Abstract
OnabotulinumtoxinA, a neurotoxin, has been studied in numerous trials as a novel preventive therapy for migraine headache. The data would support that it may be effective at reducing headache days in patients suffering from chronic migraine (≥15 headache days/month, with eight or more of those migraine headache days). The mechanism by which onabotulinumtoxinA exerts its effects on migraine is not yet understood. It is known to inhibit acetylcholine release at the neuromuscular junction, but this probably does not explain the observed antinociceptive properties noted in preclinical and clinical trials. This review will discuss the known mechanisms of action of botulinum toxin type A, and will review the available randomized, placebo-controlled trials that have looked at its efficacy as a migraine preventative. We also describe the onabotulinumtoxinA injection sites used at our institution.
Introduction
Migraine is a debilitating primary headache disorder characterized by recurrent headaches typically described as unilateral, pulsating, moderate, or severe. They are aggravated by physical activity and associated with nausea and/or sensitivity to light and sound.Citation1 Though it is often conceptualized as a continuum or spectrum disorder, migraine is divided into two subtypes based on attack frequency: episodic and chronic. In episodic migraine, attacks occur on less than 15 days each month, whereas in chronic migraine a patient has at least 15 headache days each month, and at least eight of those are migraine headaches (see ).Citation2,Citation3 Each year, 3%–14% of episodic headache sufferers convert to chronic headache sufferers.Citation1 Compared with episodic migraine sufferers, patients with chronic migraine have a lower socioeconomic status, reduced health-related quality of life, greater psychiatric and medical comorbidities, and increased occupational disability.Citation4
The main aim of preventive migraine therapy is to reduce the frequency, duration, and/or severity of migraine attacks. There are many preventive therapies available, including β-adrenergic blockers, antidepressants, calcium channel blockers, and anticonvulsants.Citation5 Unfortunately, many of the current options are of limited benefit and can be associated with potentially serious side effects.Citation6 There is therefore great demand for alternative preventive therapies that are effective and well tolerated, with limited systemic effects.Citation6
Botulinum toxin type A (BT-A) was first reported as a potential migraine therapy by Binder et alCitation7 in 1991, when they observed that the patients receiving pericranial BT-A injections for facial hyperfunctional lines experienced relief of migraine headache symptoms. A nonrandomized, open-label study suggesting that BT-A could possibly be a safe and effective migraine treatment soon followed.Citation7,Citation8 Since then, great interest has prevailed in this pharmaceutical as a potential treatment for migraine. While investigating its efficacy as a migraine preventive, there have also been efforts to establish the mechanism by which botulinum toxin might exert an analgesic effect.
The goal of this review is to discuss the known mechanisms of action of BT-A, and then discuss the evidence behind its use in the treatment of migraine. As pointed out by Dressler and BeneckeCitation9 in a recent review, there is some discrepancy in the abbreviations used in the literature for these types of discussions. For our purposes, we will use the neurotoxin abbreviation (BoNT-A) for discussion of mechanism of action at the level of the neuromuscular junction, and then use the therapeutic preparation botulinum toxin type A abbreviation (BT-A) for discussions of preclinical and clinical trials. At the time of this article, only BT-A in the form of onabotulinumtoxinA has been approved by the US Food and Drug Administration (FDA) for the treatment of chronic migraine. Therefore, our discussion of clinical efficacy will focus primarily on published data from randomized, double-blind, placebo-controlled trials examining the use of onabotulinumtoxinA in migraine prophylaxis.
Pharmacology of botulinum toxin
Strains from the gram-positive anaerobic bacteria Clostridium botulinum are known to produce seven serologically distinct neurotoxins: A, B, C1, D, E, F, and G (C2 is not considered a neurotoxin).Citation10 Currently, only the A and B serotypes are used for commercial purposes, though others have been used experimentally in humans.Citation11,Citation12 Because of differences in production, the commercially available botulinum toxin preparations each have distinct pharmacokinetics. The potency units are specific to each product, and the doses cannot be compared or converted from one botulinum product to another. To reinforce these individual potencies and prevent medication errors, the FDA has revised the classification of botulinum toxin products to include the following generic names: onabotulinumtoxinA (marketed as BOTOX®/ BOTOX® Cosmetic, Allergan Inc, Irvine, CA), rimabotulinumtoxinB (MYOBLOC®, Solstice Neurosciences Inc, Louisville, KY), abobotulinumtoxinA (Dysport®, Medicis Pharmaceuticals, Scottsdale, AZ), and incobotulinumtoxinA (XEOMIN®, Merz Pharmaceuticals, Greensboro, NC).Citation13,Citation14
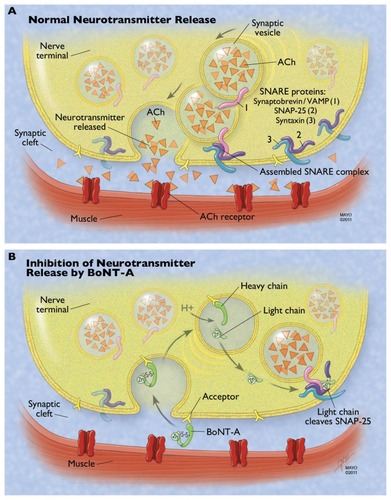
Therapeutic preparations of BT-A come as powders that have to be reconstituted with 0.9% NaCl/H2O before use. Most BT-A preparations consist of the botulinum toxin (neurotoxin and nontoxic complexing proteins) and excipients. Citation9 IncobotulinumtoxinA is slightly different, in that it has no complexing proteins.Citation15 With food-borne botulism, the complexing proteins are thought to help protect the protein from acids and proteases in the gastrointestinal tract, and may help the toxin cross the intestinal barrier to enter circulation. The role of complexing proteins in commercial preparations of injectable botulinum toxin is not clear.Citation15 The active neurotoxin molecule is made up of a heavy chain (100 kDa) and a light chain (50 kDa), joined by a disulfide bond (see ). The heavy chain acts as the binding and translocation domain, and the light chain is the enzymatically active zinc (Zn++)-dependent endopeptidase.Citation16,Citation17
Figure 1 Mechanism of botulinum neurotoxin type A (BoNT-A) at the neuromuscular junction. (A) Normal neurotransmitter release requires fusion of the vesicle membrane to the membrane of the presynaptic nerve terminal. This process is guided by the fusion of three proteins that make up the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex: the vesicle-associated membrane protein (VAMP or synaptobrevin) and the membrane-bound syntaxin and synaptosomeassociated protein of 25 kDa (SNAP-25). ACh is released from the vesicle, diffuses across the synaptic cleft, and binds to the acetylcholine (Ach) receptor, resulting in normal muscular contraction. (B) The heavy chain of BoNT-A binds to a ganglioside acceptor in the plasma membrane of the presynaptic nerve terminal. This leads to receptormediated endocytosis of the neurotoxin. The acidic environment of the synaptic vesicle or endosome leads to a conformational change in the toxin and eventual reduction of the linking disulfide bond, freeing the light chain. The light chain translocates to the cytosol and cleaves the C-terminal of the SNAP-25 protein. This inhibits SNARE complex formation and therefore inhibits neurotransmitter release.
