23 modulating the immune system, improving defenses and/or decreasing inflammation,Citation24–Citation27 and regulating metabolism,Citation28 especially in lipids.Citation29 It also has very strong antioxidant and oncostatic effects,Citation30–Citation32 and most of these functions are exerted through the G protein-coupled membrane receptors, MT1 and MT2.Citation18
What happens to depressed patients? Many studies have been done so far investigating melatonin impairment in depression, and have focused mainly on circadian melatonin rhythm profiles, plasma melatonin, urinary 6-sulphatoximelatonin (aMT6s), the main melatonin metabolite, and the expression of enzymes implicated in melatonin synthesis.
There are reports showing lower plasma melatonin levels in depressed patients compared with controls.Citation33–Citation35 There are also some hints that maximum nocturnal plasma melatonin levels are lower in patients with major unipolar or bipolar depression in the coexistence of an abnormal dexamethasone suppression test (DST) compared with controls and patients with a normal DST. The intensity of depression is negatively correlated with maximum nocturnal plasma melatonin levels,Citation34,Citation36 as well as sensitivity of pineal β-adrenoceptors. As shown in a study of depressed patients that correlated overnight urinary melatonin before and after administration of atenolol, a β-adrenoceptor antagonist, the greater the decrease in melatonin after atenolol, the more severe the depression.Citation35 Moreover, the duration in hours that melatonin was present in the plasma correlates with depressive symptomatology. Citation37 Some studies focusing on the melancholic subtype of depression found a smaller rise in melatonin and lower serum melatonin compared with controls,Citation38,Citation39 while postpartum depression was related to higher plasma melatonin levels.Citation33 Furthermore, a history of depression was related to melatonin impairment,Citation33 and postmenopausal women showed a longer duration of aMT6s excretion if they had a family history of depression, as well as delayed offset of aMT6 excretion with current major depression and a later nocturnal peak with both current and past depression.Citation40 We still do not know the exact mechanism by which melatonin impairment occurs in patients with depression, but studies in animals have given us some hints, ie, administration of melatonin seemed to have antidepressant efficacy in mice, preventing changes in behavior, coat state, and an increase in cortisol levels when they were subjected to unpredictable stressors.Citation41 Moreover, the melatonin receptor, MT1 has been investigated, and MT1 knockout mice showed an increased immobility time in the forced swim test (a depressive-like behavior) compared with wild-type mice.Citation42 MT1 receptors are widespread in the human hypothalamus and in some parts of the pituitary gland, as well as in the pineal gland, and are colocalized in some corticotropin-releasing hormone neurons.Citation43 These areas are implicated in neuroendocrine modulation and in the regulation of the circadian system,Citation44 suggesting again that melatonin can be involved in the pathogenesis of the neuroendocrine impairment found in depressed patients. Recent research has attempted to investigate deeper into the biological mechanisms that lie behind melatonin impairment in depression. A study conducted in 2010 undertook a genetic investigation in 181 patients with recurrent depression and 149 controls. The investigators analyzed for the presence of two different single nucleotide polymorphisms, rs4446909 and rs5989681, in the promoter B region of the acetylserotonin methyltransferase (ASMT) enzyme for melatonin synthesis, and found three different genotype forms for each of these single nucleotide polymorphisms, ie, rs4446909 was present in AA, AG, and GG form, and rs5989681 instead was present in CC, GC, and GG forms. They showed that the AA type (for rs4446909) and the GG type (for the rs5989681) led to increased expression of the enzymes compared with the other genotypes and, interestingly, that they were both associated with a decreased risk of having recurrent depression. Moreover, it was shown that depressed patients had significant decreased ATMS expression compared with the controls.Citation45 These studies, taken together, point even more to the involvement of melatonin in the pathogenesis of depression.
Circadian rhythms
Depressed patients have shown abnormalities in many circadian systems, including sleep/wake cycles, with generally early morning waking, changes in sleep architecture with shortened rapid eye movement latency, and the rapid eye movement-sleep phase advanced to the first third of the sleep cycle, as well as diurnal mood changes, seasonal changes, variation in the temperature nadir time, peak cortisol levels, and time of melatonin onset.Citation3,Citation46 Circadian impairments have been hypothesized to be involved in the development of seasonal affective disorder;Citation47 specifically phase shift theories have been proposed, and light therapy has proven its efficacy in both seasonal and nonseasonal affective disorders. Citation48 Understanding the basis of circadian biology is the key to explain those alterations. How does our body create and regulate circadian rhythms? Regulatory centers, such as the suprachiasmatic nucleus, contain the so-called “clock cells” with active clock genes in the nucleus. These cells, through autoregulatory transcription-(post)translational feedback loops, generate electric impulses in the form of synchronized neuronal signals toward sympathetic and parasympathetic nuclei. The latter send nerve connections to many organs, regulating adrenal corticosteroid excretion, amongst other processes. These hormones, in turn, regulate the circadian rhythm with a negative feedback by resetting the time of the clock cells.Citation49 Clock genes are found also in the adrenal glands, and mutations in these can alter daily corticosteroid excretion.Citation50 In 1985, Linkowski et al found early timing of adrenocorticotropic hormone (ACTH) cortisol secretion, as well as higher mean plasma cortisol levels, in 18 depressed patients (eight unipolar and 10 bipolar) compared with eight controls.Citation51 Another study compared biological variables, such as core body temperature, cortisol, norepinephrine, thyroid-stimulating hormone, and melatonin, in three groups of patients with major affective disorder, ie, 16 depressed patients, 15 depressed patients who recovered after 3 weeks of antidepressant treatment, and 16 controls. They found higher levels of cortisol and lower levels of nocturnal melatonin in depressed patients than in the controls.Citation52 Moreover, they found a negative correlation between the amplitude of circadian rhythms and Hamilton depression scores, ie, the lower the amplitude, the more severe the depression, and recovery was associated with reversal of circadian impairment. Clock genes can also be modulated by different hormones; ACTH is known to increase PER1 (period 1) mRNA in the adrenal glands, stimulating the excretion of cortisol, while melatonin itself can dampen these ACTH-correlated effects, as shown by a recent study.Citation53 This mechanism may partially explain the previously elicited alterations found in depressed patients. Since melatonin is known to be low in these patients, cortisol levels may be high because of this lack of inhibitory melatonin effect. Another characteristic of depression is the association of severity of the illness with phase angles of different circadian variables, such as dim light melatonin onset and the average midpoint of sleep,Citation54 dim light melatonin onset and core body temperature,Citation55 and dim light melatonin onset and cortisol acrophase (the 24-hour peak).Citation55 Other circadian abnormalities found in depressed patients were a tendency toward eveningness, a later sleep onset and midpoints, and a delayed dim light melatonin onset.Citation55 These findings highlight the presence of neuroendocrine abnormalities, such as circadian misalignments, in depressed patients.Citation56
HPA axis and glucocorticoid receptors
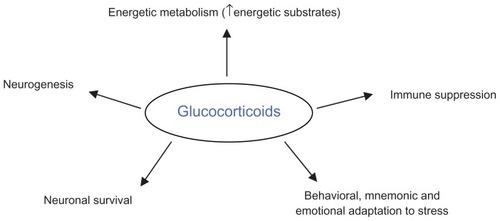
In addition to the melatonergic and circadian disturbances found in major depression, clinical studies have also demonstrated impairments in the hypothalamo-pituitary-adrenal (HPA) axis. Glucocorticoids are secreted by the adrenal gland through a neuroendocrine pathway, ie, the hypothalamus stimulates the pituitary gland via corticotropin-releasing hormone (CRH) which in turn stimulates the adrenal gland to release glucocorticoids via ACTH. The main function of these steroid hormones is to regulate energy metabolism, thereby increasing gluconeogenesis, lipolysis, and protein degradation. These hormones also have a crucial anti-inflammatory action.Citation57 They exert many other functions, like inducing behavioral adaptations in stressful situations; when exposed to environmental, psychological, or biological stressors, our bodies produce cortisol, which causes adaptive behavior, like focused attention, alertness, and immune system suppression.Citation57 In addition, cortisol has been shown to regulate memory, emotional appraisal of events, neurogenesis, and neuronal survival ().Citation58
Glucocorticoids exert their function through the glucocorticoid receptor (GR) and the mineralocorticoid receptor.Citation59 These are widespread in body tissues, but particular attention has been focused on the ones implicated in the negative feedback regulation of the HPA axis. These are found in the paraventricular nucleus of the hypothalamus, in the CRH/vasopressin neurons of the anterior pituitary, and in the hippocampus and upstream regulator of the HPA axis, as well as in other areas of the brain.Citation60,Citation61 The HPA axis is regulated also by biological stimuli, such as proinflammatory cytokines, ie, interleukin (IL)-1 and IL-6,Citation62,Citation63 and psychologically stressful situations.Citation64 A huge number of findings show that many depressed patients (up to 80% when severely depressed) are shown to have hyperactivation of the HPA axis, impairment comparable with a sustained stress response, in the absence of a stressor.Citation65–Citation69 High cortisol levels in the cerebrospinal fluid, plasma, and urine,Citation70 impairment in the negative feedback regulation of the HPA axis,Citation68,Citation71 and hyperplasia of the adrenal and pituitary glands are also found in depressed patients.Citation72,Citation73 GR-mediated negative feedback has been widely investigated using specific tests which demonstrated nonsuppression of cortisol secretion following administration of a synthetic DST; dexamethasone pretreatment also showed a lack of the inhibition of ACTH responses to CRH expected in healthy subjects (dexamethasone/CRH test).Citation68,Citation74 While DST and the dexamethasone/CRH test suggest impaired feedback inhibition at the level of the pituitary,Citation59 impaired responsiveness to hydrocortisone challenge in depressed patients may represent feedback alterations in the brain,Citation75 although this latter finding is inconsistent.Citation76 Furthermore, DST and dexamethasone/CRH tests are a biomarker of treatment success; resolution of disturbances in negative feedback in patients who are nonsuppressors before treatment is associated with efficacious antidepressant treatment, with up to 75% of nonsuppressor patients switching to suppressor status coincident with a treatment response.Citation77,Citation78
Furthermore, another analysis of HPA reactivity to stress is the so-called “cortisol awakening response” measured in salivary cortisol samples taken after awaking. Depressed patients with current or remitted depression show a higher cortisol awakening response compared with controls, and this has been suggested to be indicative of increased biological vulnerability to depression.Citation79 Studies conducted so far (as reviewed elsewhere)Citation80,Citation81 have shown that the noradrenergic system exerts an inhibitory action on the HPA axis, decreasing the release of ACTH, probably through α-1 receptors. Moreover, it has been found that normal diurnal fluctuation of the activity of the adrenal cortex requires integrity of the serotonergic system, particularly referring to the suprachiasmatic nucleus, anterior hypothalamus, and limbic system. Because these two systems are impaired in depressed patients, as shown by norepinephrine and serotonin abnormalities,Citation5 it is possible that these neurological alterations can contribute to the endocrine impairments we have spoken of so far. This connection between noradrenergic and serotonergic impairment and HPA axis activity suggests a possible link between monoamines and the neuroendocrine abnormalities found in depressed patients.
