Abstract
Objective
To evaluate the efficacy, safety and economics of levetiracetam (LEV) for epilepsy.
Materials and methods
PubMed, Scopus, the Cochrane Library, OpenGrey.eu and ClinicalTrials.gov were searched for systematic reviews (SRs), meta-analyses, randomized controlled trials (RCTs), observational studies, case reports and economic studies published from January 2007 to April 2018. We used a bubble plot to graphically display information of included studies and conducted meta-analyses to quantitatively synthesize the evidence.
Results
A total of 14,803 records were obtained. We included 30 SRs/meta-analyses, 34 RCTs, 18 observational studies, 58 case reports and 2 economic studies after the screening process. The included SRs enrolled patients with pediatric epilepsy, epilepsy in pregnancy, focal epilepsy, generalized epilepsy and refractory focal epilepsy. Meta-analysis of the included RCTs indicated that LEV was as effective as carbamazepine (CBZ; treatment for 6 months: 58.9% vs 64.8%, OR=0.76, 95% CI: 0.50–1.16; 12 months: 54.9% vs 55.5%, OR=1.24, 95% CI: 0.79–1.93), oxcarbazepine (57.7% vs 59.8%, OR=1.34, 95% CI: 0.34–5.23), phenobarbital (50.0% vs 50.9%, OR=1.20, 95% CI: 0.51–2.82) and lamotrigine (LTG; 61.5% vs 57.7%, OR=1.22, 95% CI: 0.90–1.66). SRs and observational studies indicated a low malformation rate and intrauterine death rate for pregnant women, as well as low risk of cognitive side effects. But psychiatric and behavioral side effects could not be ruled out. LEV decreased discontinuation due to adverse events compared with CBZ (OR=0.52, 95% CI: 0.41–0.65), while no difference was found when LEV was compared with placebo and LTG. Two cost-effectiveness evaluations for refractory epilepsy with decision-tree model showed US$ 76.18 per seizure-free day gained in Canada and US$ 44 per seizure-free day gained in Korea.
Conclusion
LEV is as effective as CBZ, oxcarbazepine, phenobarbital and LTG and has an advantage for pregnant women and in cognitive functions. Limited evidence supports its cost-effectiveness.
Registered number
PROSPERO (No CRD 42017069367).
Background
Epilepsy ranks fourth after tension-type headache, migraine and Alzheimer disease in the world’s neurological disorders burden.Citation1 A systematic review (SR) and meta-analysis of international studies reported that the point prevalence of active epilepsy was 6.38 per 1,000 people, while the lifetime prevalence was 7.60 per 1,000 people. The annual cumulative incidence of epilepsy was 67.77 per 100,000 people, while the incidence rate was 61.44 per 100,000 person-years.Citation2 As a fairly common clinical condition affecting all ages and requiring long-term, sometimes lifelong, treatment, epilepsy incurs high health care costs for the society.Citation1 In 2010, the total annual cost for epilepsy was 13.8 billion and the total cost per patient was €5,221 in Europe.Citation3 Meanwhile, in the USA, epilepsy-related costs ranged from $1,022 to $19,749 per person annually.Citation4 What is more, drug-refractory epilepsy was a major cost driver,Citation5 with main costs from anticonvulsants, hospitalization and early retirement.Citation6
Currently, antiepileptic drugs (AEDs) are the main treatment method for epilepsy patients, and it was reported that approximately two-thirds of epileptic seizures were controlled by AEDs.Citation7 Conventional AEDs such as carbamazepine (CBZ) and sodium valproate (VPA) have been proven to have good therapeutic effects and low treatment cost. However, some adverse events (AEs) related to these drugs, such as Stevens–Johnson syndrome, menstrual disorder and memory deterioration seriously affect the tolerance and compliance of patients. Compared with conventional AEDs, new AEDs have the potential to be safer, but also more expensive.Citation8