Method of action
Action at the neuromuscular junction
The most well-known mechanism of action is at the presynaptic nerve terminal, where the toxin prevents the calcium-dependent release of acetylcholine.Citation18 There are four main steps in this process: binding to the acceptor on the plasma membrane, internalization of the botulinum toxin, separation of the light and heavy chain with escape of the light chain into the cytosol, and light-chain inhibition of the docking/ fusion of the synaptic vesicles containing acetylcholine.Citation19 These steps are illustrated in and are described in further detail in this section.
The C-terminal of the heavy chain is responsible for interacting with a high-affinity ganglioside acceptor outside of the nerve terminal.Citation18 This acceptor is felt to be an intraluminal vesicular protein that has become exposed to the outside of the nerve terminal as part of the recycling/exocytosis of vesicles. Citation18 This induces endocytosis of the botulinum neurotoxin (BoNT) into the nerve terminal, where it is contained within a membrane-bound synaptic vesicle. The fact that BoNT may depend on the recycling of vesicles to gain entry has been proposed as an explanation for why BoNT seems to be more effective in blocking neuromuscular junctions when the target muscle is active.Citation17,Citation18,Citation20,Citation21 Next, the acidic environment of the vesicle induces a conformational change of the toxin, with eventual breaking of the disulfide bond. This allows the light chain to escape the vesicle into the cytosol.Citation18,Citation22 The light chain then cleaves one of three core proteins that comprise the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex and that are involved in vesicle docking/fusion during regulated exocytosis. The three core SNARE proteins include vesicle-associated membrane protein (VAMP; also known as synaptobrevin), syntaxin, and synaptosomal-associated protein of 25 kDa (SNAP-25).Citation23 Each immunologically distinct BoNT serotype has a unique site of action within the SNARE complex (BoNT-C1 actually has two sites of action). Synaptobrevin/VAMP is cleaved by BoNT types B, D, F, and G. Syntaxin is cleaved by BoNT type C1. SNAP-25 is cleaved by BoNT types A, E, and C1.Citation24 By inhibiting the release of acetylcholine, BoNT leads to chemical denervation and muscle paralysis. Recovery from toxin-induced paralysis involves resprouting of new terminals from the motor neuron, followed by slow recovery of the original nerve terminal’s ability to release acetylcholine.Citation25 For BoNT-A, the clinical effect terminates in about 2–6 months in humans.Citation26
Antinociceptive action
Early in the use of botulinum toxin for motor conditions, investigators noted that the pain relief preceded, and in some cases was greater than, the objective motor benefit; suggesting that the effect on pain probably involved more than just neuromuscular blockade.Citation19,Citation27 The antinociceptive action of BT-A, and the effect on migraine, is poorly understood; but the effect may be multifactorial with effects on muscle fibers, autonomic fibers, and possibly pain fibers. BT-A appears to have an effect on peripheral sensitization and may also have an effect (either direct or indirect) on the central processing of pain.Citation27,Citation28
Action at other cholinergic nerve terminals
The neuromuscular blockade just described is a result of blocking the α-motor neurons innervating the extrafusal muscle fibers, which are the fibers responsible for the actual contractile property of the muscle. However, BT-A is also known to block transmission of γ-motor neurons to the intrafusal fibers in muscle spindles.Citation17 As muscle spindles provide afferent information about muscle stretch to the central nervous system (CNS), some authors have suggested that reducing their input may attenuate the hyperactivity of the muscle involved in tonic muscle contractions, and may in this way contribute to some of the pain relief observed.Citation29,Citation30
In addition to the somatic motor neurons (α and γ), BT-A interferes with transmission of acetylcholine in the autonomic nervous system. By blocking the preganglionic nerve terminals of the sympathetic and parasympathetic nerve terminals, as well as many of the postganglionic fibers, botulinum toxin has a complex effect on autonomic function. In botulism, this effect can lead to symptoms of anhidrosis, xerophthalmia, xerostomia, orthostatic hypotension, gastrointestinal paralysis, and urinary retention. Therapeutically, this effect has made botulinum toxin a useful therapy for hyperhidrosis and hypersalivation. Many of the autonomic nerve terminals affected by botulinum toxin have other neuropeptides colocalized with acetylcholine, such as substance P, somatostatin, enkephalins, norepinephrine, adenosine triphosphate, neuropeptide Y, and nitric oxide.Citation30 Some authors hypothesize that the effect of BT-A on the autonomic nervous system, particularly the effect on autonomic vascular control, may allow the toxin to interfere with neurogenic inflammation and associated pain.Citation30
Noncholinergic nerve terminals
In vitro and in vivo studies have demonstrated that if BT-A is introduced into noncholinergic nerve terminals, it can block the calcium-dependent release of neurotransmitters other than acetylcholine.Citation17,Citation27,Citation28 BT-A has been found to block the release of inflammatory pain mediators such as substance P from cultured dorsal root ganglion neurons. It has also been shown to reduce potassium-stimulated, but not basal, release of calcitonin gene-related peptide (CGRP) from cultured trigeminal ganglia neurons.Citation31,Citation32 It has also been shown to block the release of glutamate from peripheral nerve terminals in inflammatory pain models.Citation33
Inflammatory and neuropathic pain models
In rat models of inflammatory pain, peripherally applied BT-A has been shown to be effective in reducing the associated pain behaviors. Cui et alCitation33 examined the effect of BT-A on the formalin model of inflammatory pain. In this model, a chemical irritant (formalin) is injected into the hind paw of a rat. This results in a reproducible biphasic response involving pain behaviors and excitation of dorsal horn neurons. Phase I (first 5 minutes after injection) is thought to be caused by direct activation of the peripheral small afferent fibers by formalin. Following a quiet phase, phase II (15–60 minutes) is attributed to sensitization of the peripheral afferents by inflammatory mediators. Pretreatment with BT-A was shown to produce a dose-dependent reduction in nociceptive behaviors during phase II.Citation33 Further study showed that this reduction in pain behavior was associated with a dose-dependent reduction of the excitability of the dorsal horn (as measured by Fos-like immunoreactivity) and of the formalin-mediated activity in the dorsal horn wide-dynamic-range neurons. There was no effect on acute pain behavior during phase I, which agrees with previous studies showing that BT-A has no direct effect on acute noninflammatory nociception.Citation27,Citation34 In other inflammatory pain models, BT-A pretreatment (6 days) also significantly reduced the enhanced sensitivity to mechanical and thermal stimuli provoked by peripheral carrageenan or capsaicin injections in rats.Citation35
Animal models have also examined the effect of BT-A in neuropathic pain models. Bach-Rojecky et alCitation36 demonstrated that a peripheral injection of BT-A significantly reduced thermal and mechanical hyperalgesia associated with the neuropathy induced by partial sciatic nerve transection in rats. Similar to other studies, there was no direct antinociceptive effect, as the BT-A had no effect on the thermal and mechanical sensation of sham-operated animals.Citation36 Pavone and LuvisettoCitation28 also found a dose-dependent reduction in mechanical allodynia and cold hyperalgesia following chronic constriction injury of the sciatic nerve. The effect on allodynia was present only if BT-A was given after nerve injury, suggesting that BT-A could reduce neuropathic symptoms but not protect against the neuropathy. A similar effect was present if the BT-A was injected intrathecally.Citation37 Not only does BT-A affect pain behaviors associated with chronic constriction injury of the sciatic nerve but also it was found to diminish the expected injury-induced upregulation of neuropeptides (eg, prodynorphin and pronociceptin) and nitric oxide synthase1 mRNA in the rat dorsal root ganglia.Citation38
Human trials may also support BT-A having an analgesic effect in neuropathic pain. In a recent study, Ranoux et alCitation39 used a randomized, double-blind, placebo-controlled trial to examine the effect of BT-A on allodynia associated with focal neuropathies. They found that a single intradermal injection of BT-A reduced the intensity and area of mechanical allodynia and cold pain thresholds on the affected side, with no change in perception thresholds. This effect seemed to persist from 2 weeks to 14 weeks after injection.
