Abstract
Objective
To identify whether the amplitude of low-frequency fluctuations (ALFF) analysis has the potential to serve as a biological marker to detect alcohol-induced spontaneous brain activities and distinguish the patients with alcohol dependence from the healthy subjects.
Methods
We utilized the ALFF analysis to report on the alcohol-induced spontaneous brain activities in 29 patients with alcohol dependence (9 female, 20 male) relative to 29 status-matched healthy subjects (11 female, 18 male). Receiver operating characteristic curve was used to test the ability of the ALFF analysis in discriminating the patients with alcohol dependence from the healthy subjects. Pearson correlation was used to evaluate the relationships between the signal value of those ALFF differences in brain areas and behavioral characteristics.
Results
Alcohol-induced brain differences located in the right inferior parietal lobule and right supplementary motor area with significant higher ALFF values, and in the left precuneus and bilateral cerebellum posterior lobe with lower ALFF values. The movement-related areas were significantly correlated with each other (P<0.05). Receiver operating characteristic curve revealed good area under the curve values (mean, 0.86±0.079; 0.774–0.951) of the ALFF differences in those specific brain areas, as well as high degree of sensitivities (mean, 80.84%±14.01% or 80%±14.56%; 62.5%–100%) and specificities (mean, 83.32%±9.31%; 70.8%–95.8% or 84.16%±8%; 75%–95.8%).
Conclusion
The ALFF analysis may serve as a biological indicator to detect the spontaneous brain activities in patients with alcohol dependence. The prefrontal–parietal–cerebellar circuit appears to be disturbed by long-term alcoholism in patients with alcohol dependence.
Introduction
Alcohol consumption, the most frequent substance addiction with a high morbidity or mortality, is a serious public problem. The pernicious effects of extravagant alcohol consumption on brain and behavior are well known. It brings numerous adverse health consequences such as esophageal/liver cancer, liver cirrhosis, and vehicle accidents.Citation1 Alcohol consumption would bring perceived relief from the negative emotions, such as stress and anxiety, which may thereby increase and/or reinforce the likelihood of future drinking behavior.Citation2 Therefore, alcohol dependence is a chronic relapsing disorder, characterized by morbid alcohol consumption.
Modern imaging methods promote scholars to investigate the neurobiology mechanisms and consequences caused by alcohol addiction. These imaging methods are widely accepted for the detection of specific regional brain alterations in diseases. Resting-state functional MRI (rs-fMRI) can be used to visualize the brain activities associated with oxygenation and blood flow changes, and the responses of regional brain blood flow to drug-related cues. Furthermore, the rs-fMRI also can be used to visualize the addictive symptoms and cognitive capacity caused by alcohol consumption. These changes cannot be identified by traditional fMRI analyses. Therefore, the rs-fMRI is suitable to detect the alteration of spontaneous neuronal brain activity and can be used for pathophysiological mechanism exploration for several diseases.Citation3 With the rapid development of these rs-fMRI technologies, neuroimaging studies have described diverse pernicious effects from alcohol dependence, including the neurochemical changes and regional functional activity in the brain,Citation4 which may yielded insights into the neurobiological mechanisms underlying alcohol dependence and may help clinicians assess the disease and aid in dynamic monitoring of the response to therapy, as well as guide care interventions. Alcohol dependence is associated with the changes of regional brain activities in several areas, which makes its neurobiological mechanism more complex. Although recent studies of structural and functional studies have increased tremendously and have identified several brain regions that are relevant to alcoholism,Citation5,Citation6 the neurobiological mechanism underlying alcohol dependence remains largely unknown.
The amplitude of low-frequency fluctuations (ALFF) analysis does not require prior hypothesis and/or knowledge, and has good test–retest reliability, which makes it useful for location of altered brain regions with abnormal spontaneous neuronal brain activity.Citation3,Citation7–Citation10 Therefore, the reliable characterization and simple calculation make the ALFF analysis a potential useful tool to study the alcohol dependence-induced intrinsic brain activities.Citation4 Recently, the use of the ALFF analysis has been successfully applied in several disorders.Citation3,Citation11–Citation13 However, to our knowledge, it has not been used in alcohol dependence. In the current study, we hypothesized that the alcohol dependence is associated with distinct intrinsic neuronal spontaneous activity in several brain areas with ALFF changes. To test the hypothesis, the current study utilized the ALFF analysis to identify altered functional brain areas in 29 patients with alcohol dependence relative to 29 status-matched healthy subjects. Next, we used Pearson correlations to evaluate the relationships between those brain areas with ALFF differences and behavioral characteristics. We also utilized receiver operating characteristic (ROC) curve to investigate whether the regional brain areas with ALFF differences have the ability to distinguish the patients with alcohol dependence from the status-matched healthy subjects.
