Abstract
Background:
Progressive cognitive decline develops in a nontrivial minority of stroke survivors. Although commonly used to identify cognitive decline in older stroke survivors, the usefulness of the Mini-Mental State Examination (MMSE) as a screening tool for post-stroke cognitive decline across a wider range of ages is not well established. This study therefore investigated the usefulness of the MMSE for this purpose.
Methods:
Twenty-seven subjects, aged 18–82 years, with a single known remote stroke were assessed using the MMSE. The frequency of cognitive impairment was determined by comparison of MMSE scores with population-based norms. Relationships between cognitive performance, motor impairments, age, gender, handedness, stroke laterality, and time since stroke also were explored.
Results:
Age-adjusted MMSE scores identified mild cognitive impairment in 22.2% and moderate-to-severe cognitive impairment in 7.4% of subjects. Raw and age-adjusted MMSE scores were inversely correlated with time since stroke, but not with other patient or stroke characteristics.
Conclusion:
A relationship between time since single known stroke and MMSE performance was observed in this study. The proportion of subjects identified as cognitively impaired in this group by Z-transformation of MMSE scores using previously published normative data for this measure comports well with the rates of late post-stroke cognitive impairment reported by other investigators. These findings suggest that the MMSE, when normatively interpreted, may identify cognitive decline in the late period following single known stroke. Additionally, the lack of a relationship between MMSE and Fugl-Meyer scores suggests that the severity of post-stroke motor impairments is unlikely to serve as a clinically useful indicator of the need for cognitive assessment. A larger study of stroke survivors is needed to inform more fully on the usefulness of normatively interpreted MMSE scores as a method of screening for post-stroke cognitive decline.
Introduction
Improvement of acute stroke-induced cognitive impairments is expected over the months to years following stroke,Citation1–Citation8 with as many as 30% of stroke survivors experiencing complete cognitive recovery by 18 months post-stroke.Citation9 Among persons who do not experience a complete recovery from post-stroke cognitive impairments, conventional clinical wisdom suggests that those individuals maintain persistent but stable cognitive impairments thereafter. However, a nontrivial minority of stroke survivors develop progressive cognitive decline over the first two years following a single known stroke.
For example, Ballard et alCitation6 performed cognitive assessments three and 15 months following stroke in 115 individuals without overt dementia in the immediate post-stroke period. Although 50% of these subjects demonstrated cognitive improvements by 15 months following stroke, 9% declined cognitively over that same time period. These subjects were without prior or subsequent known strokes, suggesting that even a single known stroke may provoke vascular dementia. Other studies offer similar evidence of cognitive decline in the months to years following stroke, with rates of dementia by two years post-stroke of 9%–31%.Citation8–Citation11 In these studies, extended periods of observation after stroke (1–2 years) revealed higher rates of cognitive impairment than did studies with relatively short post-stroke observation periods (less than one year).
Other patient or stroke characteristics may facilitate the identification of persons at risk for post-stroke cognitive decline. Advanced age appears to be a risk factor for dementia following stroke,Citation8 with a one-year post-stroke prevalence of dementia of 7% in those aged <65 years and 53% in those aged >85 years.Citation12 Multivariate analyses of large stroke cohorts demonstrate associations between long-term post-stroke cognitive impairment and educational level,Citation13 lower socioeconomic status,Citation14 ethnicity (Afro-Caribbean, Asian),Citation14 stroke severity,Citation13 left hemispheric lesion,Citation14 prior cerebrovascular disease,Citation13 dysphasia,Citation13 visual field defect,Citation14 and urinary incontinence.Citation14 These studies suggest that some patient and/or stroke characteristics, as well as medical comorbidities (eg, prior cerebrovascular disease, incontinence), may serve to prompt clinicians to evaluate patients with such characteristics for post-stroke cognitive decline.