Pineal-HPA axis
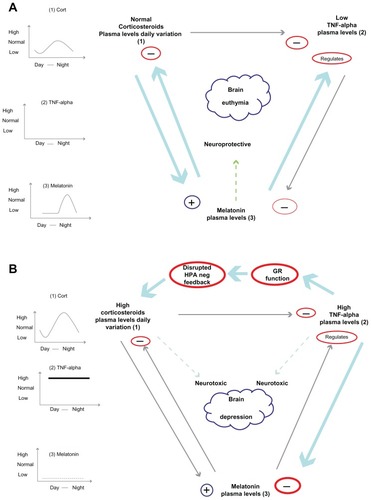
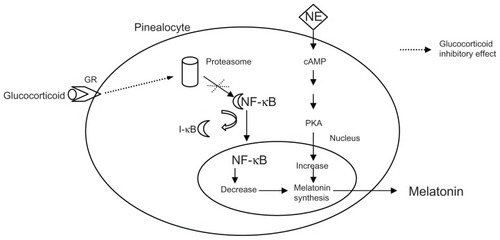
There is evidence of reciprocal interference between melatonin and cortisol, but it is not yet known if this cross talk is directly involved in the neuroendocrine impairment found in depressed patients. Studies conducted in animals give us some clues, in that rats show hypertrophy of the adrenal and pituitary glands following pinealectomy.Citation82,Citation83 Also, under acute or chronic stress, they show a decreased adrenocortical response when treated chronically with melatonin, compared with controls. Moreover, melatonin-treated rats have an increase in HPA axis sensitivity to the glucocorticoid suppression test.Citation84 These findings suggest that there is a modulatory role of melatonin on HPA axis activity and a positive effect in restoring negative glucocorticoid feedback, in particular when the animal is under stress. One study of the neuroendocrine effects of agomelatine, a new antidepressant, compared with melatonin in transgenic rats with impaired GR found that melatonin increased GR mRNA expression in the dentate gyrus, compared with wild-type animals.Citation85 Moreover, it was found that melatonin had an inhibitory action on GR function in mouse thymocytes, in particular reducing GR receptor nuclear translocation.Citation86 Furthermore, rats treated with corticosterone showed a two-fold increase in nocturnal melatonin in vivo, and extracted pineal glands from treated rats showed a significant increase in melatonin enzyme activity compared with controls.Citation87,Citation88 A possible pathway was shown in a studyCitation89 where isolated pineal glands from rats were cultured with three different substances, ie, ALLN, a proteasome inhibitor, an antagonist of the nuclear factor κB (NF-κB), or corticosterone, with or without a GR antagonist, all before stimulation with norepinephrine. As shown in , corticosterone increased the norepinephrine-mediated elevation of melatonin and N-acetylserotonin, and this effect was inhibited by GR antagonists; the potentiating effect of corticosteroids was then mimicked by treatment with an NF-κB antagonist as well as with ALLN. Physiologically, proteasomes are necessary for the translocation of NF-κB into the nucleus since they degrade inhibitory factors (I-kB) which keep NF-κB bound to the cytosol. These results show a clear pathway by which corticosterone increases melatonin production through inhibition of nuclear translocation of NF-κB.
Figure 3 Schematic representation of intracellular interaction between glucocorticoid and melatonin.
Accordingly, one placebo-controlled study conducted in 12 blind human subjects analyzed the effect of a single dose of melatonin on neuroendocrine parameters of sleep and found that melatonin could alter nocturnal cortisol and ACTH levels depending on the period of sleep. In the first half, ACTH was higher than placebo and even higher in the second half, while cortisol levels were lower in the first half and increased in the second half.Citation90 In summary, it seems that melatonin has an inhibitory action on adrenal and pituitary volume and a positive action on GR expression in the brain, thus increasing HPA negative feedback. On the other hand, glucocorticoids have a positive modulating effect on melatonin production. The pineal gland, expressing the GR, seems to monitor glucocorticoid levels and control their excessive elevation, as happens under stress conditions. This cross talk suggests that neuroendocrine impairments may work together in the development of the whole constellation of neurobiological alterations found in depression.
Melatonin and the inflammatory system
In the past ten years, an increasing amount of evidence suggests that activation of the inflammatory system is involved in the pathogenesis of depression. Firstly, depressed patients have high levels of cytokines, with increased levels of IL-6 being the most frequently observed,Citation91–Citation95 and an elevation in IL-1-β and tumor necrosis factor alpha has also been reported.Citation96–Citation99 Furthermore, major depression is strongly associated with increased levels of acute phase proteins, including C-reactive protein.Citation100,Citation101 Inflammatory markers not only increase the risk for depression and correlate positively with severity of depressive symptomsCitation102 but also modulate responsiveness to antidepressants.Citation103,Citation104 Secondly, clinical administration of cytokines or agents which increase production of proinflammatory cytokines can induce depressive symptoms in patients with no previous mental health issues,Citation105–Citation107 while inflammatory-induced depression can also be treated with antidepressants.Citation108 Thirdly, activation of the immune system and administration of proinflammatory cytokines to laboratory animals induces behavior that is similar to depression in humans.Citation109 Activation of the inflammatory system and cytokine secretion is one possible mechanism that could bring about neuroendocrine abnormalities in depression.
Cytokines are a large and diverse family of small signal molecules, best known for their immunomodulatory effect, resulting in production of other cytokines (chemotaxis) and an increase in the number of surface receptors for other molecules, activation of leukocytes, or suppression of their own effect. The most prevalent group of cytokines is composed of various subtypes of interleukins; while some stimulate immune cell proliferation and differentiation, others are predominantly inhibitory. One functional group of proinflammatory cytokines includes tumor necrosis factor alpha, IL-1, IL-6, and type I interferon-α/β. Cytokines can activate the HPA axis, causing an elevation in systemic glucocorticoid levels and inhibiting GR function at multiple levels, including GR translocation and induction of GR isoforms with a reduced capacity to bind ligand.Citation110,Citation111 IL-6 has been reported to induce a prolonged increase in plasma concentrations of ACTH and cortisol in healthy men.Citation112 A number of studies have demonstrated that treatment with proinflammatory cytokines induces a decrease in GR function, as shown by lower sensitivity to the effects of glucocorticoids on functional endpoints and decreased GR affinity for ligand. Moreover, studies performed in peripheral cells and tissues of patients with inflammatory diseases such as asthma, ulcerative colitis, acquired immunodeficiency syndrome, and rheumatoid arthritis, especially those showing resistance to the therapeutic effects of glucocorticoids, have also demonstrated reductions in GR function and affinity that are similar to those induced by cytokines.Citation106,Citation113 Indeed, major depression has also been associated with evidence of immune inflammation and increased levels of proinflammatory cytokines. It has been shown that the proinflammatory cytokine IL-1 directly blocks GR translocation and function in vitro,Citation106 an effect that is virtually opposite to that of antidepressants in the same experimental system.Citation114,Citation115 These experiments have shown that the effects of IL-1 are mediated by stimulating p38 mitogen-activated protein kinase signal transduction.
Apart from the HPA axis, neuroimmune endocrine interactions also involve the pineal gland, which influences the development and function of the immune system, while membrane-bound melatonin receptors are found in lymphoid glands and immune cells.Citation116,Citation117 In addition to mediating immune reactions and GR function, cytokines have been shown to alter sleep architecture significantly.Citation112 Inflammatory agents can also regulate melatonin synthesis. It is known that tumor necrosis factor leads to inhibition of AANAT transcription and production of N-acetylserotonin and melatonin in cultured glands. Furthermore, pinealocytes express receptors for lipopolysaccharide, which can trigger the NF-κB pathway and inhibit melatonin synthesis. Negative modulation of norepinephrine-induced melatonin production by tumor necrosis factor alpha is a transient phenomenon in the sequence of the inflammatory response, while a self-regulatory response in the pineal gland would allow restoration of the nocturnal melatonin surge.Citation118 This regulatory mechanism is disrupted when very high systemic tumor necrosis factor alpha levels are present, resulting in abnormalities in the secretion of circadian melatonin, mainly related to an absence of the diurnal rhythm. Therefore, the melatonin cycle impairment reported in depressionCitation40,Citation55,Citation56,Citation119 may occur at the onset of an inflammatory response. AANAT is considered to play a key role in the regulation of melatonin biosynthesis because changes in its activity are paralleled by alterations in melatonin levels.Citation120 The interaction of endogenous norepinephrine with β-adrenoceptors has been suggested to increase AANAT activity and melatonin release.Citation121 β-adrenoceptor stimulation has been shown to increase gene expression and protein production of tumor necrosis factor alpha as well as IL-1β and IL-6.Citation122 However, the available data suggest that enhanced adrenergic tonus leads to immunosuppression, primarily via alpha 2-receptor-mediated mechanisms.Citation123 Consequently, chronic β-receptor blockade reduces plasma levels of IL-6.Citation124 Also, stress-induced activation of NF-κB in peripheral blood mononuclear cells appears to be dependent on norepinephrine and can be brought down by α1-adrenoceptor blockade.Citation125 It has been reported that mice kept under constant light or receiving injections of β-adrenergic blockers (propranolol) to inhibit melatonin synthesis had an inability to mount a primary antibody response to sheep red blood cells, decreased cellularity in the thymus and spleen, and a depressed autologous mixed lymphocyte reaction. All of these effects were reversed by melatonin administration when given in the late afternoon.Citation126 β-adrenoceptor blockers, which depress melatonin secretion, exert immunosuppressive effects only when given in the evening,Citation35 when the immunoenhancing effect of melatonin is highest. Exogenous melatonin reverses beta-blocker-induced immunosuppression and enhances immune parameters.