Materials and methods
Subjects
A total of 29 patients with alcohol dependence (9 female, 20 male; education, 9.52±2.87 years; age, 48.62±6.81 years; mean ± SD) and 29 status-matched healthy subjects (11 female, 18 male; education, 8.48±3.1 years; age, 48.48±7.05 years) participated in the present study. The life history of psychiatric disorders, daily alcohol consumption, Severity of Alcohol Dependence Questionnaire (SADQ), mean years of alcohol consumption, and Alcohol Use Disorders Identification Test (AUDIT) were recorded by an experienced psychiatrist who had worked for more than 10 years.
Patients with alcohol dependence met the diagnostic criteria as defined by The Diagnostic and Statistical Manual of Mental Disorders, version IV (DSM-IV). All recruited volunteers met the following inclusion criteria as in previous studies,Citation14,Citation15 first-time visitors who had not taken any medications treatment before, had no history of other substance dependence or abuse, had no sleep disorders and major psychiatric disorders, had no foreign implants, and had no pathological brain MRI findings. The present study was approved by the Medical Research Ethical Committee of The Affiliated Huai’an No. 1 People’s Hospital of Nanjing Medical University in accordance with the Declaration of Helsinki. The written informed consent from all volunteers was collected.
MRI parameters
We performed the MRI scan on a 3.0 Tesla MR scanner (Siemens, Munich, Germany). First, 176 high-resolution 3D T1-weighted anatomical images of in sagittal orientation (repetition time/echo time =1,950/2.3 ms, gap/thickness =0/1 mm, field of view =244×252 mm2, acquisition matrix =248×256, flip angle =9°) were collected. Next, we collected 240 functional images (repetition time/echo time =3,000/25 ms, gap/thickness =0.5/5.0 mm, flip angle =90°, acquisition matrix =32×32, field of view =210×210 mm2) covering the whole brain.
Data analysis
First, we deleted the first 10 time points of the functional images. The remaining data were dealt with standards for form transformation. The data preprocessing of the remaining data was made up of the following steps: including the slice timing, head motion correction, spatial normalization to the Montreal Neurological Institute space, and smoothing with Gaussian kernel of 6×6×6 mm3. The data of volunteers with >1.5 mm maximum translation or/and >1.5 degree of rotation in any direction were discarded. After this, the remaining images were resampled at a resolution of 3×3×3 mm3 during the step of spatial normalization. Linear regression was applied to remove the effects of spurious covariates, including the Friston 24 head motion parameters, white matter signal, and cerebrospinal fluid signal. Next, the functional images were entered into temporal bandpass filter (0.01–0.1 Hz) and linearly detrended. The calculation details of the ALFF analysis have been presented in previous studies.Citation3,Citation10,Citation16
ROC curve and brain-behavior correlation analysis
Recently, ROC curve has been increasingly applied to identify whether one image analysis may serve as a biological indicator to distinguish one group from another group.Citation4,Citation11,Citation14 The mean β values of the ALFF differences were extracted for ROC curve to identify whether the ALFF analysis may server as a biological indicator to distinguish the patients with alcohol dependence from healthy subjects. Pearson correlation analysis was used to evaluate the relationships between the ALFF differences in brain areas and behavioral characteristics. The statistical threshold was set at P<0.05.
Statistical analysis
Data are presented as mean ± SD. The demographic characteristics (age, AUDIT score, and years of education) were analyzed with independent sample unpaired t-tests. The categorical data (sex distribution) were compared using Chi-squared (χ2) test. The statistical threshold was set at P<0.05.