From a practical standpoint, particularly in the busy clinical practices of neurologists, physiatrists, and primary care physicians caring for stroke survivors, screening for post-stroke cognitive decline presents several challenges. First, in a time-limited setting, it is often impractical to administer more than a brief measure of general cognition, such as the Mini-Mental State Examination (MMSE).Citation15 Formal neuropsychological testing is often useful for the identification and quantification of post-stroke cognitive impairments, but obtaining support for such testing is inconsistently available, especially in many managed care environments.Citation16 As such, the task of assessing post-stroke cognitive performance is often relegated to primary care physicians, neurologists, psychiatrists, and physiatrists, and therefore the office-based assessment of cognition is frequently limited to the MMSE.
While the MMSE is not a substitute for formal neuropsychological testing, it appears to be a useful measure for the assessment of post-stroke cognitive decline. For example, Laukka et alCitation17 suggest that the MMSE may be a useful measure with which to identify forthcoming vascular dementia in adults ≥75 years of age, and Madureira et alCitation18 found the MMSE to be a useful screening measure of cognition among older persons in the post-acute (three-month) period following stroke. However, the usefulness of the MMSE measure for the identification of post-stroke cognitive impairment across a broader age range and in the late (ie, more than one year) period following stroke has not been established.
Additionally, the types of stroke-related impairments associated with incipient post-stroke dementia noted above (eg, dysphasia, visual field defect, severity of initial stroke, urinary incontinence) are often challenging to identify and quantify in a brief office visit, particularly in non-neurological clinical settings. When such are identified, clinicians may be more likely to perform cognitive screening tests, assuming that the presence and severity of other stroke sequelae may serve as a gauge of the likelihood and/or severity of post-stroke cognitive impairments. However, it is possible that the relationship between cognitive and other stroke-related impairments may be an artifact of age, with older persons experiencing more frequent impairments in a variety of neurological and functional domains, regardless of whether there are causal relationships between such impairments. Accordingly, it would be useful to understand more fully the relationship between post-stroke motor and cognitive impairments in the late period following stroke, and particularly whether the former serve as a proxy with which to identify stroke survivors in need of more detailed cognitive assessment.
The present study was undertaken to address these issues by investigating the usefulness of the MMSE as a screening tool for post-stroke cognitive decline among younger stroke survivors, and particularly the utility of interpreting MMSE performance according to population-based norms for this purpose. Additionally, relationships between cognitive performance, motor performance, time since stroke, and a limited set of easily identified patient and stroke characteristics were investigated for the purpose of determining whether these variables serve usefully to identify survivors of remote strokes in need of cognitive assessment.
Materials and methods
This study was approved by the HealthONE Alliance Institutional Review Board, and all subjects provided informed consent for study participation.
Subjects
Individuals who experienced a single known stroke at least 12 months prior to study participation were recruited nationally via printed and Internet media for participation in a study examining the effects of constraint-induced movement therapy on chronic post-stroke upper extremity motor impairments. Participants were enrolled on the basis of the onset and persistence of moderate-to-severe upper extremity motor impairments following a single known stroke, with moderate-to-severe upper extremity motor impairment, defined as movement from a resting position limited to wrist extension of no more than 20°, metacarpophalangeal and interphalangeal joint extension of no more than 20°, but preserved ability to grasp a washcloth using any method of prehension. Subjects were also required to have the ability to sit at the bedside for 10 minutes without support, to follow directions using written, verbal, or demonstration instructions, and to have no other serious and/or uncontrolled medical conditions. Findings from the constraint-induced movement therapy protocol into which these subjects subsequently entered are described elsewhere.Citation19–Citation21 Medical records were reviewed for the purpose of determining stroke type and laterality.
Outcome measures
Subjects completed pretreatment assessments using the MMSECitation15 and the Fugl-Meyer evaluation of physical performance.Citation22 The MMSE is a brief cognitive assessment measure used commonly by physicians and allied health care providers in clinical practice. MMSE scores range between 0 and 30, with higher scores reflecting better performance. This measure was administered and scored using the method described by Folstein et al.Citation15 In order to account for the effect of age prior to interpreting MMSE scores, adjusted MMSE scores were calculated using the population-based norms reported by Crum et al.Citation23 Mild cognitive impairment was defined as an MMSE score ≥1 standard deviation (SD) below age-adjusted performance expectations,Citation24 and moderate or greater cognitive impairment was defined as an MMSE score ≥2 SD below age-adjusted performance expectations.