Possible central action of BT-A
While studying the effect of BT-A on paclitaxel-induced neuropathy in rats, Favre-Guilmard et alCitation40 noted that injection of BT-A in the hindpaw almost completely abolished the mechanical hyperalgesia not only in the injected paw but also in the contralateral paw. This bilateral antinociceptive effect remained stable over the 6 days of observation. The mechanism behind this bilateral effect was unclear, but the authors pointed out that a systemic diffusion effect was unlikely, as a contralateral injection of BT-A did not elicit an antihyperalgesic effect in their model of carrageenan-induced hyperalgesia or in previous models of formalin-induced pain.Citation40 They proposed that the mechanism might involve more complex processes such as central sensitization. Bach- Rojecky et alCitation41 observed a similar bilateral antinociceptive effect while studying the effect of a single injection of BT-A on streptozotocin-induced diabetic neuropathy in rats. Other studies have found that peripheral injections of BT-A in the cranial region can affect associated brainstem nuclei. For instance, BT-A injected into the cat lateral rectus muscle can have dose-dependent structural and functional effects on the abducens nuclei, including a reduction in the firing rate.Citation42 Using a rat model of infraorbital nerve constriction, Kitamura et alCitation43 found that BT-A injected 3 days following nerve injury not only reduced mechanical allodynia but also reduced exaggerated neurotransmitter release from trigeminal ganglion neurons.
Given the high-affinity binding of botulinum toxin to the peripheral cholinergic nerve terminals, many authors have hypothesized that any central effect of botulinum toxin was indirect; either related to an alteration in sensory input from the muscle spindles or as an effect of denervation on CNS plasticity.Citation41,Citation44 The possibility that BT-A could reach the CNS had been suggested in the 1970s, but it was felt that the retrograde axonal transport system was so slow that the toxin was likely to be inactive before it reached the cell body.Citation28 However, in the last few years, studies have shown that at high doses, at least some amount of catalytically active BoNT-A may undergo retrograde transport to the motor nuclei supplying that nerve terminal.Citation45 As axonal transport to the CNS within motor neurons would not necessarily explain a reduction in pain, a more recent study looked for similar evidence of retrograde transport within sensory neurons. Matak et alCitation46 used a rat model of formalin-induced facial pain and examined the effects of BT-A injected into the rat whisker pad or sensory trigeminal ganglion. They found that BT-A at either location reduced the phase II pain. When colchicine was given to block microtubule-dependent transport, the antinociceptive effect was prevented. They also found BT-A-truncated SNAP-25 in the medullary dorsal horn of the trigeminal nucleus caudalis 3 days following injection into the whisker pad.
Summary
The precise mechanism of BT-A as a migraine preventive is still unknown but remains an area of great interest and ongoing research. Based on the available in vitro and in vivo models, it has been proposed that BT-A inhibits the release of pain-related neurotransmitters and neuropeptides such as substance P, CGRP, and glutamate from the peripheral termini of primary trigeminal and cervical afferents. This reduces peripheral sensitization. Because central sensitization results from ongoing input from pain fibers, the inhibition of these peripheral signals indirectly inhibits central sensitization. In addition, peripherally injected BT-A may be retrogradely transported along axons of peripheral nerves, allowing inhibitory effects at the level of the dorsal root ganglion and dorsal horn.Citation28 Whatever the mechanism of action, it is the analgesic effects and low systemic side effects observed in clinical trials that have led to the growing use of botulinum toxin for migraine headache.
Clinical efficacy studies
As mentioned previously, the present discussion analyzes data from randomized, double-blind, placebo-controlled studies published in the English literature researching onabotulinumtoxinA specifically in adult migraine prophylaxis (see ).Citation47–Citation57 Although small case series have shown encouraging results using onabotulinumtoxinA in pediatric chronic migraine,Citation58,Citation59 to this date no placebo-controlled studies have been published in English literature.
Table 2 Randomized, double-blind, placebo-controlled trials evaluating onabotulinumtoxinA efficacy in migraine prophylaxis
Episodic migraine
In the first randomized, double-blind, placebo-controlled study, Silberstein et alCitation47 attempted to confirm Binder et al’sCitation6 findings. Subjects having two to eight moderate-to-severe migraines per month (with or without aura) were randomized to placebo or onabotulinumtoxinA (25 U or 75 U). Subjects received symmetrical injections into the frontalis, temporalis, corrugator, and procerus muscles. The mean migraine frequencies per month at baseline in the placebo, 25 U, and 75 U treatment groups were 4.8, 4.3, and 4.0, respectively. The study’s primary efficacy variable was met. There was a significantly greater reduction in the number of moderate-tosevere migraines in the 25 U group than in the placebo group at Month 2 (placebo, −0.37; 25 U onabotulinumtoxinA, −1.57; P = 0.008) and Month 3 (placebo, −0.98; 25 U onabotulinumtoxinA, −1.88; P = 0.042) after injection. For unclear reasons, the 75 U dose did not perform as well as the 25 U dose. The authors suspected that this was secondary to the lower migraine frequency at baseline in the 75 U treatment group.