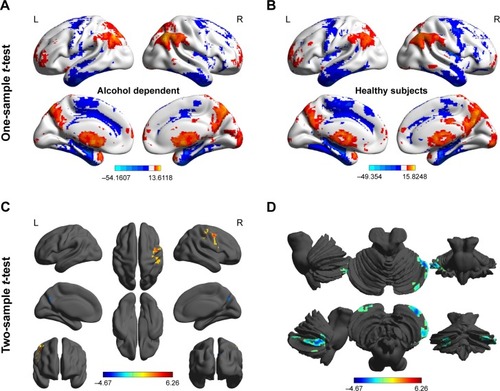
Before comparing the ALFF differences between groups, we used one-sample t-tests to construct within-group statistical maps of ALFF analysis to identify the network distributions of each group (P<0.001, false discovery rate (FDR) corrected). Then, independent sample unpaired t-tests were utilized to study the ALFF differences in regional brain areas between patients with alcohol dependence and healthy controls with nuisance covariates (age, sex, and years of education) of no interest. AlphaSim correction (threshold of individual voxel of P<0.01, cluster level of P<0.05 with contiguous voxel volume ≥1,620 mm3) was used to determine the statistical differences.
Results
Sample characteristics
The behavioral characteristics of the alcohol dependence and the healthy subjects are presented in . Patients with alcohol dependence did not significantly differ from healthy subjects in mean age (t=0.076, P=0.94), sex distribution (χ2=0.305, P=0.581), and mean education (t=1.318, P=0.193). The mean AUDIT score was higher in patients with alcohol dependence than healthy controls (t=20.353, P<0.001). In the alcohol dependence group, the mean duration of drink history was (27.93±10.28 years, range: 7–45 years), the mean SADQ score was (20.34±6.89), and the mean daily alcohol consumption was (mean ± SD, 239.66±107.22 mL).
Table 1 Characteristics of alcohol dependents and healthy subjects
ALFF differences
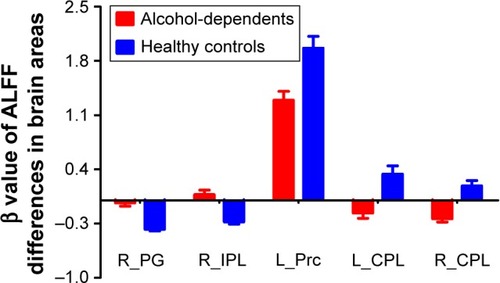
Before comparing the ALFF differences between patients with alcohol dependence and healthy controls, one-sample t-test were used to construct within-group statistical maps for alcohol dependence group () and healthy subjects () separately (P<0.001, FDR corrected). We found that the locations of the ALFF differences in brain areas of patients with alcohol dependence differ from healthy subjects in several areas (). Next, we performed t-test to investigate the ALFF differences between groups. Patients with alcohol dependence relative to healthy subjects demonstrated differences in brain areas in the right inferior parietal lobule (Brodmann’s area [BA] 40) and right supplementary motor area (BA 6) with higher ALFF values, and the left precuneus (BA 7) and bilateral cerebellum posterior lobe with lower ALFF values (, ).
Table 2 The ALFF differences between patients with alcohol dependence and healthy subjects
Figure 1 Altered ALFF areas in patient with alcohol dependence relative to healthy subject.
Abbreviations: ALFF, amplitude of low-frequency fluctuation; L, left; R, right.
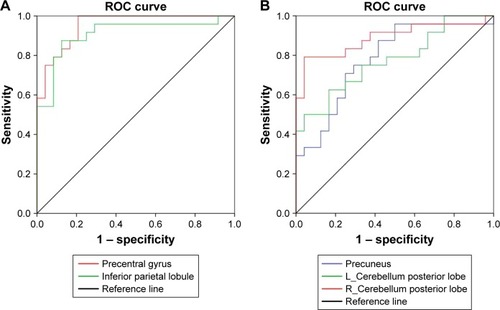
ROC curve
The mean β value of those altered ALFF values in brain areas was extracted () for ROC curve analysis. The area under the curve (AUC) value is considered as good to excellent if it is >0.8, fair if between 0.7 and 0.8, and poor to failed if lower than 0.7.Citation17 Our findings demonstrated that the ROC curve revealed good AUC values (mean ± SD, 0.86±0.079; range: 0.774–0.951) of those specific brain areas with ALFF differences. Further diagnostic analysis exhibited that those specific areas with ALFF differences alone discriminated the alcohol dependents from the healthy subjects with high degree of sensitivities (mean ± SD, 80.84%±14.01% or 80%±14.56%; range: 62.5%–100%) and specificities (mean ± SD, 83.32%±9.31%; range: 70.8%–95.8% or 84.16%±8%; range: 75%–95.8%) ( and ).