The Fugl-Meyer assessment generated a score for upper extremity performance (FM-UE) based on motor skill, coordination, and speed of upper extremity movement; FM-UE scores range from 0 to 66, with lower scores reflecting more severe impairment. The Fugl-Meyer assessment also generates a total motor performance score (FM-T) based on the FM-UE and also joint range of motion, pain, and sensory function, as well as lower extremity function. For the purpose of this study, FM-T scores ranged from 0 to 126 points, again with lower scores reflecting more severe impairment. All administrations of the Fugl-Meyer assessment were completed by one occupational therapist following Fugl-Meyer testing guidelinesCitation22 and employed a standardized assessment environment (ie, the same chair, testing equipment, and testing procedures used for every subject). Determination of handedness was also made during the course of Fugl-Meyer assessment. Test-retest reliability on both the FM-UE and FM-T were determined by repeat assessment of 10 randomly selected patients; for both measures, the Pearson product moment correlation was r = 0.96 (P < 0.05).
Statistical analyses
All statistical analyses were performed using Statistica 6.0 (Statsoft Inc, Tulsa, OK). Pearson product moment correlation coefficients were calculated for age versus MMSE (raw and age-adjusted), age versus Fugl-Meyer (FM-UE or FM-T), time since stroke versus MMSE (raw and age-adjusted), time since stroke versus Fugl-Meyer (FM-UE or FM-T), and MMSE (raw and age-adjusted) versus Fugl-Meyer (FM-UE or FM-T). Student t-tests were used to investigate differences in MMSE and FM-T scores as a function of gender, laterality of stroke, and cerebral dominance. These analyses were cross-validated by dividing the study group into those with and without cognitive impairment (ie, age-adjusted performance ≥1 SD below norm-based expectations) and then using Student t-tests to investigate between-group differences in age, time since stroke, FM-UE, and FM-T. χ2 analyses were used to investigate differences in gender, cerebral dominance, and laterality of stroke among subjects with and without cognitive impairment.
Results
Twenty-seven subjects (10 of whom were female) were included. The study group is described in (continuous variables of interest) and (categorical variables of interest). Mild cognitive impairment was observed in 6/27 subjects (22.2%), and moderate or greater cognitive impairment was observed in 2/27 subjects (7.4%). Time since stroke was inversely correlated with both raw and age-adjusted MMSE scores (r = −0.65, P < 0.001, and r = −0.59, P < 0.002, respectively), but not with FM-UE or FM-T scores. Age was not correlated with raw or age-adjusted MMSE scores, but age was inversely correlated with FM-UE and FM-T scores (both r = −0.47, P < 0.02). Neither raw nor age-adjusted MMSE scores correlated with FM-UE or FM-T scores. Relationships between age-adjusted MMSE scores, FM-T scores, and age are presented in , and relationships between age-adjusted MMSE scores, FM-T scores, and time since stroke are presented in . Raw and age-adjusted MMSE scores did not differ as a function of gender, cerebral dominance, or laterality of stroke. Similarly, FM-UE or FM-T scores did not differ as a function of gender, cerebral dominance, or laterality of stroke.
Figure 1 Relationships between age-adjusted Mini-Mental State Examination (MMSE) scores and age, and Fugl-Meyer total (FM-Total) scores and age.
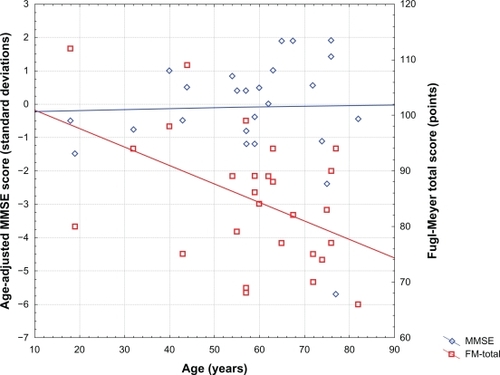
Figure 2 Relationship between age-adjusted Mini-Mental State Examination (MMSE) scores and time since stroke, and between Fugl-Meyer total (FM-Total) scores and time since stroke.