Evers et alCitation48 used different onabotulinumtoxinA doses with a specific focus on different injection sites. Sixty subjects having two to eight migraine attacks were enrolled. Subjects were treated with either 100 U in the frontal and neck muscles or with 16 U in the frontal muscles and placebo in the neck muscles, or with placebo in all muscles. The primary efficacy parameter was not met. The rate of patients with at least a 50% reduction of migraine frequency was 30% in the group receiving 100 U, 30% in the group receiving 16 U, and 25% in the group receiving placebo (P = 0.921). Regarding secondary efficacy parameters, the only significant difference observed was in the sum score of all accompanying symptoms. In the 16 U group, but not in the 100 U group, the accompanying symptoms (photophobia, phonophobia, nausea, and vomiting) were significantly reduced by 29% in Month 3 compared with a reduction of 5% in the placebo group (P = 0.048; posthoc test). Similar to Silberstein et al’sCitation47 study, it was the low-dose treatment group in which Evers et alCitation48 were able to observe a significant finding, leading them to consider the total dosage of onabotulinumtoxinA a less important variable than other parameters such as injection sites and patient selection.
In a series of three sequential randomized, double-blind studies, Elkind et alCitation49 further explored onabotulinumtoxinA for migraine prophylaxis in subjects suffering four to eight moderate-to-severe migraines per month. In study I, patients were randomized to placebo or onabotulinumtoxinA into frontal, glabellar, and temporal muscle at a dose of 7.5 U, 25 U, or 50 U. In study II, completers who received placebo or onabotulinumtoxinA 7.5 U were randomized to receive two treatments of either 25 U or 50 U onabotulinumtoxinA, whereas patients who had received onabotulinumtoxinA 25 U or 50 U continued the same dose for two additional treatments. Completers entered study III, where they were randomized to placebo or continued treatment with 25 U or 50 U. All treatments across the three studies were administered at 4 month intervals. The primary efficacy measure was not met. Migraine frequency was not different among treatment groups at any visit in any of the studies (assessed as change from baseline, all P ≥ 0.201). There was no statistically significant effect of onabotulinumtoxinA at any time. When comparing their results with more encouraging results obtained in chronic daily headache (CDH) trials (with many chronic migraine patients),Citation60 Elkind et alCitation49 suggested that perhaps those with a greater frequency of migraine attacks are more responsive to onabotulinumtoxinA than subjects with episodic migraine.
Aurora et alCitation50 performed a phase II trial using the “follow-the-pain” injection protocol. Instead of having the onabotulinumtoxinA injection sites and doses already fixed (referred to as the “fixed-site, fixed-dose” approach), they allowed the investigator to define the injection sites and doses based on pain distribution and severity (except the occipitalis muscle, where dosing was fixed). A total of 369 subjects were randomized to three treatments with onabotulinumtoxinA 110–260 U or placebo at 90 day intervals. OnabotulinumtoxinA did not show superiority over placebo in the primary efficacy measure. At Day 180, the mean change from baseline in migraine frequency per 30 day period was −2.4 in the onabotulinumtoxinA group compared with −2.2 in the placebo group (P > 0.999). There were no statistically significant differences between treatment groups. In a post hoc analysis, however, in the group of patients experiencing a higher baseline frequency (≥12 headache days), patients receiving onabotulinumtoxinA experienced a significant reduction in migraine frequency, with a mean change from baseline of −4 headache episodes compared with −1.9 for the placebo patients (P = 0.048). Although the Day 180 time point was the only time point at which the between-group difference reached statistical significance, a consistent trend was observed within this subgroup of patients favoring onabotulinumtoxinA throughout the study.
In a small 3 month study, 32 subjects with four to eight migraine attacks per month were randomized to symmetrical injections of 50 U of onabotulinumtoxinA in three pericranial muscle regions (frontalis, temporalis, and glabellar) versus placebo.Citation51 The primary efficacy parameter was frequency of attacks per 4 weeks, and the effect on this was difficult to determine due to inconsistencies in the text of the manuscript. The secondary efficacy parameter was defined as severity of attacks, and the authors state that 75% of patients in the onabotulinumtoxinA group reported a marked improvement in the intensity of headaches from a “moderate-severe” category to “complete relief-mild” category, whereas none in the placebo group noted this improvement (P < 0.05).
Relja et alCitation54 used a 30 day placebo run-in to divide 495 patients into placebo responders (n = 173) and placebo nonresponders (n = 322). They then injected 225 U, 150 U, or 75 U of onabotulinumtoxinA or placebo using a fixedsite, fixed-dose seven-site approach in pericranial and neck muscles. Patients received additional treatments at Day 90 and Day 180 and returned for follow-up visits at 30 day intervals following each treatment through Day 270. At Day 180, the primary endpoint, the mean change from baseline in the frequency of migraine episodes in the placebo nonresponders stratum per 30 day period was −1.6, −1.7, −1.5, and −1.4 in the onabotulinumtoxinA 225 U, 150 U, 75 U, and placebo groups, respectively. The differences between the groups were not statistically significant (P = 0.817). A strong placebo response was evident in this trial. According to the authors, this could have been secondary to the uneven randomization scheme with a greater number of active treatment arms instead of an even 1:1 randomization.
Saper et alCitation55 compared different injection sites and doses of onabotulinumtoxinA in patients with four to eight moderate-to-severe headaches per month. A total of 232 patients were randomized, with 45 assigned to placebo and 187 assigned to onabotulinumtoxinA (n = 44 frontal, n = 45 temporal, n = 49 glabellar, n = 49 all three sites or “FTG”). The primary efficacy variable was frequency of migraine headaches, with Day 60 specified as the primary endpoint. Following intervention, baseline frequency ratings were 5.6 (FTG), 5.7 (frontal), 5.9 (temporal), 5.3 (glabellar), and 5.5 (placebo) migraine headaches per month (P = 0.399). No statistically significant among-group differences were observed for decreases from baseline in the frequency of migraines of any severity at the 30, 60, or 90 day follow-up visit (all P ≥ 0.411). According to the authors, low onabotulinumtoxinA dose, lack of posterior head and neck muscle injections, allowance of other preventive medication use, and exclusion of patients with chronic migraine may all have been reasons for a negative trial.