Table 3 ROC curve for ALFF differences in brain areas between alcohol dependent and healthy subjects
Pearson correlation analysis
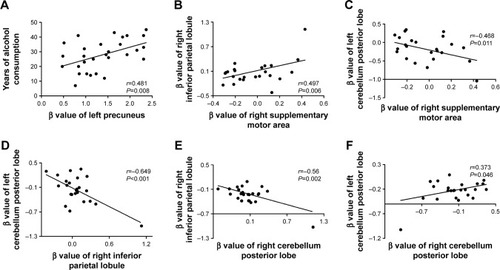
In the alcohol dependence group, the mean years of alcohol consumption displayed a positive correlation with the β value of left precuneus (r=0.481, P=0.008; ); other significant correlations between the β value of brain areas with ALFF differences and the clinical features were not found (P>0.05). However, several correlations among the β value of ALFF differences in brain areas were found (). The β value of the right supplementary motor area showed a positive correlation with that of the right inferior parietal lobule (r=0.497, P=0.006; ), and a negative correlation with that of the left cerebellum posterior lobe (r=−0.468, P=0.011; ). The β value of the left and right cerebellum posterior lobes, respectively, displayed a negative correlation with that of the right inferior parietal lobule (left, r=−0.649, P<0.001, ; right, r=−0.56, P=0.002, ). The β value of the left and right cerebellum posterior lobe also showed a positive correlation with each other (r=0.373, P=0.046; ).
Figure 4 Pearson correlation among characteristics of alcohol dependent and β value of ALFF differences in brain areas.
Abbreviation: ALFF, amplitude of low-frequency fluctuation.
Discussion
The current study is the first to utilize the ALFF analysis to identify altered functional brain areas in 29 patients with alcohol dependence relative to 29 status-matched healthy subjects. ROC curve was applied to identify the ability of those ALFF differences in distinguishing the two groups. The present study revealed the following main results: 1) alcohol dependence was associated with right supplementary motor area and right inferior parietal lobule with significant higher ALFF differences, and left precuneus and bilateral cerebellum posterior lobe with lower ALFF differences; 2) ROC curve revealed good AUC values of those specific brain areas, and further diagnostic analysis demonstrated that those specific brain areas alone discriminated the patients with alcohol dependence from the healthy subjects with high degree of sensitivities and specificities; 3) in the alcohol dependence group, the mean years of alcohol consumption displayed a positive correlation with the β value of the left precuneus, and several correlations among those specific brain areas were found.
Morphological–anatomical studies have found decreased gray matter volumes in the frontal lobeCitation18–Citation21 and cerebellumCitation20,Citation22 in patients with alcohol dependence. These changes have been shown to be predictive of relapse risk, suggesting a significant role of decreased gray matter volumes in the frontal lobe and cerebellum in clinical outcomes in alcohol dependence.Citation21 Similarly, resting-state functional connectivity studies also showed consistent findings. Distinct areas of the cerebellum posterior lobe have shown altered functional brain connectivity with the prefrontal and parietal lobes.Citation23,Citation24 Herting et alCitation25 found differences of contralateral functional connectivity between the prefrontal cortex and the lateral cerebellum in healthy adolescents, but this brain connectivity is atypical in high risk alcohol-naïve youth subjects with a history of family alcoholism. These disturbed functional connectivities also have been shown in the contralateral frontocerebellar regions,Citation23,Citation24 suggesting that white matter fibers decussate and cross over to the contralateral hemisphere between the cerebellum and the cerebral cortex.Citation26 The frontocerebellar connectivity was shown to be associated with fractional anisotropy value in the superior longitudinal fasciculus and anterior limb of the internal capsule. Notably, the superior longitudinal fasciculus has projections into the frontal cortex, while the anterior limb of the internal capsule carries the fibers that project from the frontal lobe to the cerebellum.Citation27 These findings showed functional and structural connectivity abnormalities in the prefrontal, parietal, and cerebellar cortex in patients with alcohol dependence. Our results of altered ALFF areas in the prefrontal lobe, inferior parietal lobule, and cerebellum posterior lobe support these findings.