Levetiracetam (LEV) is a novel AED that has been approved as an adjunctive therapy for adults with focal epilepsy since 1999 in the US. In 2006, it was licensed as monotherapy for adults and adolescents above 16 years of age with newly diagnosed focal-onset seizures with or without secondary generalization in Europe. Also, it has been indicated as an adjunctive therapy for partial-onset seizures in patients above 4 years of age in China since 2007. Although the precise mechanism of LEV is still unclear, current researches suggest that its pharmacological mechanism is different from those of other AEDs. It may bind to the synaptic vesicle protein 2A (SV2A), which presents on the synaptic vesicles and some neuroendocrine cells. SV2A may participate in the exocytosis of synaptic vesicles and regulate the release of neurotransmitters, especially the release of excitatory amino acids, and thus depress the epilepsy discharge.Citation9,Citation10 Other possible mechanisms of LEV include the following: selective inhibition of voltage-dependent N-type calcium channels in hippocampal pyramidal cells and reduction of the negative allosteric agents’ inhibition, such as zinc ions and B-carbolines, on glycine and γ-aminobutyric acid neurons, which results in indirectly increasing central nervous system inhibition.Citation11
LEV is almost completely absorbed after oral administration and the absorption is unaffected by food. The bioavailability is nearly 100% and the steady-state concentrations are achieved in 2 days if LEV is taken twice daily. Sixty-six percent of LEV is renally excreted unchanged and its major metabolic pathway is enzymatic hydrolysis of the acetamide group, which is independent of liver CYP/CYP450; so, no clinically meaningful drug–drug interactions with other AEDs were found.Citation12 One published SR of LEV suggested LEV has an equal efficacy compared with conventional AEDs and it is well tolerated for long-term therapy without significant effect on the immune system.Citation13 But in recent years, apart from the most frequent AEs of LEV, such as nausea, gastrointestinal symptoms, dizziness, irritability and aggressive behavior, some rare AEs of LEV have been reported, including eosinophilic pneumonia, rhabdomyolysis, thrombocytopenia, elevated kinase and reduced sperm quality.Citation14–Citation17
Thus, we conducted a mapping review to evaluate the efficacy, safety and economic profiles of LEV compared with all other AEDs for epilepsy, to provide evidence-based information for the rational use of LEV and research agendas.
Materials and methods
Search strategy
We searched PubMed, Scopus, Cochrane Central Register of Controlled Trials, ClinicalTrials.gov and OpenGrey.eu from Jan 1, 2007 to April 30, 2017 and updated the search results till April 23, 2018. The following keywords were used in search terms: “anticonvulsant*”, “anticonvulsive”, “antiepileptic*”, “antiepilepsirin*”, “epileps*”, “epileptic*”, “seizure*”, “convulsion*”, “trial”, “comparative effectiveness research”, “cohort study”, “case-control study”, “case report*”, “case series”, “cost-benefit analysis”, “cost-effectiveness analysis”, “cost-utility analysis”, “cost-minimization analysis”, “systematic review”, “meta-analysis” and “health technology assessment”. The search terms “Keppra”, “Levetiracetam”, “Desitrend”, “Spritam”, “Kepcet”, “Kevtam” and “Levitam” were used to search relevant literature to LEV. The study was registered on PROSPERO (No CRD 42017069367).
Study selection and outcome measures
Four independent investigators manually screened the references of all retrieved records for potentially eligible studies through the title and abstract screening in the first stage and the full-text screening in the second. For the title and abstract screening, studies appearing to meet the inclusion criteria or with insufficient information to make a clear judgment, judged by either authors or both, were included in the full-text screening process. We obtained full texts of all these studies for the full-text screening. We included studies if they 1) enrolled patients diagnosed with epilepsy, 2) compared the efficacy, safety or economic profiles of LEV, without restricting to dosage and duration and 3) SR, meta-analysis, randomized controlled trials (RCTs), observational studies, case reports and economic studies were considered. We resolved the disagreements through discussion, and if necessary, a third party was consulted and discussed.
The primary efficacy outcomes focused on seizure freedom. The secondary efficacy outcomes included 50% responder rate, quality of life (QoL), discontinuation due to AEs, serious AEs, total AEs, single AEs and cost-effectiveness.
Data extraction and quality assessment
Data extraction was performed by two independent investigators according to a predesigned data collection form. Extracted information included authors, publication year, search time frame, number of LEV trials, participant characteristic (seizure type, gender and age), intervention information (the dosage and duration), treatment duration, outcome of interest and dropout rate.