To conclude, although results from Silberstein et al’sCitation47 original study in episodic migraine were encouraging, the available data from randomized, double-blind, placebo-controlled studies do not convincingly show onabotulinumtoxinA to be effective in the prevention of episodic migraine. Based on the current evidence, a report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology has labeled onabotulinumtoxinA as probably ineffective in episodic migraine treatment.Citation61
Chronic migraine
In a small study from Freitag et al,Citation53 41 patients were randomized to onabotulinumtoxinA 100 U or placebo using a fixedsite, fixed-dose paradigm at the glabella, temporal, frontal, suboccipital, and trapezius muscles. Patients were excluded if they were overusing analgesics or caffeine. The primary efficacy parameter was the change in monthly migraine frequency per 4 week assessment period over 4 months compared with baseline. OnabotulinumtoxinA injections were found to be superior to placebo in terms of reduction of headache attack frequency, reducing headaches from 13.8 to 10.1 per month (P = 0.001, correlation coefficient = 0.695), compared with the placebo arm that actually noted a rise in headache frequency from 14.6 to 15.4 per month (P = 0.046, correlation coefficient = 0.475). In addition, six of 18 (33%) completers on onabotulinumtoxinA had at least a 50% reduction in migraine episodes compared with three of 18 (16.7%) placebo patients.
The PREEMPT 1 (Phase III Research Evaluating Migraine Prophylaxis Therapy-1) trialCitation56 had a 24 week, double-blind, parallel-group, placebo-controlled phase followed by a 32 week, open-label phase. A total of 679 patients were randomized 1:1 to onabotulinumtoxinA or placebo injected every 12 weeks. Two cycles of injections were administered. Two-thirds of patients were overusing acute medications, though patients using frequent opiates were avoided at enrollment. No pharmacologic prophylactics were allowed. OnabotulinumtoxinA or placebo was administered using fixed-site, fixed-dose injections across seven head and neck muscle areas (corrugator, procerus, frontalis, temporalis, occipitalis, cervical paraspinal, and trapezius). At the investigator’s discretion, an additional 40 U could be administered using a follow-the-pain strategy. The primary endpoint was mean change from baseline in frequency of headache episodes for the 28 day period ending with Week 24. No significant between-group difference for onabotulinumtoxinA versus placebo was seen for this (−5.2 vs −5.3; P = 0.344). A high placebo response rate was observed. Significant between-group differences for onabotulinumtoxinA were observed for the secondary endpoints headache days (P = 0.006) and migraine days (P = 0.002) at all time points (including Week 24). The trial design of PREEMPT 2 was identical to its predecessor’s and randomized 705 patients to either onabotulinumtoxinA or placebo.Citation57 Similar to PREEMPT 1, most patients overused pain medications at baseline. The primary endpoint in PREEMPT 2 was the mean change from baseline in frequency of headache days for the 28 day period ending with Week 24. OnabotulinumtoxinA was significantly superior to placebo for this endpoint (−9.0 headache days with onabotulinumtoxinA vs −6.7 placebo, P < 0.001; mean intergroup difference −2.3 [95% confidence interval −3.25, −1.31]).
Pooled analyses from PREEMPT 1 and 2 (1384 patients) demonstrated a mean decrease from baseline in frequency of headache days, with statistically significant between-group differences favoring onabotulinumtoxinA over placebo at Week 24 (−8.4 vs −6.6; P < 0.001) and at all other time points.Citation62 Significant differences favoring onabotulinumtoxinA were also observed for a number of secondary efficacy variables, including reductions in moderate or severe headache days (P < 0.001), cumulative hours of headache on headache days (P < 0.001), headache episodes (P < 0.009), the proportion of patients with a severe headache impact test-6 score (P < 0.001), and migraine episodes (P < 0.004).
To conclude, available randomized, double-blind, placebo-controlled trials suggest that onabotulinumtoxinA is effective in chronic migraine prophylaxis.Citation53,Citation62 It is important to note, however, that the therapeutic gain over placebo for many of the PREEMPT trials’ outcomes was modest, even if statistically significant.Citation56,Citation57,Citation62 As an example, in the pooled analysis, treatment with onabotulinumtoxinA led to a statistically significant reduction of headache days per 28 days compared with placebo (8.4 vs 6.6), but the absolute difference between the two groups was small (1.8 days);Citation62 supporting the utility of onabotulinumtoxinA injection as moderately superior to placebo for the treatment of chronic migraine. This, the high cost of the toxin, and the need for an experienced clinician to administer onabotulinumtoxinA have led some authors to consider it as a second-line chronic migraine prophylactic therapy.Citation63
Still, the excellent tolerability of onabotulinumtoxinA makes it an extremely attractive alternative for patients who fail to tolerate, and therefore discontinue, traditional oral prophylactics. In comparison with topiramate, for example, Mathew and JaffriCitation64 reported that patients receiving onabotulinumtoxinA had fewer adverse effects leading to discontinuation (7.7%) than patients in the topiramate group (24.1%). In another study, more than half of the study population had discontinued oral migraine prophylaxis within 3 months after commencing onabotulinumtoxinA.Citation65 Because it is injected, compliance appears to be less of an issue when comparing onabotulinumtoxinA with oral prophylactics. Citation62 OnabotulinumtoxinA may be a useful treatment option for headache patients demonstrating poor compliance, adherence, or adverse event profile with oral prophylactic regimens.Citation66
Unspecified migraine subtype or CDH
A small study looked at the effect of higher doses of onabotulinumtoxinA on migraine,Citation52 without specifying episodic or chronic migraine. Forty-nine patients with more than five migraine attacks per month were randomized to receive either onabotulinumtoxinA or placebo into corrugator, frontalis, temporalis, sternocleidomastoid, occipitalis, and posterior neck muscles. The proportion of episodic versus chronic migraine patients is uncertain. The two standardized dosing schemes based on patient weight were 135 U for those <65 kg and 205 U for those ≥65 kg. The primary outcome measure was the average frequency of headache days measured during 30 day blocks for 3 months. The secondary outcome measure was the severity of attacks. No significant differences were observed between control and test groups at baseline on these measures. Importantly, a high dropout rate of 17 (34%) led to a small sample size to be analyzed, which may have attenuated the power to detect any potential main effects of onabotulinumtoxinA in the prevention of migraine.