Melatonin on the one hand promotes inflammation but on the other counteracts it. These effects can in fact be dependent on the interaction between melatonin and NF-κB; the hormone has been reported to inhibit NF-κB in various animal models.Citation127,Citation128 As a matter of fact, melatonin is also able to activate NF-κB, thus regulating the expression of adhesion molecules on circulating leukocytes.Citation129,Citation130 NF-κB, a determinant of inflammatory responses, is constitutively expressed in the pineal gland, which possesses receptors to trigger the NF-κB pathway.Citation118 Activation of NF-κB exaggerates the inflammatory response including the release of the proinflammatory cytokines, tumor necrosis factor alpha, IL-1, and IL-6, while inhibition of pineal NF-κB leads to enhancement of melatonin production.Citation118 In turn, melatonin inhibits translocation of NF-κB to the nucleusCitation128 and inflammation mediated by NF-κB. It has been suggested that NF-κB inhibition can be achieved through activation of transcription factor Nrf2 which protects cells and tissues from oxidative stress by activating protective antioxidants and detoxifying enzymes. Reports that Nrf2 disruptions are associated with increased NF-κB further support this hypothesis.Citation131,Citation132 The mechanism mentioned above further expands and integrates the concept of melatonin being a powerful antioxidant with anti-inflammatory properties.
In addition, not only cytokines but also glucocorticoids transmit signals through a common NF-κB pathway to induce and turn off inflammatory responses, respectively,Citation133 which suggests an even more profound effect of melatonin on the inflammatory response. Melatonin has been shown to abolish several effects of exogenous corticoids inducing immune depression,Citation126 and is believed to work as an antiadrenocortical or antistress factor.Citation84 The melatonin/corticoid relationship is significant because high absolute levels of corticoids and disorganization of the normal rhythm of corticoid release are also involved in the pathogenesis of depression. In line with these findings, melatonin acts against the negative effects of stress on immune homeostasis; characteristics such as sleep duration can also entail variations in inflammatory markers. There is also evidence of elevated levels of IL-6 and C-reactive protein in short sleeping women.Citation134 Moreover, patients with major depression show abnormal IL-6 production across the melatonin cycle,Citation93 indicating a possible modulating effect of melatonin on the immune system. In line with this, humoral and cellular immunities are significantly influenced by melatonin,Citation135 through specific receptors, MT1/MT2, and high affinity nuclear receptors (RZR) found on leukocytes. Furthermore, the last can synthesize melatonin, having the enzyme necessary for its production.Citation136 Gender, age, the effects of maturation or activation on the immune system, and stressful conditions are all factors influencing the effects of melatonin on the immune system,Citation137 which from one side promotes and from the other counteracts inflammation simultaneously because of its differential proinflammatory and anti-inflammatory roles.Citation136 Pineal activity induces feedback of an inflammatory response, and factors secreted by activated immune cells act as messages which are understood by the pineal gland, closing the regulatory loop of the immune-pineal axis.
Another main pathway by which cytokines can induce neuroendocrine abnormalities in depression involves tryptophan metabolism. Tryptophan either leads to the synthesis of serotonin and melatonin or to the kynurenine pathway. Tryptophan via indoleamine-2,3-dioxygenase is converted into kynurenine, which in turn can take two different pathways, ie, one leading to a neuroprotective metabolite, kynurenic acid, and the other through kynurene-3-mono-oxygenase to neurotoxic metabolites (3-hydroxykynurenine and then quinolinic acid).Citation6 Proinflammatory cytokines have a positive effect on kynurene-3-mono-oxygenase, shifting tryptophan metabolism towards a neurotoxic pathway.Citation138–Citation140 External or psychosocial factors as well as internal inflammatory conditions may trigger depression through an inflammatory process.Citation141 Furthermore, lipopolysaccharide-treated mice show depressive-like behavior that was prevented by administration of anti-inflammatory drugs which attenuated cytokine expression induced by lipopolysaccharide or directly by a kynurene-3-mono-oxygenase antagonist. Those treatments also normalized the kynurenine/tryptophan ratio, while direct administration of kynurenine induced depressive-like behavior in healthy mice.Citation142 These results reinforce the idea that kynurene-3-mono-oxygenase is a central enzyme in the development of depressive-like behavior induced by inflammation. All these findings taken together contribute to the idea that depression is the symptomatic manifestation of a multifactorial disease which involves, in addition to well known psychological and social factors, underlying abnormalities in the complex web of neuroendocrine pathways and possibly the intercommunications between all these systems.
Therapeutic actions of antidepressants
How do antidepressants exert their therapeutic action? Although the exact etiopathogenesis of depression is not clear, pharmacotherapy has to date targeted and modulated various sites of action believed to be impaired in this major disease, ie, monoamine levels, serotonin transporter, receptor abnormalities, neuropeptide systems, glutamatergic neurotransmission, HPA axis, and circadian rhythm misalignment. Citation143 In this section, we focus attention on the possible effects of antidepressants on the main neuroendocrine abnormalities found in depression, in particular melatonin, cortisol, and immune system impairment.
Effects on melatonin
The effects of antidepressant medication on melatonin synthesis, metabolism, and circulating levels have been extensively studied. Almost every class of antidepressant has been tested, ie, tricyclic antidepressants, tetracyclic antidepressants, monoamine oxidase inhibitors, serotonin-norepinephrine reuptake inhibitors, and selective serotonin reuptake inhibitors.
Studies on isolated rat pineal glands incubated with desipramine (a tricyclic antidepressant) showed increased levels of melatonin and increased activity of N-acetyltransferase compared with controls.Citation144,Citation145 A similar effect was found with venlafaxine (a serotonin-norepinephrine reuptake inhibitor) on acute treatment.Citation146 However, melatonin levels were attenuated by subchronic treatment, possibly because of adaptive desensitization of the pinealocyte β-adrenoceptors that maintain normal pineal function after modification of overall pineal synaptic function.Citation145,Citation146 Another study showed that fluoxetine (a selective serotonin reuptake inhibitor) had a positive effect on AA-NAT gene expression in the hippocampus and striatum, indicating a possible pathway via antidepressants which modulate melatonin synthesis.Citation147 Moreover, fluvoxamine (a selective serotonin reuptake inhibitor) seems to have an inhibitory effect on hepatic cytochrome peroxidase (CYP450), which is implicated in melatonin catabolism,Citation148,Citation149 but this was not confirmed by other antidepressants, except for paroxetine, a selective serotonin reuptake inhibitor, given to supratherapeutic concentrations. Another possible target of selective serotonin reuptake inhibitors seems to be hepatic tryptophan pyrrolase (the main enzyme degrading tryptophan), that paroxetine has been shown to inhibitCitation150 which, in turn, can increase circulating tryptophan levels, leading indirectly to an increase in the pineal substrate for melatonin synthesis.