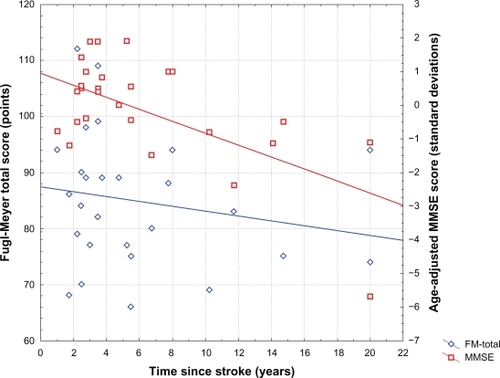
Table 1 Study group characteristics (continuous variables)
Table 2 Study group characteristics (categorical variables)
After dividing subjects into groups with and without cognitive impairment, there were no significant differences between these groups with respect to age, gender, cerebral dominance, laterality of stroke, FM-UE, or FM-T scores. However, time since injury was significantly longer among subjects with MMSE-determined cognitive impairment (10.3 ± 8.4 years) when compared with subjects performing within normal limits for age on this measure (4.6 ± 3.2 years, t = 2.6, P < 0.02).
Discussion
The present findings suggest that the MMSE, particularly when interpreted using age-adjusted normative data, may be useful in the identification of post-stroke cognitive impairment among both younger and older adult stroke survivors. This suggestion is consistent with the conclusions of other investigatorsCitation25–Citation27 and the American Heart Association.Citation28 Our findings clarify these suggestions by demonstrating that the usefulness of the MMSE for this purpose relies upon Z-transforming scores on this measure. Age influences MMSE performance, and the magnitude of the effect of age on MMSE performance increases with advancing age. Accordingly, interpreting MMSE scores in a manner that adequately controls for the potential confound of age-related performance decrements necessitates Z-transforming raw MMSE scores using the best available normative data.Citation23 In this study, age-adjusted MMSE scores identified 22.2% of subjects in this study with cognitive impairment of at least mild severity, 50% of whom were ≤60 years of age (see ). Moderate or greater cognitive impairment (ie, vascular dementia) was identified in 7.4% of subjects, consistent with frequencies identified in studies using more extensive neuropsychological testing batteries.Citation8–Citation11 By comparison, using raw MMSE cutoff scores of ≤25 or ≤24 would identify only 18.5% or 7.4%, respectively, of subjects in this group as cognitively impaired. The even more conservative cutoff score of <20 (for “organicity”), originally proposed by Folstein et al,Citation15 would identify only 1/27 (3.7%) of subjects in this sample as cognitively impaired. Therefore, we suggest that using raw MMSE score cutoff values to establish cognitive impairment is not appropriate, and may explain why some other groups conclude (perhaps erroneously) that this measure underestimates the frequency of post-stroke cognitive decline.Citation29–Citation32 Conversely, applying a less conservative raw MMSE cutoff score of ≤26 to our sample overidentifies subjects (29.6%) as having cognitive impairments of at least mild severity. Collectively, these observations suggest that the MMSE may be useful as an assessment for clinically significant post-stroke cognitive decline, and that the interpretation of MMSE scores for this purpose is best undertaken by comparing individual scores with published normative data.Citation23
Cognitive performance as assessed by both raw and age-adjusted MMSE scores was inversely correlated with time since stroke, but was not correlated with the severity of post-stroke motor impairments, age, gender, cerebral dominance, or laterality of stroke. By contrast, the severity of post-stroke motor impairment was correlated with age, but was not correlated with time since stroke or the other patient or stroke characteristics assessed in this study. The pattern of relationships between cognitive performance, motor function, age, and time since stroke observed in this study is complex. These relationships are considered individually and collectively in the service of considering their potential application to the care and future study of stroke survivors.