“Chronic daily headache” (CDH) is a descriptive term, generally defined by ≥15 headache days per month,Citation67 that encompasses multiple headache diagnoses, including chronic migraine, chronic tension-type headache, new daily persistent headache, hemicrania continua, and others. Randomized, placebo-controlled studies evaluating onabotulinumtoxinA in CDH have yielded mixed results and are difficult to analyze, given the heterogeneity of the headache syndromes represented. For instance, a study by Ondo et alCitation67 noted that only 14 of their 60 enrolled CDH subjects had chronic migraine, and the rest had chronic tension-type headache. This makes it difficult to draw strong conclusions from the study’s somewhat positive results.Citation60,Citation68 Other investigators used onabotulinumtoxinA in a fixed-site approach for CDH prophylaxis in 702 enrolled patients, 53% of whom had a confirmed “transformed” (chronic) migraine diagnosis while the rest (47%) had an unspecified or alternative CDH subtype.Citation69 The primary efficacy endpoint (mean change from baseline in the frequency of headache-free days for the 30 day period ending on Day 180) was not met, although at Day 240 the decrease in headache frequency was significantly greater for the onabotulinumtoxinA 225 U and 150 U groups compared with placebo. No subgroup statistical analysis was done based on CDH subtype. Another attempt to establish onabotulinumtoxinA as a probable effective CDH prophylactic using a follow-the-pain approach in a dose of 105–260 U failed to reach statistical significance for the primary efficacy measure (mean change from baseline in the frequency of headache-free days in a 30 day period), although a significantly higher percentage of onabotulinumtoxinA patients had a ≥50% headache day frequency decrease from baseline per 30 day period at Day 180 (32.7% vs 15.0%, P = 0.027), the secondary efficacy measure.Citation60 In this study, most patients (61% of 355) reported a history of migraine. However, precise CDH subtypes were not known.
Safety and tolerability
OnabotulinumtoxinA has been fairly well tolerated across studies. Reported adverse events with onabotulinumtoxinA in these randomized trials were transient, and were most frequently related to muscle weakness or pain at injections sites. Muscle weakness around the face and neck, for instance, has led to a higher number of patients in the onabotulinumtoxinA groups reporting neck pain, muscular weakness, eyelid ptosis, and diplopia. In the pooled analysis of the PREEMPT trials, only neck pain occurred, with an incidence of >5% (6.7% onabotulinumtoxinA group vs 2.2% placebo group). The most frequently reported side effects leading to discontinuation of onabotulinumtoxinA injections were neck pain (0.6%), muscular weakness (0.4%), headache (0.4%), and migraine (0.4%).Citation62
The safety of onabotulinumtoxinA in migraine management during pregnancy and lactation has not been established. Most randomized, double-blind, placebo-controlled studies using onabotulinumtoxinA in migraine prophylaxis have excluded pregnant and lactating women. With the exception of a woman who discontinued the study at Day 145 because of pregnancy and subsequently delivered a healthy child,Citation49 no other pregnancy outcome has been reported in these trials. At present, onabotulinumtoxinA belongs to the pregnancy category C (risk cannot be ruled out) of the FDA and is not recommended during pregnancy.Citation70
Outside of the studies included in this review, distant spread of onabotulinumtoxinA beyond the site of injection and systemic effects resembling botulism (dysphagia, breathing difficulties, generalized muscle weakness, among others) appear to be rare but have been reported even at low doses.Citation71 Because of this, the FDA has required a boxed warning for all botulinum toxin products (see ).
Disability and health-related quality of life
It is well recognized that migraine attacks not only impair the individual’s ability to function during the attack but also may affect an individual’s functioning and overall well-being between attacks.Citation1 Many of the randomized, controlled efficacy trials attempted to assess whether onabotulinumtoxinA treatment impacted the patients’ migraine-related disability and health-related quality of life. The measuring instruments were not consistent throughout the efficacy studies, however, making comparison among them problematic. In some cases, the questionnaire used may not have been a validated tool or its source was not properly cited, further complicating the interpretation of their results.
Based on limited data available, there is some suggestion that onabotulinumtoxinA may improve the health-related quality of life in patients with chronic migraine. The PREEMPT trials looked at this using the Migraine-specific Quality of Life Questionnaire Version 2.1. Pooled analysis of the data from both trials showed that treatment with onabotulinumtoxinA significantly improved health-related quality of life (P < 0.001), as measured by changes from baseline in all three role function domains (restrictive, preventive, and emotional) at both time points evaluated (Weeks 12 and 24).Citation62 Pooled analysis also demonstrated a clinically meaningful between-group difference for onabotulinumtoxinA versus placebo observed at Week 24 in mean change from baseline in total Headache Impact Test-6 score (2.4; P < 0.001), a score that measures headache-related disability. Freitag et alCitation53 also noted a positive trend when examining the effect of onabotulinumtoxinA on Migraine Disability Assessment Scores, but the differences did not meet statistical significance.
Comparator studies
Small studies comparing onabotulinumtoxinA with other first-line migraine preventives are promising. A randomized, double-blind study of 59 migraineurs showed that patients receiving onabotulinumtoxinA with an oral placebo had similar improvements in migraine disability scores, and similar reductions in headache days to patients receiving divalproex sodium and placebo injections.Citation72 Another study of 72 patients showed that 250 U of onabotulinumtoxinA had similar efficacy to 25 mg or 50 mg of amitriptyline for chronic migraine.Citation73 Finally, two recent randomized, placebocontrolled trials (59 and 60 patients) compared up to 200 U of onabotulinumtoxinA with topiramate and concluded that onabotulinumtoxinA had similar benefit to topiramate in the treatment of chronic migraine.Citation64,Citation74
Dosing and administration
Ample variation in onabotulinumtoxinA dose, injection sites, and strategy (“fixed-site, fixed-dose” or “follow-the-pain”) has been seen throughout the trials. The large multicenter PREEMPT trials used a fixed-site, fixed-dose injection paradigm, with additional follow-the-pain sites considered depending on individual symptoms.Citation75 The PREEMPT paradigm has proven to be safe, well tolerated, and more effective than placebo, and has been recommended by some authors as the evidence-based approach to optimize clinical outcomes for patients with chronic migraine.Citation56,Citation57,Citation75 Other authors have disputed the need for all of the injections sites included in the PREEMPT trials, and advocate further studies to identify the best injection strategy.Citation76
Until future studies establish the optimal dose, injection sites, and protocol for onabotulinumtoxinA in chronic migraine prophylaxis, the standardized 155 U injection protocol tested in the PREEMPT trials is a great and welcomed advance. Unfortunately, the recommended 155 U dose creates obvious problems in resource utilization and cost effectiveness. In the US, onabotulinumtoxinA is available in single-use 50 U, 100 U, or 200 U vials. If one were to routinely use the 155 U protocol, this could lead to a significant waste of nonreusable product.
At our institution, most chronic migraine patients get a 150 U protocol (, ) in a fixed-site, fixed-dose fashion targeting similar injection sites to those in the PREEMPT injection protocol. This is occasionally combined with an additional follow-the-pain site on an individual case basis. There is no indisputable evidence yet supporting that adding the follow-the-pain strategy is superior to the 155 U fixed-site, fixed-dose protocol alone. OnabotulinumtoxinA injections are administered typically every 12 weeks. More frequent administration is avoided to minimize the formation of neutralizing antibodies, which can affect onabotulinumtoxinA efficacy.Citation26
Figure 3 OnabotulinumtoxinA injection sites used by the authors (see for dosing).