Patients with alcohol dependence had impaired coordination while moving and impaired balance.Citation28 Poor regulation of coordinating movement and emotional changes are core characteristics of alcohol dependence.Citation14 The cerebellum posterior lobe is associated with regulation of nerve function and coordinating movement, emotion, and cognition, and is particularly vulnerable to alcoholism-related damage.Citation10,Citation28,Citation29 The frontal–temporal– basal ganglia and cerebellar circuits have been shown to overlap motor control function and are associated with motor behavior, which is disrupted by alcohol intoxication.Citation15,Citation30 The disturbed functional activities in these brain areas may be the main reasons for impaired driving behavior in alcoholics. Our results revealed that the alcohol dependence was associated with altered regional brain activities in the movement-related areas, including the supplementary motor area, cerebellum posterior lobe, and inferior parietal lobule. Furthermore, these movement-related areas were significantly correlated with each other. These areas have been shown to have altered functional connectivity in several studies in patients with alcohol dependence,Citation11,Citation14,Citation30,Citation31 and may therefore support our results.
The precuneus is thought to be engaged in visuospatial imagery, collection and evaluation of information, and self-processing operations,Citation32–Citation34 suggesting a key role in the advanced cognitive function. Disturbed regional brain activity or functional connectivity in the precuneus was not only found in primate brain suffer acute exposure to cue of alcohol,Citation35 but also in subjects with alcohol use disorder and/or after heavy drinking.Citation15,Citation36,Citation37 The increased brain connectivity between the precuneus and the cerebellum was also found in chronic alcohol consumption.Citation38 The metabolic activity in the precuneus was higher, and may require about 35% glucose, which is more than that needed in other areas.Citation39–Citation41 Our data showed that the β value of the precuneus with lower ALFF value displayed a positive correlation with mean years of alcohol consumption. The functional brain activity decrease in the precuneus may be interpreted as functional impairment caused by long-term alcoholism.
Conclusion
In summary, the ALFF analysis may serve as a biological indicator in the detection of regional spontaneous brain activities in patients with alcohol dependence with high degree of sensitivities and specificities. The prefrontal– parietal–cerebellar circuit appears to be disturbed by long-term alcoholism in patients with alcohol dependence. The findings of the present study could expand our understanding of the neurobiological mechanisms underlying alcohol dependence, and may help us develop targeted intervention and prevention strategies.
Acknowledgments
This study was supported by the Key Research and Development Projects in Shaanxi Province in the Field of Social Development (2017SF-081). This was not an industry-supported study.
Disclosure
The authors report no conflicts of interest in this work.
References
- WiseRABrain reward circuitry: insights from unsensed incentivesNeuron200236222924012383779
- BakerTBPiperMEMcCarthyDEMajeskieMRFioreMCAddiction motivation reformulated: an affective processing model of negative reinforcementPsychol Rev20041111335114756584
- DaiXJLiuCLZhouRLLong-term total sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: a resting-state fMRI studyNeuropsychiatr Dis Treat20151176177225834451
- VolkowNDFowlerJSWangGJThe addicted human brain: insights from imaging studiesJ Clin Invest2003111101444145112750391
- EverittBJBelinDEconomidouDPellouxYDalleyJWRobbinsTWReview. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addictionPhilos Trans R Soc Lond B Biol Sci200836315073125313518640910
- KoobGFVolkowNDNeurocircuitry of addictionNeuropsychopharmacology201035121723819710631
- SimpsonJRSnyderAZGusnardDARaichleMEEmotion-induced changes in human medial prefrontal cortex: I. During cognitive task performanceProc Natl Acad Sci U S A200198268368711209065
- MasonMFNortonMIvan HornJDWegnerDMGraftonSTMacraeCNWandering minds: the default network and stimulus-independent thoughtScience2007315581039339517234951
- YanHZhangYChenHWangYLiuYAltered effective connectivity of the default mode network in resting-state amnestic type mild cognitive impairmentJ Int Neuropsychol Soc201319440040923425569
- DaiXJNieXLiuXGender differences in regional brain activity in patients with chronic primary insomnia: evidence from a resting-state fMRI studyJ Clin Sleep Med201612336337426715399