The correlation between cognitive performance and time since stroke suggests a time-related decline in cognition in the late period following stroke. Importantly, that decline is not accounted for by age, post-stroke motor impairment, or the other subject and stroke characteristics evaluated in this study. Although the association between increased severity of cognitive impairment and time since stroke observed in this study is likely to be multifactorial, two interpretations are immediately forthcoming.
First, it is possible that the cognitive performance of these subjects simply reflects their pre-stroke cognitive baseline, persistent and stable cognitive impairments since the time of stroke, or both, and that the apparent relationship between time since stroke and cognitive impairment is spurious. The strength of the association between time since stroke and both raw MMSE and age-adjusted MMSE scores suggests that the likelihood of a Type I error in this analysis is small, but this possibility cannot be dismissed entirely in light of the relatively small sample size of the present study.
Second, and more likely, our present findings suggest that a nontrivial minority of stroke survivors develop progressive cognitive decline in the late post-stroke period. That decline may result from the cumulative effects of additional (including otherwise clinically “silent”) cerebrovascular disease,Citation33–Citation37 the induction of Alzheimer’s-type neuropathology by cerebrovascular disease,Citation38–Citation41 or both of these and/or other factors.Citation42–Citation46 This interpretation is concordant with findings from other similar studies,Citation24,Citation37,Citation47–Citation49 and suggests that a single known stroke is probably understood most usefully as an overt manifestation of an underlying cerebrovascular process that in a substantial minority of individuals will result in gradual cognitive decline.
In contrast with post-stroke cognitive performance, motor performance remained relatively more stable as a function of time since stroke. However, motor performance demonstrated a clear age-related decline. The quality of motor function varies with normal aging,Citation50 and clinically apparent motor decline begins in the fifth decade of life. By contrast, the Crum et alCitation23 data suggest that significant age-related decline in MMSE scores is not expected until the eighth decade of life. These observations might suggest that age may more strongly influence motor performance than cognitive performance among relatively younger stroke survivors. Given that the mean age in the present study was 58.5 ± 16.8 years, the present observation of a relationship between age and post-stroke motor performance, but not between age and MMSE scores, is not entirely unexpected.
It is also important to note that the severity of motor impairments experienced by the subjects in this study were just short of plegia of the affected limb or hemibody. The lack of correlation between post-stroke cognitive performance and motor performance is therefore even more important to highlight here. If in this group there is no significant association between motor and cognitive performance, then severity of motor impairments seems unlikely to serve usefully as an indicator of post-stroke cognitive impairments.
The present study suffers from several limitations, including its development as a secondary analysis of cognition in a sample of stroke survivors recruited for a different purpose (constraint-induced movement therapy of post-stroke motor impairments), cross-sectional rather than longitudinal assessment of cognition and motor function, nonblinded assessments, lack of a matched comparison sample, lack of extensive demographic data (eg, educational levels, ethnicity, primary language, socioeconomic status), absence of overall stroke severity metrics (eg, National Institutes of Health Stroke ScaleCitation51), lack of ascertainment of potential confounds such as neuropsychiatric conditions (ie, depression, anxiety, substance use) and neuroactive medications on cognitive and motor performance, and lack of assessment with the formal neuropsychological testing needed to establish the validity of the rates of cognitive impairment identified by Z-transformed MMSE scores. Of particular note, the recruitment strategy for the constraint-induced movement therapy study may at least in part contribute to the lack of correlation between motor and cognitive performance in the present sample. As noted earlier, subjects were required to be able to follow directions using written, verbal, or demonstration instructions. This requirement reduces the likelihood of enrolling subjects with functionally significant language impairments, and would tend to bias MMSE scores towards the less impaired range. Accordingly, these subjects were less likely than the general stroke population to demonstrate an association between motor and cognitive (including language) abilities. It is possible that, if subjects with more overt impairments of language had been included in the present study, a correlation between motor and cognitive performance might have been observed. Conversely, the finding of an association between time since stroke and cognitive performance despite the apparent selection bias against patients with aphasia is that much more noteworthy, because it suggests that post-stroke language disturbances alone are unlikely to explain the MMSE scores observed in these subjects.