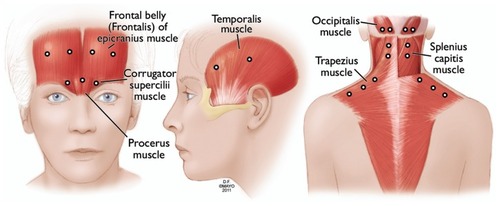
Table 3 Standard fixed-site, fixed-dose 150 U protocol for onabotulinumtoxinA currently used by the authors at the time of this manuscript’s publication
Conclusion
Based on the available data, onabotulinumtoxinA has not been convincingly shown to be effective in the prevention of episodic migraine. In chronic migraine prophylaxis, however, available randomized, double-blind, placebo-controlled trials suggest that onabotulinumtoxinA is effective. The injected form and excellent tolerability of onabotulinumtoxinA makes it an extremely attractive alternative for chronic migraine patients who have demonstrated poor tolerance or poor compliance with traditional oral prophylactics. Although the mechanisms through which onabotulinumtoxinA may exert its benefit remain uncertain, onabotulinumtoxinA is a welcome addition to the available treatment options for chronic migraine, which is often a disabling and difficult-to-manage condition.
Disclosure
Carrie E Robertson has received honoraria for continuing education courses related to neuroimaging. She reports no conflicts of interest. Ivan Garza receives compensation as an author for Up-to-Date and as headache section editor for Current Neurology and Neuroscience Reports. He reports no conflicts of interest.
References
- LiptonRBBigalMEMigraine: epidemiology, impact, and risk factors for progressionHeadache200545 suppl 1S31315833088
- IHSThe International Classification of Headache Disorders: 2nd editionCephalalgia200424 suppl 1916014979299
- OlesenJBousserMGDienerHCNew appendix criteria open for a broader concept of chronic migraineCephalalgia200626674274616686915
- ManackANBuseDCLiptonRBChronic migraine: epidemiology and disease burdenCurr Pain Headache Rep2011151707821063918
- SilbersteinSDTreatment recommendations for migraineNat Clin Pract Neurol20084948248918665146
- BinderWJBrinMFBlitzerAPogodaJMBotulinum toxin type A (BOTOX) for treatment of migraineSemin Cutan Med Surg20012029310011474749
- BinderWJBrinMFBlitzerABotulinum toxin type A (BOTOX) for treatment of migraine headaches: an open-label studyOtolaryngol Head Neck Surg2000123666967611112955
- BinderWJBlitzerABrinMFTreatment of hyperfunctional lines of the face with botulinum toxin ADermatol Surg19982411119812059834739
- DresslerDBeneckeRPharmacology of therapeutic botulinum toxin preparationsDisabil Rehabil200729231761176818033601
- AktoriesKBarmannMOhishiIBotulinum C2 toxin ADP-ribosylates actinNature198632260773903923736664
- BhidayasiriRTruongDDExpanding use of botulinum toxinJ Neurol Sci1520052351–219
- SampaioCCostaJFerreiraJJClinical comparability of marketed formulations of botulinum toxinMov Disord200419 suppl 8S12913615027065
- FDA UFDA gives update on botulinum toxin safety warnings; established names of drugs changedFDA News Release
- AlbaneseATerminology for preparations of botulinum neurotoxins: what a difference a name makesJama20113051899021205970
- FrevertJDresslerDComplexing proteins in botulinum toxin type A drugs: a help or a hindranceBiologics2010432533221209727
- ForanPGMohammedNLiskGOEvaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neuronsJ Biol Chem200327821363137112381720
- RosalesRLBigalkeHDresslerDPharmacology of botulinum toxin: differences between type A preparationsEur J Neurol200613 suppl 121016417591
- DollyJOLawrenceGWMengJNeuro-exocytosis: botulinum toxins as inhibitory probes and versatile therapeuticsCurr Opin Pharmacol20099332633519394272
- BrinMFFahnSMoskowitzCLocalized injections of botulinum toxin for the treatment of focal dystonia and hemifacial spasmAdv Neurol1988505996083400513
- EleopraRTugnoliVDe GrandisDThe variability in the clinical effect induced by botulinum toxin type A: the role of muscle activity in humansMov Disord199712189948990059
- FrassonEPrioriARuzzanteBNerve stimulation boosts botulinum toxin action in spasticityMov Disord200520562462915726575
- WeyJJTangSSWuTYDisulfide bond reduction corresponds to dimerization and hydrophobi-city changes of Clostridium botulinum type A neurotoxinActa Pharmacol Sin20062791238124616923346
- ForanPGDavletovBMeunierFAGetting muscles moving again after botulinum toxin: novel therapeutic challengesTrends Mol Med20039729129912900216
- DollyJOAokiKRThe structure and mode of action of different botulinum toxinsEur J Neurol200613 suppl 41917112344
- MeunierFASchiavoGMolgoJBotulinum neurotoxins: from paralysis to recovery of functional neuromuscular transmissionJ Physiol Paris2002961–210511311755789
- BrinMFBotulinum toxin: chemistry, pharmacology, toxicity, and immunologyMuscle Nerve Suppl19976S1461689826987
- AokiKRReview of a proposed mechanism for the antinociceptive action of botulinum toxin type ANeurotoxicology200526578579316002144
- PavoneFLuvisettoSBotulinum neurotoxin for pain management: insights from animal modelsToxins201022890291322069581
- MenseSNeurobiological basis for the use of botulinum toxin in pain therapyJ Neurol2004251 suppl 1I1714991335
- ArezzoJCPossible mechanisms for the effects of botulinum toxin on painClin J Pain2002186 supplS12513212569959
- WelchMJPurkissJRFosterKASensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxinsToxicon200038224525810665805
- DurhamPLCadyRRegulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapyHeadache20044413542 discussion 42–3314979881
- CuiMKhanijouSRubinoJAokiKRSubcutaneous administration of botulinum toxin A reduces formalin-induced painPain120041071–212513314715398
- BlerschWSchulte-MattlerWJPrzywaraSBotulinum toxin A and the cutaneous nociception in humans: a prospective, double-blind, placebo-controlled, randomized studyJ Neurol Sci20022051596312409185
- Bach-RojeckyLLackovicZAntinociceptive effect of botulinum toxin type a in rat model of carrageenan and capsaicin induced painCroat Med J200546220120815849840
- Bach-RojeckyLReljaMLackovicZBotulinum toxin type A in experimental neuropathic painJ Neural Transm2005112221521915657640
- MarinelliSLuvisettoSCobianchiSBotulinum neurotoxin type A counteracts neuropathic pain and facilitates functional recovery after peripheral nerve injury in animal modelsNeuroscience2010171131632820826198