Studies in depressed patients seem to confirm the idea that antidepressants modulate melatonin (). A large number have tested desipramine and consistently found that it increases circulating melatonin levels and urinary aMT6s concentrations after treatment compared with controls after one day,Citation20 one week,Citation151,Citation152 3 weeks,Citation153 and 6 weeks.Citation20 Interestingly, one study found that long-term treatment (6 weeks) led to normalization of urinary aMT6s after an initial increase in the short term. This normalization can be explained again by an adaptive mechanism on the part of β1-adrenoceptors in the pineal gland to the constant high levels of norepinephrine in the synaptic cleft.Citation152 Similar results were found following 3–6 weeks of treatment with a monoamine oxidase inhibitor, tranylcypromine or clorgyline, with regard to urinary aMT6s in 27 depressed patients.Citation154 These findings were confirmed also for mirtazapine (a tetracyclic antidepressant) given in the long term.Citation155 Furthermore, a recent study by Carvalho et al showed an increase in melatonin production after treatment with fluoxetine and duloxetine, a serotonin-norepinephrine reuptake inhibitor, in drug-free depressed patients compared with placebo; both groups had the same improvement in emotional state, suggesting a pharmacological effect of antidepressants on melatonin which may not be directly related to their therapeutic action.Citation156 In contrast with those findings, another studyCitation157 measured plasma melatonin before and after 8 weeks of treatment with clomipramine (a tricyclic antidepressant) in 20 depressed patients compared with 14 healthy subjects. Surprisingly, they showed that depressed patients had higher levels of both diurnal and nocturnal melatonin compared with controls. Further, like previous studies, they showed that clomipramine significantly increased diurnal melatonin but controversially decreased nocturnal secretion; however, this latter finding was not significant. Finally, pineal reactivity to antidepressants seems to be a potential biomarker of patient response to treatment, as shown by a study in 24 depressed outpatients treated with either the selective serotonin reuptake inhibitor, fluvoxamine, or the tricyclic antidepressant, imipramine, for 6 weeks. When measuring urinary aMT6s, the results divided responders into those who showed an increase in the metabolite and nonresponders who showed a decrease.Citation158
Table 1 Antidepressant effect on melatonin in depressed patients
Similar results were found observing melatonin modulation by antidepressants given to healthy subjects (). Two interesting studies investigated the effect of desipramine in the short and long term and shed some light on the possible mechanisms of interaction between antidepressants and melatonin. DesipramineCitation19 induced an initial increase in plasma melatonin during the first week of treatment, while for the other days it caused a progressive decrease until it reached normal levels again. Moreover, there was a rebound effect after treatment withdrawal. The authors suggested that desipramine caused adaptive inhibition of the presynaptic junction firing rate leading to a desensitization of presynaptic a2-adrenoceptors implicated in the control of the negative feedback on the presynaptic portion; when desipramine is withdrawn, there is an increased firing rate at the prejunctional synapses, which, in the presence of reduced negative feedback, leads to increased norepinephrine outflow, and thus in plasma melatonin secretion.Citation19 A second study indicates that the effect of desipramine on melatonin is due more to increased norepinephrine availability at the synaptic junction induced by the antidepressant than to other effects on melatonin metabolism. Eight male volunteers administered desipramine at 4 pm showed an increase in plasma melatonin at 9 pm, 10 pm, 11 pm, and midnight and an increase in nocturnal aMT6s at 11 pm and midnight, but no significant effect on total aMT6s in one day. Moreover, in the later hours, there was no significant increase in plasma melatonin levels, perhaps because of adaptive desensitization of pineal β-adrenoceptors to high levels of norepinephrine or because the peak capacity of the pineal gland to synthesize melatonin was reached.Citation159 Like desipramine, fluvoxamine also had a positive effect on melatonin after acute administration,Citation160 and the same results were found for both acute and chronic administration of oxaprotiline, a serotonin-norepinephrine reuptake inhibitor.Citation161 A positive effect was found also for mirtazapineCitation162 and clomipramine,Citation163 but the latter was associated also with a normalization of pineal activity after long-term treatment. Here the increase in melatonin also seemed to be associated with an improvement in emotional state. The monoamine oxidase inhibitors, tranylcypromine and pirlindole, the tetracyclic antidepressant maprotiline, and the serotonin S2-receptor antagonist, mianserin, were compared with fluvoxamine, each given for 3 weeks, but only the latter had an effect on melatonin.Citation164 Interestingly, total melatonin excretion was increased by desipramine, advancing onset, and by fluvoxamine, delaying offset. The acrophase was also modulated, being delayed with fluvoxamine and advanced with desipramine. Similarly opposite effects in melatonin metabolism were seen whereby increased aMT6s levels followed desipramine and decreased levels followed fluvoxamine. These studies confirm the hypothesis that the effect of desipramine is exerted mainly on melatonin synthesis, while fluvoxamine modulates its catabolism.Citation165
Table 2 Antidepressant effect on melatonin in healthy subjects
In summary, antidepressants appear to modulate melatonin via three main mechanisms: pineal β-adrenoceptor stimulation by elevated levels of norepinephrine in the synaptic junction, though this effect seems susceptible to adaptive desensitization of receptors; modulation of melatonin catabolism; serotonergic stimulation of the suprachiasmatic nuclei by projections from the raphe nuclei which modulate the response of the suprachiasmatic nuclei to light signals from the retinohypothalamic tract. Antidepressants also may modulate melatonin by a direct effect on expression of the enzymes involved in its synthesis, and the increase in circulating serotonin by some antidepressants like monoamine oxidase inhibitors and fluoxetine can be another mechanism.Citation166,Citation167 Fewer studies have investigated the effects of selective serotonin reuptake inhibitors on melatonin. Fluoxetine increases melatonin levels after effective antidepressant treatment.Citation156,Citation168 In contrast, paroxetine caused no alterations in melatonin in eight healthy volunteers. Citation169 Tan et al showed no significant differences with selective serotonin reuptake inhibitors, but it is noteworthy that they positively associated the amplitude of melatonin secretion and improvement in recovery from depression after fluoxetine treatment.Citation170
Effects on circadian rhythm
Can antidepressants influence biological rhythms? This issue is summarized in . Some hints come from studies in rats which tested mainly the effects of activation of the serotonergic system on the suprachiasmatic nucleus. Serotonergic agonists and selective serotonin reuptake inhibitors, like fluoxetine, seem to have both a photic (night-time phase shifts) and nonphotic influence (daytime phase shifts and night- time photic resetting attenuation). The first probably occurs through 5-HT(3) and 5-HT(2C) receptor interactions, while the second seems related to direct modulation of clock genes, possibly through 5-HT(1A) receptors, ie, downregulation of Per1 and Ror-beta expression and upregulation of Rev-erb-alfa, correlated with daytime behavioral phase advance, while night-time photic resetting attenuation is associated with altered expression of Per1, Per2, and Ror-beta.Citation171 A consistent phase advance in neuronal firing was showed in rat slices after treatment with fluoxetine and tryptophan.Citation172
Table 3 Antidepressant effects on circadian rhythm
Effects on the HPA axis
Glucocorticoids exert their physiological action through GR. We know that impairment of the HPA axis is implicated in the etiology of depression, and because there is evidence that antidepressants may have an effect on these receptors, we believe that this is part of their therapeutic action (). Several studies have demonstrated that GR is upregulated by antidepressants; rats treated with desipramine or imipramine showed an increase in hippocampal and hypothalamic GR mRNA levels,Citation173 and in vitro antidepressant treatment for 24 hours increased GR expression, promoted GR nuclear translocation, and enhanced GR function in mouse fibroblasts. Citation114,Citation174 Similar results were found for animal neuronal cells treated with either selective serotonin reuptake inhibitors or tricyclic antidepressants.Citation175 Such treatment also showed modulation of GR function in peripheral red blood cells.Citation176 Furthermore, recent studies have shown an effect of antidepressants on circulating levels of corticosteroids. Fluoxetine effectively reduced cortisol levels when given to alcohol-treated rats.Citation177 Also, perinatal exposure to maternal selective serotonin reuptake inhibitors can induce a lower basal cortisol level in the newborn.Citation178 Long-term mirtazapine treatment decreases levels of cortisol and dehydroepiandrosterone, an androgen secreted by the adrenal gland, a decrease in which can be used to assess HPA axis function,Citation179 and there seems to be a relationship between dehydroepiandrosterone reduction and the therapeutic effect of an antidepressant.Citation180,Citation181 Also, one study found that restoring of HPA axis hyperactivity in 194 depressed patients was associated with remission, and, moreover, the integrity of negative feedback in the HPA axis, as assessed by DST, predicted the response to an antidepressant. Citation182 In accordance with this, a recent study found that resistance to treatment was associated with an abnormal HPA axis negative feedback response, as assessed by prednisolone activation of the GR and mineralocorticoid receptor.Citation183 Some controversial results come from two studiesCitation184,Citation185 measuring the effects of venlafaxine or sertraline on cortisol and showing no effect or even increased levels; however, these studies are limited by their small sample sizes, while previous studies refer to much bigger sample sizes, and are thus more reliable.
Table 4 Antidepressant effects on HPA axis
Effects on the immune system
There are many studies showing a direct effect of antidepressants on inflammatory cytokines. Results come from studies in cells, animals, and humans (). Two studies conducted in animal glial cells showed a decrease in proinflammatory cytokines after exposure to an antidepressant and stimulation with interferon gamma, fluvoxamine, reboxetine, and imipramine, along with decreased levels of nitric oxideCitation186 when treated with lipopolysaccharide, while amitriptyline and nortriptyline decreased levels of IL-1 and tumor necrosis factor alpha.Citation187 Another study in encephalitogenic T cell clones, splenocytes, and peritoneal macrophages from rats showed that venlafaxine induced a decrease in generation of IL-12, tumor necrosis factor alpha, and interferon gamma.Citation188 Moreover, experiments conducted in stimulated peripheral white blood cells are in accordance with these findings, ie, imipramine, mianserin, clomipramine, sertraline, and citalopram reduced proinflammatory cytokine levels and increased anti-inflammatory cytokine levels.Citation189–Citation191 Accordingly, whole blood from healthy controls and treatment-resistant patients incubated with lipopolysaccharide and treated with antidepressants showed markedly reduced levels of proinflammatory cytokines compared with untreated blood.Citation192,Citation193 Finally, studies in depressed patients confirm the hypothesis that antidepressants have an anti-inflammatory effect. In patients with major depression, long-term treatment with selective serotonin reuptake inhibitors decreased tumor necrosis factor alpha, C-reactive protein, and leukocyte levels similar to those found in controls.Citation2,Citation98 In one study, 48 depressed patients received either bupropion, mirtazapine, citalopram, paroxetine, or venlafaxine for 6 weeks, and again there was a significant decrease in proinflammatory cytokines.Citation195 Furthermore, desipramine and fluoxetine seem to have an inhibitory effect on indoleamine- 2,3-dioxygenase activity.Citation196 More details on the effects of antidepressants on the immune system have been reviewed by Janssen et al.Citation197
Table 5 Antidepressant effects on immune system
Agomelatine
Promising results have been reported for agomelatine, a new antidepressant with both serotonergic and melatonergic activity. Agomelatine inhibits the serotonergic system through its 5HT-2C receptor antagonist properties, and stimulates the melatonergic system via melatonin receptor agonist binding. Serotonergic blockade leads to enhancement of the frontocortical adrenergic and dopaminergic pathways responsible for its antidepressant activity, and melatonergic stimulation accounts for its chronobiotic re-entrainment features.Citation198–Citation200 Studies in rats,Citation201,Citation202 healthy subjectsCitation203,Citation204 and depressed patientsCitation205–Citation207 had interesting findings with regard to the capacity of agomelatine to re-entrain abnormal circadian rhythms. Moreover, it has a better side effect profile,Citation208 in particular concerning sexual impairment in comparison with other antidepressants,Citation209 and low discontinuation symptoms after withdrawal compared with the selective serotonin reuptake inhibitor, paroxetine.Citation210
Conclusion
Major depression is a multifactorial and complex disorder, with social, psychological, and biological components. In this review, we have focused mainly on the neuroendocrine basis of these abnormalities. It is clear that melatonin, the HPA axis, immune system, and circadian rhythms are profoundly altered in depressed patients compared with healthy subjects. Moreover, we show how all those systems are interconnected (). Melatonin, in addition to being a well known circadian rhythm modulator, has been shown to regulate immune system activity, having proinflammatory and anti-inflammatory actions, to downregulate the HPA axis and cortisol secretion, and to modulate excessive elevations in corticosteroids. Glucocorticoids, in addition to their established immunosuppressive action, seem to have an upregulatory effect on melatonin synthesis. Cytokines, on the other hand, have been shown to inhibit GR function, and inflammation has shown a negative effect on melatonin synthesis. These interconnections may play an important role in the pathogenesis of depression. The immune system and the HPA axis, along with elevation of corticosteroids, act as important biological defense systems under conditions of stress. It seems that prolonged and chronic activation of these mechanisms leads to neurotoxic alterations in the brain that may eventually trigger depression; also, a genetic predisposition to this is shown, ie, polymorphism in melatonin promoter enzymes. We tried to determine in the literature if antidepressants could play a role in these neuroendocrine alterations. We identified that these agents work separately on each of these interconnected systems. In fact, they have antiinflammatory actions, stimulate melatonin synthesis, restore negative glucocorticoid feedback by upregulation of GR, and modulate circadian rhythm. It is possible then that all these modifications influence each other synergistically. Inhibition of inflammation itself may first restore melatonin levels, having the least anti-inflammatory properties, and, secondly, benefit negative feedback in the HPA axis. Restoration of GR may positively influence the anti-inflammatory action of glucocorticoids, their stimulation of melatonin secretion by the pineal gland, and perhaps their influence on negative feedback by adrenal gland clock genes. An increase in melatonin on the other hand may influence the other systems. It seems that antidepressants, in addition to increasing primary monoamine levels in the synaptic cleft, work at different neuroendocrine levels, each closely related to others. Further research will help to clarify the complex mechanisms underlying depression and the actions of antidepressants upon all the neuroendocrine systems.