- WangLChenYYaoYPanYSunYSleep deprivation disturbed regional brain activity in healthy subjects: evidence from a functional magnetic resonance-imaging studyNeuropsychiatr Dis Treat20161280180727110113
- HuangXZhongYLZengXJDisturbed spontaneous brain activity pattern in patients with primary angle-closure glaucoma using amplitude of low-frequency fluctuation: a fMRI studyNeuropsychiatr Dis Treat2015111877188326251603
- LiHJDaiXJGongHHNieXZhangWPengDCAberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRINeuropsychiatr Dis Treat20151120721425653530
- TuXWangJLiuXZhengJAberrant regional brain activities in alcohol dependence: a functional magnetic resonance imaging studyNeuropsychiatr Dis Treat20181484785329606878
- LuoXGuoLDaiXJAbnormal intrinsic functional hubs in alcohol dependence: evidence from a voxelwise degree centrality analysisNeuropsychiatr Dis Treat2017132011202028814870
- ZangYFHeYZhuCZAltered baseline brain activity in children with ADHD revealed by resting-state functional MRIBrain Dev2007292839116919409
- El KhouliRHMacuraKJBarkerPBHabbaMRJacobsMABluemkeDARelationship of temporal resolution to diagnostic performance for dynamic contrast enhanced MRI of the breastJ Magn Reson Imaging2009305999100419856413
- BühlerMMannKAlcohol and the human brain: a systematic review of different neuroimaging methodsAlcohol Clin Exp Res201135101771179321777260
- DemirakcaTEndeGKämmererNEffects of alcoholism and continued abstinence on brain volumes in both gendersAlcohol Clin Exp Res20113591678168521599718
- FeinGdi SclafaniVCardenasVAGoldmannHTolou-ShamsMMeyerhoffDJCortical gray matter loss in treatment-naïve alcohol dependent individualsAlcohol Clin Exp Res200226455856411981133
- RandoKHongKIBhagwagarZAssociation of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective studyAm J Psychiatry2011168218319221078704
- MakrisNOscar-BermanMJaffinSKDecreased volume of the brain reward system in alcoholismBiol Psychiatry200864319220218374900
- HabasCKamdarNNguyenDDistinct cerebellar contributions to intrinsic connectivity networksJ Neurosci200929268586859419571149
- O’ReillyJXBeckmannCFTomassiniVRamnaniNJohansen-BergHDistinct and overlapping functional zones in the cerebellum defined by resting state functional connectivityCereb Cortex201020495396519684249
- HertingMMFairDNagelBJAltered fronto-cerebellar connectivity in alcohol-naïve youth with a family history of alcoholismNeuroimage20115442582258920970506
- KellyRMStrickPLCerebellar loops with motor cortex and prefrontal cortex of a nonhuman primateJ Neurosci200323238432844412968006
- WakanaSJiangHNagae-PoetscherLMvan ZijlPCMoriSFiber tract-based atlas of human white matter anatomyRadiology20042301778714645885
- SullivanEVRosenbloomMJPfefferbaumAPattern of motor and cognitive deficits in detoxified alcoholic menAlcohol Clin Exp Res200024561162110832902
- DaiXJGongHHWangYXGender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: a resting-state fMRI studySleep Med201213672072722503940
- Rzepecki-SmithCIMedaSACalhounVDDisruptions in functional network connectivity during alcohol intoxicated drivingAlcohol Clin Exp Res201034347948720028354
- ZhengHKongLChenLZhangHZhengWAcute effects of alcohol on the human brain: a resting-state FMRI studyBiomed Res Int2015201594752925705701
- CavannaAETrimbleMRThe precuneus: a review of its functional anatomy and behavioural correlatesBrain2006129Pt 356458316399806
- AddisDRMcintoshARMoscovitchMCrawleyAPMcandrewsMPCharacterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approachNeuroimage20042341460147115589110
- LundstromBNIngvarMPeterssonKMThe role of precuneus and left inferior frontal cortex during source memory episodic retrievalNeuroimage200527482483415982902
- TelesfordQKLaurientiPJFriedmanDPKraftRADaunaisJBThe effects of alcohol on the nonhuman primate brain: a network science approach to neuroimagingAlcohol Clin Exp Res201337111891190023905720
- SchachtJPAntonRFMyrickHFunctional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic reviewAddict Biol201318112113322574861
- VergaraVMLiuJClausEDHutchisonKCalhounVAlterations of resting state functional network connectivity in the brain of nicotine and alcohol usersNeuroimage2017151455427864080
- ChanraudSPitelALPfefferbaumASullivanEVDisruption of functional connectivity of the default-mode network in alcoholismCereb Cortex201121102272228121368086
- FranssonPMarrelecGThe precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysisNeuroimage20084231178118418598773
- RaichleMEMacleodAMSnyderAZPowersWJGusnardDAShulmanGLA default mode of brain functionProc Natl Acad Sci U S A200198267668211209064
- UtevskyAVSmithDVHuettelSAPrecuneus is a functional core of the default-mode networkJ Neurosci201434393294024431451