In summary, the present findings suggest that the MMSE may serve as a useful screening measure of post-stroke cognitive performance across a wide age range, particularly when MMSE scores are interpreted with respect to population-based norms rather than raw MMSE cutoff scores. Additionally, the present study findings suggest that clinicians should remain vigilant for the development of progressive cognitive decline throughout the post-stroke period, and that such vigilance should be maintained regardless of a patient’s age and/or severity of post-stroke motor impairments. Given the morbidity and mortality risks posed by post-stroke cognitive impairmentCitation52–Citation54 and promise of emerging therapies for the treatment of vascular dementia,Citation55–Citation61 routine screening for cognitive impairments among stroke survivors is necessary if such treatments are to be offered early in the course of vascular dementia, when preservation of function may yield the greatest benefits for affected persons and their families. The present findings suggest that identification of cognitive impairments rests upon direct assessment of cognition, and that recognition of other patient or stroke characteristics are neither suitable substitutes nor reliable prompts for post-stroke cognitive assessment. Prospective studies are needed to validate the present findings, including direct comparison of the rates of cognitive impairment identified by Z-transformed MMSE scores versus formal neuropsychological testing, and to investigate further their clinical implications.
Acknowledgements
The authors gratefully acknowledge the support provided for this study by the Spalding Community Foundation, the HealthONE Alliance, and HealthONE Spalding Rehabilitation Hospital, Aurora, CO.
Disclosure
The authors report no conflicts of interest in this work.
References
- WadeDTSkilbeckCHewerRLSelected cognitive losses after stroke. Frequency, recovery and prognostic importanceInt Disabil Stud198911134392670878
- KinsellaGFordBAcute recovery from patterns in stroke patients: Neuropsychological factorsMed J Aust19802126636667219301
- EnderbyPWoodVAWadeDTHewerRLAphasia after stroke: A detailed study of recovery in the first 3 monthsInt Rehabil Med1987841621652440824
- WadeDTWoodVAHewerRLRecovery of cognitive function soon after stroke: A study of visual neglect, attention span and verbal recallJ Neurol Neurosurg Psychiatry198851110133351509
- DesmondDWMoroneyJTSanoMSternYRecovery of cognitive function after strokeStroke19962710179818038841333
- BallardCRowanEStephensSKalariaRKennyRAProspective follow-up study between 3 and 15 months after stroke: Improvements and decline in cognitive function among dementia-free stroke survivors >75 years of ageStroke200334102440244414512580
- HochstenbachJBden OtterRMulderTWCognitive recovery after stroke: A 2-year follow-upArch Phys Med Rehabil200384101499150414586918
- PatelMCoshallCRuddAGWolfeCDNatural history of cognitive impairment after stroke and factors associated with its recoveryClin Rehabil200317215816612625656
- ThamWAuchusAPThongMProgression of cognitive impairment after stroke: One year results from a longitudinal study of Singaporean stroke patientsJ Neurol Sci2002203–2044952
- KaseCSWolfPAKelly-HayesMKannelWBBeiserAD’AgostinoRBIntellectual decline after stroke: The Framingham StudyStroke19982948058129550515
- PrencipeMFerrettiCCasiniARSantiniMGiubileiFCulassoFStroke, disability, and dementia: Results of a population surveyStroke19972835315369056607
- LoweryKBallardCRodgersHCognitive decline in a prospectively studied group of stroke survivors, with a particular emphasis on the >75’sAge Ageing200231Suppl324327
- PohjasvaaraTErkinjunttiTYlikoskiRHietanenMVatajaRKasteMClinical determinants of poststroke dementiaStroke199829175819445332