Acknowledgments
LAC is funded by a Young Investigator Award from National Alliance for Research on Schizophrenia and Depression, the European College of Neuropsychopharmacology, the Partek- British Council Partnership and the Seventh Framework Programme Collaborative Project, grant agreement 22963 (Mood Inflame). MA is funded by an ERASMUS placement grant within the LLP/ERASMUS placement program. JR is funded by a ZPORR grant from the European Union.
Disclosure
The authors have no relevant financial interest to disclose in this work.
References
- KesslerRCEpidemiology of women and depressionJ Affect Disord200374151312646294
- O’BrienSMScottLVDinanTGCytokines: abnormalities in major depression and implications for pharmacological treatmentHum Psychopharmacol200419639740315303243
- GermainAKupferDJCircadian rhythm disturbances in depressionHum Psychopharmacol200823757158518680211
- CarvalhoLAParianteCMIn vitro modulation of the glucocorticoid receptor by antidepressantsStress200811641142419065455
- LeeSJeongJKwakYParkSKDepression research: where are we nowMol Brain20103820219105
- ZunszainPAAnackerCCattaneoACarvalhoLAParianteCMGlucocorticoids, cytokines and brain abnormalities in depressionProg Neuropsychopharmacol Biol Psychiatry201135372277920406665
- AnackerCZunszainPACarvalhoLAParianteCMThe glucocorticoid receptor: pivot of depression and of antidepressant treatment?Psychoneuroendocrinology201136341542520399565
- AxelrodJWurtmanRJSnyderSHControl of hydroxyindole O-methyltransferase activity in the rat pineal gland by environmental lightingJ Biol Chem196524094995414275158
- HardelandRMelatonin: signaling mechanisms of a pleiotropic agentBiofactors200935218319219449447
- MarondeEStehleJHThe mammalian pineal gland: known facts, unknown facetsTrends Endocrinol Metab200718414214917374488
- HankinsMWLucasRJThe primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigmentCurr Biol200212319119811839270
- BrainardGCSlineyDHanifinJPSensitivity of the human circadian system to short-wavelength (420-nm) lightJ Biol Rhythms200823537938618838601
- JasserSABlaskDEBrainardGCLight during darkness and cancer: relationships in circadian photoreception and tumor biologyCancer Causes Control200617451552316596305
- DeurveilherSSembaKIndirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural stateNeuroscience2005130116518315561433
- LeanderPVrangNMollerMNeuronal projections from the mesencephalic raphe nuclear complex to the suprachiasmatic nucleus and the deep pineal gland of the golden hamster (Mesocricetus auratus)J Comp Neurol1998399173939725702
- YingSWRusakBEffects of serotonergic agonists on firing rates of photically responsive cells in the hamster suprachiasmatic nucleusBrain Res19946511–237467922588
- QuinteroJEMcMahonDGSerotonin modulates glutamate responses in isolated suprachiasmatic nucleus neuronsJ Neurophysiol199982253353910444653
- ReiterRJTanDXFuentes-BrotoLMelatonin: a multitasking moleculeProg Brain Res201018112715120478436
- CowenPJGreenARGrahame-SmithDGBraddockLEPlasma melatonin during desmethylimipramine treatment: evidence for changes in noradrenergic transmissionBr J Clin Pharmacol19851967998052992560
- PalazidouEPapadopoulosARatcliffHDawlingSCheckleySANoradrenaline uptake inhibition increases melatonin secretion, a measure of noradrenergic neurotransmission, in depressed patientsPsychol Med19922223093151319597
- SugdenDComparison of circadian expression of tryptophan hydroxylase isoform mRNAs in the rat pineal gland using real-time PCRJ Neurochem20038651308131112911638
- AgezLLaurentVGuerreroHYPevetPMasson-PevetMGauerFEndogenous melatonin provides an effective circadian message to both the suprachiasmatic nuclei and the pars tuberalis of the ratJ Pineal Res20094619510519090912
- SotthibundhuAPhansuwan-PujitoPGovitrapongPMelatonin increases proliferation of cultured neural stem cells obtained from adult mouse subventricular zoneJ Pineal Res201049329130020663047
- SrinivasanVSpenceDWTrakhtIPandi-PerumalSRCardinaliDPMaestroniGJImmunomodulation by melatonin: its significance for seasonally occurring diseasesNeuroimmunomodulation20081529310118679047
- MaldonadoMDMurillo-CabezasFCalvoJRMelatonin as pharmacologic support in burn patients: a proposed solution to thermal injury-related lymphocytopenia and oxidative damageCrit Care Med20073541177118517312564
- MaldonadoMDMurillo-CabezasFTerronMPThe potential of melatonin in reducing morbidity-mortality after craniocerebral traumaJ Pineal Res200742111117198533
- CernysiovVGerasimcikNMauricasMGirkontaiteIRegulation of T-cell-independent and T-cell-dependent antibody production by circadian rhythm and melatoninInt Immunol2010221253419946015
- AgilARosadoIRuizRFigueroaAZenNFernandez-VazquezGMelatonin improves glucose homeostasis in young Zucker diabetic fatty ratsJ Pineal ResJuly 262011 [Epub ahead of print.]