- MikaJRojewskaEMakuchWThe effect of botulinum neurotoxin A on sciatic nerve injury-induced neuroimmunological changes in rat dorsal root ganglia and spinal cordNeuroscience201117535836621111791
- RanouxDAttalNMorainFBouhassiraDBotulinum toxin type A induces direct analgesic effects in chronic neuropathic painAnn Neurol200864327428318546285
- Favre-GuilmardCAuguetMChabrierPEDifferent antinociceptive effects of botulinum toxin type A in inflammatory and peripheral polyneuropathic rat modelsEur J Pharmacol20096171–3485319576881
- Bach-RojeckyLSalkovic-PetrisicMLackovicZBotulinum toxin type A reduces pain supersensitivity in experimental diabetic neuropathy: bilateral effect after unilateral injectionEur J Pharmacol20106331–3101420123097
- CaleoMAntonucciFRestaniLMazzocchioRA reappraisal of the central effects of botulinum neurotoxin type A: by what mechanism?J Neurochem20091091152419154335
- KitamuraYMatsukaYSpigelmanIBotulinum toxin type a (150 kDa) decreases exaggerated neurotransmitter release from trigeminal ganglion neurons and relieves neuropathy behaviors induced by infraorbital nerve constrictionNeuroscience200915941422142919409226
- CaleoMSchiavoGCentral effects of tetanus and botulinum neurotoxinsToxicon10200954559359919264088
- AntonucciFRossiCGianfranceschiLLong-distance retrograde effects of botulinum neurotoxin AJ Neurosci200828143689369618385327
- MatakIBach-RojeckyLFilipovicBLackovicZBehavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin ANeuroscience201118620120721539899
- SilbersteinSMathewNSaperJJenkinsSBotulinum toxin type A as a migraine preventive treatment. For the BOTOX Migraine Clinical Research GroupHeadache200040644545010849039
- EversSVollmer-HaaseJSchwaagSBotulinum toxin A in the prophylactic treatment of migraine – a randomized, double-blind, placebo-controlled studyCephalalgia2004241083884315377314
- ElkindAHO’CarrollPBlumenfeldAA series of three sequential, randomized, controlled studies of repeated treatments with botulinum toxin type A for migraine prophylaxisJ Pain200671068869617018329
- AuroraSKGawelMBrandesJLBotulinum toxin type a prophylactic treatment of episodic migraine: a randomized, double-blind, placebo- controlled exploratory studyHeadache200747448649917445098
- AnandKSPrasadASinghMMBotulinum toxin type A in prophylactic treatment of migraineAm J Ther200613318318716772757
- VoAHSatoriRJabbariBBotulinum toxin type-a in the prevention of migraine: a double-blind controlled trialAviat Space Environ Med2007785 supplB11311817547312
- FreitagFGDiamondSDiamondMUrbanGBotulinum toxin type A in the treatment of chronic migraine without medication overuseHeadache200848220120918042229
- ReljaMPooleACSchoenenJA multicentre, double-blind, randomized, placebo-controlled, parallel group study of multiple treatments of botulinum toxin type A (BoNTA) for the prophylaxis of episodic migraine headachesCephalalgia200727649250317428299
- SaperJRMathewNTLoderEWA double-blind, randomized, placebo-controlled comparison of botulinum toxin type a injection sites and doses in the prevention of episodic migrainePain Med20078647848517716321
- AuroraSKDodickDWTurkelCCOnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trialCephalalgia201030779380320647170
- DienerHCDodickDWAuroraSKOnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trialCephalalgia201030780481420647171
- ChanVWMcCabeEJMacGregorDLBotox treatment for migraine and chronic daily headache in adolescentsJ Neurosci Nurs200941523524319835236
- AhmedKOasKHMackKJGarzaIExperience with botulinum toxin type A in medically intractable pediatric chronic daily headachePediatr Neurol201043531631920933173
- MathewNTFrishbergBMGawelMBotulinum toxin type A (BOTOX) for the prophylactic treatment of chronic daily headache: a randomized, double-blind, placebo-controlled trialHeadache200545429330715836565
- NaumannMSoYArgoffCEAssessment: botulinum neurotoxin in the treatment of autonomic disorders and pain (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of NeurologyNeurology200870191707171418458231
- DodickDWTurkelCCDegryseREOnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical programHeadache20105069213619925625
- GarzaISchwedtTJChronic migraineUpToDate, Wolters Kluwer Health2011 Desktop 19.1Accessed June 18, 2011
- MathewNTJaffriSFA double-blind comparison of onabotulinumtoxina (BOTOX) and topiramate (TOPAMAX) for the prophylactic treatment of chronic migraine: a pilot studyHeadache200949101466147819912346
- RahimtoolaHBuurmaHTijssenCCMigraine prophylactic medication usage patterns in The NetherlandsCephalalgia200323429330112716348
- CadyRSchreiberCBotulinum toxin type A as migraine preventive treatment in patients previously failing oral prophylactic treatment due to compliance issuesHeadache200848690091318047501
- OndoWGVuongKDDermanHSBotulinum toxin A for chronic daily headache: a randomized, placebo-controlled, parallel design studyCephalalgia2004241606514687015
- SilbersteinSDStarkSRLucasSMBotulinum toxin type A for the prophylactic treatment of chronic daily headache: a randomized, double-blind, placebo-controlled trialMayo Clin Proc20058091126113716178492
- SilbersteinSDStarkSRLucasSMBotulinum toxin type A for the prophylactic treatment of chronic daily headache: a randomized, double-blind, placebo-controlled trial. Mayo Clinic proceedingsMayo Clinic9200580911261137
- AllerganBotox/Botox Cosmetic package insert:17
- WoodcockJResponse to citizen petition on botulinum toxin (Docket No. FDA-2008-P-0061)FDA2009
- BlumenfeldAMSchimJDChippendaleTJBotulinum toxin type A and divalproex sodium for prophylactic treatment of episodic or chronic migraineHeadache200848221022018047502
- MagalhaesEMenezesCCardealMMeloABotulinum toxin type A versus amitriptyline for the treatment of chronic daily migraineClin Neurol Neurosurg2010112646346620399553
- CadyRKSchreiberCPPorterJAA multi-center double-blind pilot comparison of onabotulinumtoxinA and topiramate for the prophylactic treatment of chronic migraineHeadache2011511213221070228
- BlumenfeldASilbersteinSDDodickDWMethod of injection of onabotulinumtoxinA for chronic migraine: a safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical programHeadache20105091406141820958294
- GerwinRTreatment of chronic migraine headache with onabotulinumtoxinACurr Pain Headache Rep201115533633821547527