- PatelMDCoshallCRuddAGWolfeCDCognitive impairment after stroke: Clinical determinants and its associations with long-term stroke outcomesJ Am Geriatr Soc200250470070611982671
- FolsteinMFFolsteinSEMcHughPR“Mini-mental state”. A practical method for grading the cognitive state of patients for the clinicianJ Psychiatr Res19751231891981202204
- SchatzPHughesLJChuteDLUnderutilization of neuropsychology in traumatic brain injury rehabilitation: Is managed care to blame?NeuroRehabilitation200116428128711790915
- LaukkaEJJonesSFratiglioniLBackmanLCognitive functioning in preclinical vascular dementia: A 6-year follow-upStroke20043581805180915192244
- MadureiraSGuerreiroMFerroJMDementia and cognitive impairment three months after strokeEur J Neurol20018662162711784347
- BoniferNAndersonKMApplication of constraint-induced movement therapy for an individual with severe chronic upper-extremity hemiplegiaPhys Ther200383438439812665409
- BoniferNMAndersonKMArciniegasDBConstraint-induced movement therapy after stroke: Efficacy for patients with minimal upper-extremity motor abilityArch Phys Med Rehabil20058691867187316181956
- BoniferNMAndersonKMArciniegasDBConstraint-induced therapy for moderate chronic upper extremity impairment after strokeBrain Inj200519532333016094779
- Fugl-MeyerARJaaskoLLeymanIOlssonSSteglindSThe post-stroke hemiplegic patient. 1. A method for evaluation of physical performanceScand J Rehabil Med19757113311135616
- CrumRMAnthonyJCBassettSSFolsteinMFPopulation-based norms for the Mini-Mental State Examination by age and educational levelJAMA199326918238623918479064
- MeyerJSXuGThornbyJChowdhuryMHQuachMIs mild cognitive impairment prodromal for vascular dementia like Alzheimer’s disease?Stroke20023381981198512154249
- BourARasquinSBoreasALimburgMVerheyFHow predictive is the MMSE for cognitive performance after stroke?J Neurol2010257463063720361295
- CummingTBBlomstrandCBernhardtJLindenTThe NIH stroke scale can establish cognitive function after strokeCerebrovasc Dis201030171420424439
- Te Winkel-WitloxACPostMWVisser-MeilyJMLindemanEEfficient screening of cognitive dysfunction in stroke patients: Comparison between the CAMCOG and the R-CAMCOG, Mini Mental State Examination and Functional Independence Measure-cognition scoreDisabil Rehabil200830181386139119230177
- Kelly-HayesMRobertsonJTBroderickJPThe American Heart Association Stroke Outcome ClassificationStroke1998296127412809626308
- PopovicIMSericVDemarinVMild cognitive impairment in symptomatic and asymptomatic cerebrovascular diseaseJ Neurol Sci20072571–2185189317328916
- SrikanthVThriftAGFryerJLThe validity of brief screening cognitive instruments in the diagnosis of cognitive impairment and dementia after first-ever strokeInt Psychogeriatr200618229530516734921
- DongYSharmaVKChanBPThe Montreal Cognitive Assessment (MoCA) is superior to the Mini-Mental State Examination (MMSE) for the detection of vascular cognitive impairment after acute strokeJ Neurol Sci20102991–2151820889166
- PendleburySTCuthbertsonFCWelchSJMehtaZRothwellPMUnderestimation of cognitive impairment by Mini-Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: A population-based studyStroke20104161290129320378863
- SrikanthVKQuinnSJDonnanGASalingMMThriftAGLong-term cognitive transitions, rates of cognitive change, and predictors of incident dementia in a population-based first-ever stroke cohortStroke200637102479248316946165
- AppelrosPAnderssonAGChanges in Mini Mental State Examination score after stroke: Lacunar infarction predicts cognitive declineEur J Neurol200613549149516722974
- IshikawaHMeguroKIshiiHTanakaNYamaguchiSSilent infarction or white matter hyperintensity and impaired attention task scores in a nondemented population: The Osaki-Tajiri projectJ Stroke Cerebrovasc Dis10222010 [Epub ahead of print].