- Sanchez-HidalgoMLuZTanDXMaldonadoMDReiterRJGregermanRIMelatonin inhibits fatty acid-induced triglyceride accumulation in ROS17/2.8 cells: implications for osteoblast differentiation and osteoporosisAm J Physiol Regul Integr Comp Physiol20072926R2208221517379847
- ReiterRJTanDXLeonJKilicUKilicEWhen melatonin gets on your nerves: its beneficial actions in experimental models of strokeExp Biol Med (Maywood)2005230210411715673559
- TanDXManchesterLCTerronMPFloresLJReiterRJOne molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species?J Pineal Res2007421284217198536
- Garcia-NavarroAGonzalez-PugaCEscamesGCellular mechanisms involved in the melatonin inhibition of HT-29 human colon cancer cell proliferation in cultureJ Pineal Res200743219520517645698
- ParryBLMeliskaCJSorensonDLPlasma melatonin circadian rhythm disturbances during pregnancy and postpartum in depressed women and women with personal or family histories of depressionAm J Psychiatry2008165121551155818829869
- Beck-FriisJKjellmanBFAperiaBSerum melatonin in relation to clinical variables in patients with major depressive disorder and a hypothesis of a low melatonin syndromeActa Psychiatr Scand19857143193304039876
- PaparrigopoulosTMelatonin response to atenolol administration in depression: indication of beta-adrenoceptor dysfunction in a subtype of depressionActa Psychiatr Scand2002106644044512392487
- Beck-FriisJLjunggrenJGThorenMvonRDKjellmanBFWetterbergLMelatonin, cortisol and ACTH in patients with major depressive disorder and healthy humans with special reference to the outcome of the dexamethasone suppression testPsychoneuroendocrinology19851021731862994141
- ParryBLMeliskaCJSorensonDLIncreased melatonin and delayed offset in menopausal depression: role of years past menopause, follicle-stimulating hormone, sleep end time, and body mass indexJ Clin Endocrinol Metab2008931546018042653
- BrownRKocsisJHCaroffSDifferences in nocturnal melatonin secretion between melancholic depressed patients and control subjectsAm J Psychiatry198514278118164014502
- FountoulakisKNKaramouzisMIacovidesAMorning and evening plasma melatonin and dexamethasone suppression test in patients with nonseasonal major depressive disorder from northern Greece (latitude 40–41.5 degrees)Neuropsychobiology200144311311711586048
- TuunainenAKripkeDFElliottJADepression and endogenous melatonin in postmenopausal womenJ Affect Disord2002691–314915812103461
- DetanicoBCPiatoALFreitasJJAntidepressant-like effects of melatonin in the mouse chronic mild stress modelEur J Pharmacol20096071–312112519249298
- WeilZMHotchkissAKGatienMLPieke-DahlSNelsonRJMelatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gatingBrain Res Bull200668642542916459197
- WuYHZhouJNBalesarRDistribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: colocalization of MT1 with vasopressin, oxytocin, and corticotropin- releasing hormoneJ Comp Neurol2006499689791017072839
- RosenwasserAMFunctional neuroanatomy of sleep and circadian rhythmsBrain Res Rev200961228130619695288
- GaleckiPSzemrajJBartoszGSingle-nucleotide polymorphisms and mRNA expression for melatonin synthesis rate-limiting enzyme in recurrent depressive disorderJ Pineal Res201048431131720433639
- BoycePBarriballECircadian rhythms and depressionAust Fam Physician201039530731020485718
- LamRWLevitanRDPathophysiology of seasonal affective disorder: a reviewJ Psychiatry Neurosci200025546948011109298
- GoldenRNGaynesBNEkstromRDThe efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidenceAm J Psychiatry2005162465666215800134
- OkamuraHSuprachiasmatic nucleus clock time in the mammalian circadian systemCold Spring Harb Symp Quant Biol20077255155618419314
- PilorzVSteinlechnerSOsterHAge and oestrus cycle-related changes in glucocorticoid excretion and wheel-running activity in female mice carrying mutations in the circadian clock genes Per1 and Per2Physiol Behav2009961576318786554
- LinkowskiPVan CauterELeclercqRACTH, cortisol and growth hormone 24-hour profiles in major depressive illnessActa Psychiatr Belg19858556156233004110
- SouetreESalvatiEBelugouJLCircadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormalityPsychiatry Res19892832632782762432
- CampinoCValenzuelaFJTorres-FarfanCMelatonin exerts direct inhibitory actions on ACTH responses in the human adrenal glandHorm Metab Res201143533734221332028
- EmensJLewyAKinzieJMArntzDRoughJCircadian misalignment in major depressive disorderPsychiatry Res2009168325926119524304
- HaslerBPBuysseDJKupferDJGermainAPhase relationships between core body temperature, melatonin, and sleep are associated with depression severity: further evidence for circadian misalignment in non-seasonal depressionPsychiatry Res2010178120520720471106
- BuckleyTMSchatzbergAFA pilot study of the phase angle between cortisol and melatonin in major depression – a potential biomarker?J Psychiatr Res2010442697420004915
- ParianteCMGlucocorticoid receptor function in vitro in patients with major depressionStress20047420921916019586
- HerbertJGoodyerIMGrossmanABDo corticosteroids damage the brain?J Neuroendocrinol200618639341116684130
- de KloetERVreugdenhilEOitzlMSJoelsMBrain corticosteroid receptor balance in health and diseaseEndocr Rev19981932693019626555
- TaskerJGDiSMalcher-LopesRMinireview: rapid glucocorticoid signaling via membrane-associated receptorsEndocrinology2006147125549555616946006
- de KloetERDerijkRHMeijerOCTherapy insight: is there an imbalanced response of mineralocorticoid and glucocorticoid receptors in depression?Nat Clin Pract Endocrinol Metab20073216817917237843
- NavarraPTsagarakisSFariaMSReesLHBesserGMGrossmanABInterleukins-1 and -6 stimulate the release of corticotropin-releasing hormone-41 from rat hypothalamus in vitro via the eicosanoid cyclooxygenase pathwayEndocrinology1991128137441846105
- KaralisKMugliaLJBaeDHilderbrandHMajzoubJACRH and the immune systemJ Neuroimmunol19977221311369042104
- ZhouDKusnecovAWShurinMRDePaoliMRabinBSExposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axisEndocrinology19931336252325308243274
- HolsboerFThe corticosteroid receptor hypothesis of depressionNeuropsychopharmacology200023547750111027914
- McQuadeRYoungAHFuture therapeutic targets in mood disorders: the glucocorticoid receptorBr J Psychiatry200017739039511059990
- HeuserIYassouridisAHolsboerFThe combined dexamethasone/CRH test: a refined laboratory test for psychiatric disordersJ Psychiatr Res19942843413567877114
- NemeroffCBThe corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directionsMol Psychiatry1996143363429118360
- ParianteCMMillerAHGlucocorticoid receptors in major depression: relevance to pathophysiology and treatmentBiol Psychiatry200149539140411274650
- GoldPWGoodwinFKChrousosGPClinical and biochemical manifestations of depression. Relation to the neurobiology of stress (1)N Engl J Med198831963483533292920
- CarrollBJFeinbergMGredenJFA specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utilityArch Gen Psychiatry198138115227458567
- AxelsonDADoraiswamyPMBoykoOBIn vivo assessment of pituitary volume with magnetic resonance imaging and systematic stereology: relationship to dexamethasone suppression test results in patientsPsychiatry Res199244163701461948
- RubinRDinanTJScottLVThe neuroendocrinology of affective disordersPfaffDArnoldAPEtgenAMFahrbachSEMossPLRubinRTHormones, Brain and BehaviourNew York, NYNew York Academic Press2001
- KunugiHIdaIOwashiTKimuraMAssessment of the dexamethasone/CRH test as a state-dependent marker for hypothalamic- pituitary-adrenal (HPA) axis abnormalities in major depressive episode: a multicenter studyNeuropsychopharmacology200631121222016123748
- YoungEAHaskettRFMurphy-WeinbergVWatsonSJAkilHLoss of glucocorticoid fast feedback in depressionArch Gen Psychiatry19914886936991652926
- CooneyJMDinanTGPreservation of hypothalamic-pituitary-adrenal axis fast-feedback responses in depressionActa Psychiatr Scand19969464494539020998
- LinkowskiPMendlewiczJKerkhofsM24-hour profiles of adrenocorticotropin, cortisol, and growth hormone in major depressive illness: effect of antidepressant treatmentJ Clin Endocrinol Metab19876511411523034952
- HeuserIJSchweigerUGotthardtUPituitary-adrenal-system regulation and psychopathology during amitriptyline treatment in elderly depressed patients and normal comparison subjectsAm J Psychiatry1996153193998540599
- BhagwagarZHafiziSCowenPJIncrease in concentration of waking salivary cortisol in recovered patients with depressionAm J Psychiatry2003160101890189114514508
- TuomistoJMannistoPNeurotransmitter regulation of anterior pituitary hormonesPharmacol Rev19853732493322869509
- PlotskyPMCunninghamETJrWidmaierEPCatecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretionEndocr Rev19891044374582558876
- WurtmanRJAltschuleMdHolmgrenUEffects of pinealectomy and of a bovine pineal extract in ratsAm J Physiol1959197110811013661402
- KonakchievaRMitevYAlmeidaOFPatchevVKChronic melatonin treatment and the hypothalamo-pituitary-adrenal axis in the rat: attenuation of the secretory response to stress and effects on hypothalamic neuropeptide content and releaseBiol Cell19978995875969673011
- KonakchievaRMitevYAlmeidaOFPatchevVKChronic melatonin treatment counteracts glucocorticoid-induced dysregulation of the hypothalamic-pituitary-adrenal axis in the ratNeuroendocrinology19986731711809630434
- BardenNShinkELabbeMVacherRRochfordJMocaerEAntidepressant action of agomelatine (S20098) in a transgenic mouse modelProg Neuropsychopharmacol Biol Psychiatry200529690891616005135
- PresmanDMHoijmanECeballosNRGalignianaMDPecciAMelatonin inhibits glucocorticoid receptor nuclear translocation in mouse thymocytesEndocrinology2006147115452545916916958
- FernandesPABothorelBClesseDLocal corticosterone infusion enhances nocturnal pineal melatonin production in vivoJ Neuroendocrinol2009212909719076264
- Couto-MoraesRPalermo-NetoJMarkusRPThe immune-pineal axis: stress as a modulator of pineal gland functionAnn N Y Acad Sci2009115319320219236342
- FerreiraZSFernandesPADumaDAssreuyJAvellarMCMarkusRPCorticosterone modulates noradrenaline-induced melatonin synthesis through inhibition of nuclear factor kappa BJ Pineal Res200538318218815725340
- FischerSSmolnikRHermsMBornJFehmHLMelatonin acutely improves the neuroendocrine architecture of sleep in blind individualsJ Clin Endocrinol Metab200388115315532014602767
- BrietzkeEStertzLFernandesBSComparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorderJ Affect Disord2009116321421719251324
- JehnCFKuhnhardtDBartholomaeAAssociation of IL-6, hypothalamus-pituitary-adrenal axis function, and depression in patients with cancerIntegr Cancer Ther20109327027520702499
- AlesciSMartinezPEKelkarSMajor depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implicationsJ Clin Endocrinol Metab20059052522253015705924
- BouhuysALFlentgeFOldehinkelAJvan den BergMDPotential psychosocial mechanisms linking depression to immune function in elderly subjectsPsychiatry Res2004127323724515296823
- TiemeierHHofmanAvan TuijlHRKiliaanAJMeijerJBretelerMMInflammatory proteins and depression in the elderlyEpidemiology200314110310712500057
- LanquillonSKriegJCBening-Abu-ShachUVedderHCytokine production and treatment response in major depressive disorderNeuropsychopharmacology200022437037910700656
- MikovaOYakimovaRBosmansEKenisGMaesMIncreased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosisEur Neuropsychopharmacol200111320320811418279
- TugluCKaraSHCaliyurtOVardarEAbayEIncreased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorderPsychopharmacology (Berl)2003170442943312955291
- OwenBMEcclestonDFerrierINYoungAHRaised levels of plasma interleukin-1beta in major and postviral depressionActa Psychiatr Scand2001103322622811240580
- FordDEErlingerTPDepression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination SurveyArch Intern Med200416491010101415136311
- DannerMKaslSVAbramsonJLVaccarinoVAssociation between depression and elevated C-reactive proteinPsychosom Med200365334735612764206
- ThomasAJDavisSMorrisCJacksonEHarrisonRO’BrienJTIncrease in interleukin-1beta in late-life depressionAm J Psychiatry2005162117517715625217
- YuYWChenTJHongCJChenHMTsaiSJAssociation study of the interleukin-1 beta (C-511T) genetic polymorphism with major depressive disorder, associated symptomatology, and antidepressant responseNeuropsychopharmacology20032861182118512700687
- JunTYPaeCUHoonHPossible association between G308A tumour necrosis factor-alpha gene polymorphism and major depressive disorder in the Korean populationPsychiatr Genet200313317918112960751
- CapuronLMillerAHCytokines and psychopathology: lessons from interferon-alphaBiol Psychiatry2004561181982415576057
- ParianteCMPearceBDPisellTLThe proinflammatory cytokine, interleukin-1alpha, reduces glucocorticoid receptor translocation and functionEndocrinology199914094359436610465310
- OrruMGBaitaASitziaRInterferon-alpha-induced psychiatric side effects in patients with chronic viral hepatitis: a prospective, observational, controlled studyEpidemiol Psichiatr Soc2005143145153 Italian16255161
- MusselmanDLLawsonDHGumnickJFParoxetine for the prevention of depression induced by high-dose interferon alfaN Engl J Med20013441396196611274622
- HuangYHenryCJDantzerRJohnsonRWGodboutJPExaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharideNeurobiol Aging200829111744175317543422
- PaceTWHuFMillerAHCytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depressionBrain Behav Immun200721191917070667
- HaddadJJSaadeNESafieh-GarabedianBCytokines and neuroimmune- endocrine interactions: a role for the hypothalamic-pituitary-adrenal revolving axisJ Neuroimmunol20021331–211912446003
- Spath-SchwalbeEHansenKSchmidtFAcute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy menJ Clin Endocrinol Metab1998835157315799589658
- MaddockCParianteCMHow does stress affect you? An overview of stress, immunity, depression and diseaseEpidemiol Psichiatr Soc2001103153162 Italian11787449
- ParianteCMPearceBDPisellTLOwensMJMillerAHSteroid-independent translocation of the glucocorticoid receptor by the antidepressant desipramineMol Pharmacol19975245715819380019
- WangXWuHMillerAHInterleukin 1alpha (IL-1alpha) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor functionMol Psychiatry200491657514699442
- PierpaoliWNeuroimmunomodulation of aging. A program in the pineal glandAnn N Y Acad Sci19988404914979629275
- Skwarlo-SontaKMajewskiPMarkowskaMOblapROlszanskaBBidirectional communication between the pineal gland and the immune systemCan J Physiol Pharmacol200381434234912769226
- da Silveira Cruz-MachadoSCarvalho-SousaCETamuraEKTLR4 and CD14 receptors expressed in rat pineal gland trigger NFKB pathwayJ Pineal Res201049218319220586888
- BrancheyLWeinbergUBrancheyMLinkowskiPMendlewiczJSimultaneous study of 24-hour patterns of melatonin and cortisol secretion in depressed patientsNeuropsychobiology1982852252327133371
- BlomekeBGolkaKGriefahnBRoemerHCArylalkylamine N-acetyltransferase (AANAT) genotype as a personal trait in melatonin synthesisJ Toxicol Environ Health A20087113–1487487618569588
- CardinaliDPVacasMIKeller SarmientoMIEtchegoyenGSPereyraENChuluyanHENeuroendocrine integrative mechanisms in mammalian pineal gland: effects of steroid and adenohypophyseal hormones on melatonin synthesis in vitroJ Steroid Biochem1987271–35655712447392
- MurrayDRPrabhuSDChandrasekarBChronic beta-adrenergic stimulation induces myocardial proinflammatory cytokine expressionCirculation2000101202338234110821806
- SchauensteinKFelsnerPRinnerIIn vivo immunomodulation by peripheral adrenergic and cholinergic agonists/antagonists in rat and mouse modelsAnn N Y Acad Sci200091761862711268390
- MayerBHolmerSRHengstenbergCLiebWPfeiferMSchunkertHFunctional improvement in heart failure patients treated with beta-blockers is associated with a decline of cytokine levelsInt J Cardiol2005103218218616080978
- BierhausAWolfJAndrassyMA mechanism converting psychosocial stress into mononuclear cell activationProc Natl Acad Sci U S A200310041920192512578963
- MaestroniGJContiAPierpaoliWRole of the pineal gland in immunity. Circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosteroneJ Neuroimmunol198613119302944914
- ChuangJIMohanNMeltzMLReiterRJEffect of melatonin on NF-kappa-B DNA-binding activity in the rat spleenCell Biol Int199620106876928969462
- MohanNSadeghiKReiterRJMeltzMLThe neurohormone melatonin inhibits cytokine, mitogen and ionizing radiation induced NF-kappa BBiochem Mol Biol Int1995376106310708747536
- CristofanonSUguccioniFCerellaCIntracellular prooxidant activity of melatonin induces a survival pathway involving NF-kappaB activationAnn N Y Acad Sci2009117147247819723091
- CeconEFernandesPAPinatoLFerreiraZSMarkusRPDaily variation of constitutively activated nuclear factor kappa B (NFKB) in rat pineal glandChronobiol Int2010271526720205557
- JinWWangHYanWDisruption of Nrf2 enhances upregulation of nuclear factor-kappaB activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injuryMediators Inflamm2008200872517419190763
- ThimmulappaRKLeeHRangasamyTNrf2 is a critical regulator of the innate immune response and survival during experimental sepsisJ Clin Invest2006116498499516585964
- SmoakKACidlowskiJAMechanisms of glucocorticoid receptor signaling during inflammationMech Ageing Dev200412510–1169770615541765
- MillerMAKandalaNBKivimakiMGender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II studySleep200932785786419639748
- Carrillo-VicoAGuerreroJMLardonePJReiterRJA review of the multiple actions of melatonin on the immune systemEndocrine200527218920016217132
- RadognaFDiederichMGhibelliLMelatonin: a pleiotropic molecule regulating inflammationBiochem Pharmacol201080121844185220696138
- Skwarlo-SontaKMelatonin in immunity: comparative aspectsNeuro Endocrinol Lett200223Suppl 1616612019354
- WichersMCMaesMThe role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depressionJ Psychiatry Neurosci2004291111714719046
- WichersMCKoekGHRobaeysGVerkerkRScharpeSMaesMIDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicityMol Psychiatry200510653854415494706
- MullerNSchwarzMJThe immune-mediated alteration of serotonin and glutamate: towards an integrated view of depressionMol Psychiatry20071211988100017457312
- MaesMYirmyiaRNorabergJThe inflammatory and neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depressionMetab Brain Dis2009241275319085093
- O’ConnorJCLawsonMAAndreCLipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in miceMol Psychiatry200914551152218195714
- RacagniGPopoliMThe pharmacological properties of antidepressantsInt Clin Psychopharmacol201025311713120305568
- ParfittAKleinDCIncrease caused by desmethylimipramine in the production of [3H]melatonin by isolated pineal glandsBiochem Pharmacol1977269904905861059
- CowenPJFraserSGrahame-SmithDGGreenARStanfordCThe effect of chronic antidepressant administration on beta-adrenoceptor function of the rat pinealBr J Pharmacol198378189966130811
- FranklinMClementEMCamplingGCowenPJEffect of venlafaxine on pineal melatonin and noradrenaline in the male ratJ Psychopharmacol199812437137410065911
- UzTAhmedRAkhisarogluMEffect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatumNeuroscience200513441309131615994025
- PastrakuljicATangBKRobertsEAKalowWDistinction of CYP1A1 and CYP1A2 activity by selective inhibition using fluvoxamine and isosafroleBiochem Pharmacol19975345315389105404
- HartterSWangXWeigmannHDifferential effects of fluvoxamine and other antidepressants on the biotransformation of melatoninJ Clin Psychopharmacol200121216717411270913
- BadawyAAMorganCJEffects of acute paroxetine administration on tryptophan metabolism and disposition in the ratBr J Pharmacol199110224294331826617
- BearnJFraneyCArendtJCheckleySAA study of the effects of desipramine treatment alone and in combination with L-triiodothyronine on 6-sulphatoxymelatonin excretion in depressed patientsBr J Psychiatry19891553413472611544
- KennedySHBrownGMEffect of chronic antidepressant treatment with adinazolam and desipramine on melatonin outputPsychiatry Res19924321771851410073
- ThompsonCMezeyGCornTThe effect of desipramine upon melatonin and cortisol secretion in depressed and normal subjectsBr J Psychiatry19851473893934075027
- GoldenRNMarkeySPRisbyEDRudorferMVCowdryRWPotterWZAntidepressants reduce whole-body norepinephrine turnover while enhancing 6-hydroxymelatonin outputArch Gen Psychiatry19884521501543122700
- SchmidDAWichniakAUhrMChanges of sleep architecture, spectral composition of sleep EEG, the nocturnal secretion of cortisol, ACTH, GH, prolactin, melatonin, ghrelin, and leptin, and the DEXCRH test in depressed patients during treatment with mirtazapineNeuropsychopharmacology200631483284416237393
- CarvalhoLGorensteinCMorenoRParianteCMarkusREffect of antidepressants on melatonin metabolite in depressed patientsJ Psychopharmacol200923331532118562432
- Rabe-JablonskaJSzymanskaADiurnal profile of melatonin secretion in the acute phase of major depression and in remissionMed Sci Monit20017594695211535940
- MillerHLEkstromRDMasonGALydiardRBGoldenRNNoradrenergic function and clinical outcome in antidepressant pharmacotherapyNeuropsychopharmacology200124661762311331141
- FraneyCAldhousMBurtonSCheckleySArendtJAcute treatment with desipramine stimulates melatonin and 6-sulphatoxy melatonin production in manBr J Clin Pharmacol198622173793741729
- DemischKDemischLBochnikHJMelatonin and cortisol increase after fluvoxamineBr J Clin Pharmacol19862256206223098271
- PalazidouESkeneDArendtJEverittBCheckleySAThe acute and chronic effects of (+) and (−) oxaprotiline upon melatonin secretion in normal subjectsPsychol Med199222161671315445
- PalazidouEPapadopoulosASitsenAStahlSCheckleySAn alpha 2 adrenoceptor antagonist, Org 3770, enhances nocturnal melatonin secretion in manPsychopharmacology (Berl)19899711151172565588
- MarkusRPFrancoDGCarvalhoLAGentilVGorensteinCAcute increase in urinary 6-sulfatoximelatonin after clomipramine, as a predictive measure for emotional improvementJ Psychopharmacol201024685586019264813
- DemischKDemischLNickelsenTRiethRThe influence of acute and subchronic administration of various antidepressants on early morning melatonin plasma levels in healthy subjects: increases following fluvoxamineJ Neural Transm1987683–42572703104537
- SkeneDJBojkowskiCJArendtJComparison of the effects of acute fluvoxamine and desipramine administration on melatonin and cortisol production in humansBr J Clin Pharmacol19943721811868186063
- CeladaPPerezJAlvarezEArtigasFMonoamine oxidase inhibitors phenelzine and brofaromine increase plasma serotonin and decrease 5-hydroxyindoleacetic acid in patients with major depression: relationship to clinical improvementJ Clin Psychopharmacol19921253093151282522
- ZolkowskaDRothmanRBBaumannMHAmphetamine analogs increase plasma serotonin: implications for cardiac and pulmonary diseaseJ Pharmacol Exp Ther2006318260461016644904
- ChildsPARodinIMartinNJEffect of fluoxetine on melatonin in patients with seasonal affective disorder and matched controlsBr J Psychiatry199516621961987728363
- NathanPJNormanTRBurrowsGDNocturnal plasma melatonin concentrations in healthy volunteers: effect of single doses of d-fenfluramine, paroxetine, and ipsapironeJ Pineal Res199621255588912230
- TanZLBaoAMZhaoGQLiuYJZhouJNEffect of fluoxetine on circadian rhythm of melatonin in patients with major depressive disorderNeuro Endocrinol Lett2007281283217277729
- CuestaMMendozaJClesseDPevetPChalletESerotonergic activation potentiates light resetting of the main circadian clock and alters clock gene expression in a diurnal rodentExp Neurol2008210250151318190911
- SprouseJBraseltonJReynoldsLFluoxetine modulates the circadian biological clock via phase advances of suprachiasmatic nucleus neuronal firingBiol Psychiatry200660889689916631132
- PeifferAVeilleuxSBardenNAntidepressant and other centrally acting drugs regulate glucocorticoid receptor messenger RNA levels in rat brainPsychoneuroendocrinology19911665055151811246
- ParianteCMMakoffALovestoneSAntidepressants enhance glucocorticoid receptor function in vitro by modulating the membrane steroid transportersBr J Pharmacol200113461335134311704655
- OkugawaGOmoriKSuzukawaJFujisekiYKinoshitaTInagakiCLong-term treatment with antidepressants increases glucocorticoid receptor binding and gene expression in cultured rat hippocampal neuronesJ Neuroendocrinol1999111188789510520140
- CarvalhoLAGarnerBADewTFazakerleyHParianteCMAntidepressants, but not antipsychotics, modulate GR function in human whole blood: an insight into molecular mechanismsEur Neuropsychopharmacol201020637938720231081
- HuJXiaYWuZLiuLTangCFluoxetine might alleviate brain damage and hypercortisolemia related to chronic alcohol in ratsJ Stud Alcohol Drugs201071229029420230727
- OberlanderTFWeinbergJPapsdorfMGrunauRMisriSDevlinAMPrenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responsesEpigenetics2008329710618536531
- FischliSJenniSAllemannSDehydroepiandrosterone sulfate in the assessment of the hypothalamic-pituitary-adrenal axisJ Clin Endocrinol Metab200893253954217986637
- SchuleCBaghaiTCEserDSchwarzMBondyBRupprechtREffects of mirtazapine on dehydroepiandrosterone-sulfate and cortisol plasma concentrations in depressed patientsJ Psychiatr Res200943553854518706658
- PaslakisGLuppaPGillesMVenlafaxine and mirtazapine treatment lowers serum concentrations of dehydroepiandrosterone-sulfate in depressed patients remitting during the course of treatmentJ Psychiatr Res201044855656020022345
- BinderEBKunzelHENickelTHPA-axis regulation at inpatient admission is associated with antidepressant therapy outcome in male but not in female depressed patientsPsychoneuroendocrinology20093419910918829172
- JuruenaMFParianteCMPapadopoulosASPoonLLightmanSCleareAJPrednisolone suppression test in depression: prospective study of the role of HPA axis dysfunction in treatment resistanceBr J Psychiatry2009194434234919336786
- HallamKTBeggDPOlverJSNormanTRAn investigation of the effect of immediate and extended release venlafaxine on nocturnal melatonin and cortisol release in healthy adult volunteersHum Psychopharmacol200823212913718172907
- AhrensTFrankhauserPLederbogenFDeuschleMEffect of single-dose sertraline on the hypothalamus-pituitary-adrenal system, autonomic nervous system, and platelet functionJ Clin Psychopharmacol200727660260618004127
- HashiokaSKlegerisAMonjiAAntidepressants inhibit interferon-gamma-induced microglial production of IL-6 and nitric oxideExp Neurol20072061334217481608
- ObuchowiczEKowalskiJLabuzekKKrysiakRPendzichJHermanZSAmitriptyline and nortriptyline inhibit interleukin-1 release by rat mixed glial and microglial cell culturesInt J Neuropsychopharmacol200691273515963243
- VollmarPNesslerSKalluriSRHartungHPHemmerBThe antidepressant venlafaxine ameliorates murine experimental autoimmune encephalomyelitis by suppression of pro-inflammatory cytokinesInt J Neuropsychopharmacol200912452553618922202
- TalerMGil-AdILomnitskiLImmunomodulatory effect of selective serotonin reuptake inhibitors (SSRIs) on human T lymphocyte function and gene expressionEur Neuropsychopharmacol2007171277478017499975
- XiaZDePierreJWNassbergerLTricyclic antidepressants inhibit IL-6, IL-1 beta and TNF-alpha release in human blood monocytes and IL-2 and interferon-gamma in T cellsImmunopharmacology199634127378880223
- Szuster-CiesielskaATustanowska-StachuraASlotwinskaMMarmurowska-MichalowskaHKandefer-SzerszenMIn vitro immunoregulatory effects of antidepressants in healthy volunteersPol J Pharmacol200355335336214506314
- MaesMSongCLinAHNegative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretionNeuropsychopharmacology199920437037910088138
- KuberaMLinAHKenisGBosmansEvan BockstaeleDMaesMAnti-inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratioJ Clin Psychopharmacol200121219920611270917
- KimYKNaKSShinKHJungHYChoiSHKimJBCytokine imbalance in the pathophysiology of major depressive disorderProg Neuropsychopharmacol Biol Psychiatry20073151044105317433516
- SutcigilLOktenliCMusabakUPro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapyClin Dev Immunol200720077639618317531
- WalshHADayaSInfluence of the antidepressants desipramine and fluoxetine on tryptophan-2,3-dioxygenase in the presence of exogenous melatoninLife Sci19986226241724239651108
- JanssenDGCaniatoRNVersterJCBauneBTA psychoneuroimmunological review on cytokines involved in antidepressant treatment responseHum Psychopharmacol201025320121520373471
- MillanMJGobertALejeuneFThe novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathwaysJ Pharmacol Exp Ther2003306395496412750432
- de BodinatCGuardiola-LemaitreBMocaerERenardPMunozCMillanMJAgomelatine, the first melatonergic antidepressant: discovery, characterization and developmentNat Rev Drug Discov20109862864220577266
- HowlandRHCritical appraisal and update on the clinical utility of agomelatine, a melatonergic agonist, for the treatment of major depressive disease in adultsNeuropsychiatr Dis Treat2009556357619966905
- MartinetLGuardiola-LemaitreBMocaerEEntrainment of circadian rhythms by S-20098, a melatonin agonist, is dose and plasma concentration dependentPharmacol Biochem Behav19965447137188853194
- VanROWeibelLOlivaresEMaccariSMocaerETurekFWMelatonin or a melatonin agonist corrects age-related changes in circadian response to environmental stimulusAm J Physiol Regul Integr Comp Physiol20012805R1582159111294784
- CajochenCKrauchiKMoriDGrawPWirz-JusticeAMelatonin and S-20098 increase REM sleep and wake-up propensity without modifying NREM sleep homeostasisAm J Physiol19972724 Pt 2R118911969140019
- KrauchiKCajochenCMoriDGrawPWirz-JusticeAEarly evening melatonin and S-20098 advance circadian phase and nocturnal regulation of core body temperatureAm J Physiol19972724 Pt 2R117811889140018
- LlorcaPMThe antidepressant agomelatine improves the quality of life of depressed patients: implications for remissionJ Psychopharmacol201024Suppl 2212620663805
- Quera-SalvaMALemoinePGuilleminaultCImpact of the novel antidepressant agomelatine on disturbed sleep-wake cycles in depressed patientsHum Psychopharmacol201025322222920373473
- KasperSHajakGWulffKEfficacy of the novel antidepressant agomelatine on the circadian rest-activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: a randomized, double-blind comparison with sertralineJ Clin Psychiatry201071210912020193645
- OlieJPKasperSEfficacy of agomelatine, a MT1/MT2 receptor agonist with 5-HT2C antagonistic properties, in major depressive disorderInt J Neuropsychopharmacol200710566167317477888
- SerrettiAChiesaATreatment-emergent sexual dysfunction related to antidepressants: a meta-analysisJ Clin Psychopharmacol200929325926619440080
- MontgomerySAKennedySHBurrowsGDLejoyeuxMHindmarchIAbsence of discontinuation symptoms with agomelatine and occurrence of discontinuation symptoms with paroxetine: a randomized, double-blind, placebo-controlled discontinuation studyInt Clin Psychopharmacol200419527128015289700