- PodgorskaAHierDBPytlewskiACzlonkowskaALeukoaraiosis and stroke outcomeJ Stroke Cerebrovasc Dis200211633634017903896
- BlassJPRatanRR“Silent” strokes and dementiaN Engl J Med2003348131277127812660392
- DecarliCVascular factors in dementia: An overviewJ Neurol Sci20042261–2192315537513
- WellerROYowHYPrestonSDMazantiINicollJACerebrovascular disease is a major factor in the failure of elimination of Abeta from the aging human brain: Implications for therapy of Alzheimer’s diseaseAnn N Y Acad Sci200297716216812480747
- ZlokovicBVDeaneRSallstromJChowNMianoJMNeurovascular pathways and Alzheimer amyloid beta-peptideBrain Pathol2005151788315779240
- MokVLeungEYChuWPittsburgh compound B binding in poststroke dementiaJ Neurol Sci20102901–213513720056250
- BourAMRasquinSMBaarsLThe effect of the APOE-epsilon4 allele and ACE-I/D polymorphism on cognition during a two-year follow-up in first-ever stroke patientsDement Geriatr Cogn Disord201029653454220606435
- KhedrEMHamedSAEl-ShereefHKCognitive impairment after cerebrovascular stroke: Relationship to vascular risk factorsNeuropsychiatr Dis Treat2009510311619557105
- RothenburgLSHerrmannNSwardfagerWThe relationship between inflammatory markers and post stroke cognitive impairmentJ Geriatr Psychiatry Neurol201023319920520601647
- NewmanGCBangHHussainSITooleJFAssociation of diabetes, homocysteine, and HDL with cognition and disability after strokeNeurology200769222054206218040011
- RajiMAReyes-OrtizCAKuoYFMarkidesKSOttenbacherKJDepressive symptoms and cognitive change in older Mexican AmericansJ Geriatr Psychiatry Neurol200720314515217712097
- HonigLSTangMXAlbertSStroke and the risk of Alzheimer diseaseArch Neurol200360121707171214676044
- KalariaRNThe role of cerebral ischemia in Alzheimer’s diseaseNeurobiol Aging200021232133010867217
- VermeerSEPrinsNDden HeijerTHofmanAKoudstaalPJBretelerMMSilent brain infarcts and the risk of dementia and cognitive declineN Engl J Med2003348131215122212660385
- HurleyBFAge, gender, and muscular strengthJ Gerontol A Biol Sci Med Sci199550 Spec No. 41–44.
- BrottTAdamsHPJrOlingerCPMeasurements of acute cerebral infarction: A clinical examination scaleStroke19892078648702749846
- MelkasSOksalaNKJokinenHPoststroke dementia predicts poor survival in long-term follow-up: Influence of prestroke cognitive decline and previous strokeJ Neurol Neurosurg Psychiatry200980886587019240049
- OksalaNKJokinenHMelkasSCognitive impairment predicts poststroke death in long-term follow-upJ Neurol Neurosurg Psychiatry200980111230123519620138
- AppelrosPNydevikITerentALiving setting and utilisation of ADL assistance one year after a stroke with special reference to gender differencesDisabil Rehabil2006281434916393832
- MaloufRBirksJDonepezil for vascular cognitive impairmentCochrane Database Syst Rev20041CD00439514974068
- MorettiRTorrePAntonelloRMRivastigmine superior to aspirin plus nimodipine in subcortical vascular dementia: An open, 16-month, comparative studyInt J Clin Pract200458434635315161118
- ErkinjunttiTRomanGGauthierSTreatment of vascular dementia – evidence from clinical trials with cholinesterase inhibitorsJ Neurol Sci20042261–2636615537522
- SmallGErkinjunttiTKurzALilienfeldSGalantamine in the treatment of cognitive decline in patients with vascular dementia or Alzheimer’s disease with cerebrovascular diseaseCNS Drugs2003171290591412962529
- WilkinsonDDoodyRHelmeRDonepezil in vascular dementia: A randomized, placebo-controlled studyNeurology200361447948612939421
- WilcockGMobiusHJStofflerAA double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500)Int Clin Psychopharmacol200217629730512409683
- OrgogozoJMRigaudASStofflerAMobiusHJForetteFEfficacy and safety of memantine in patients with mild to moderate vascular dementia: A randomized, placebo-controlled trial (MMM 300)Stroke20023371834183912105362