Two investigators independently assessed the methodological quality of included studies. We assessed the quality of included SRs using the Assessment of Multiple Systematic Reviews tool (range, 0–11).Citation18 We assessed the risk of bias in the eligible RCTs with the Cochrane risk of bias assessment tool.Citation19 The methodological quality of eligible observational studies was evaluated with the Newcastle–Ottawa Scale.Citation20 We evaluated the quality of the eligible pharmacoeconomic study with consolidated health economic evaluation reporting standard.Citation21 We did not conduct quality assessment of case reports. In the case of missing data, we contacted the authors of eligible studies for clarifications. All disagreements about data extraction and quality assessment were resolved through discussion among all authors.
Statistical analysis
We compared the treatment effect through meta-analyses in an intention-to-treat manner (following the allocation of participants in studies) of newly included RCTs. Results of RCTs evaluating similar interventions in similar participants were pooled. We calculated the OR for categorical outcomes. We performed meta-analyses of newly included RCTs with RevMan 5.3 software using random-effect model. Statistical heterogeneity was assessed with the Mantel–Haenszel chi-squared test and quantified with the I2 test. P<0.05 was considered statistically significant. Analyses of evidence mapping were conducted in R version 3.4.3. We used a bubble plot to graphically display the evidence regarding seizure type, control vs LEV and outcome measures. Seizure type was classified based on the type of patients and type of epilepsy. Controls were classified based on the class of antiepileptic drug. Outcomes were classified into efficacy and safety outcomes. The number of included studies in SRs and the number of included patients in RCTs were presented as the size of the circles. We described the safety outcomes of observational studies and pooled the numbers of case reports by classification of diseases.
Results
Study selection
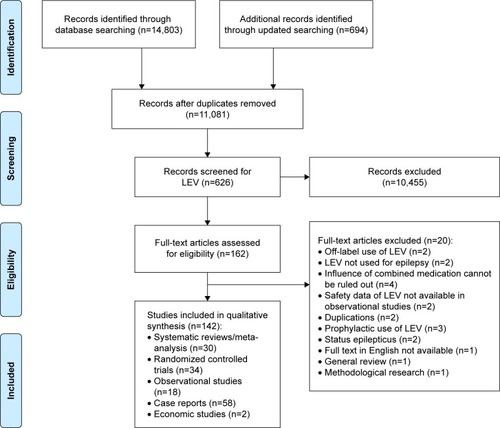
The initial search identified 14,803 relevant records and the updated search identified 694 records. Also, 11,801 records remained after duplicates were removed. Of these, 10,455 records were excluded after LEV search and title/abstract screening and 162 reports were eligible for full-text review. After full-text review, we included 142 reports: 30 SRs/meta-analyses,Citation22–Citation51 34 RCTs,Citation52–Citation85 18 observational studies,Citation86–Citation103 58 case reportsCitation104–Citation161 and 2 economic studiesCitation162,Citation163 ().
Study characteristics and quality assessment
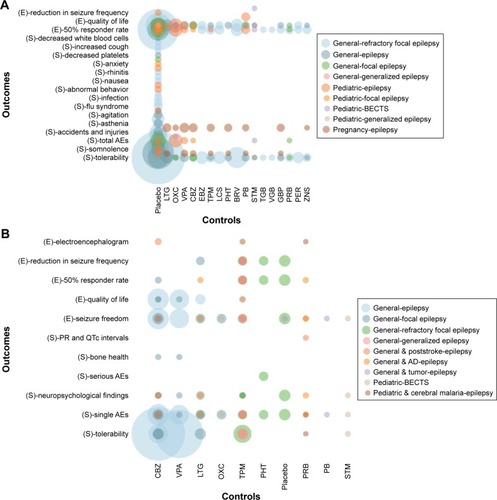
The included SRs were published between 2007 and 2018, enrolling patients with pediatric epilepsy, epilepsy in pregnancy, focal epilepsy, generalized epilepsy and refractory focal epilepsy. Twenty SRs compared LEV with placebo,Citation22–Citation35,Citation38,Citation40,Citation44,Citation46,Citation49,Citation50 19 SRs compared LEV with other AEDsCitation23,Citation24,Citation30,Citation34,Citation36–Citation43,Citation45–Citation51 and 8 SRs were network meta-analyses that compared LEV with other AEDsCitation23,Citation30,Citation37,Citation45–Citation48,Citation50 as well as placebo.Citation23,Citation30,Citation46,Citation50 Outcome measures included seizure freedom, 50% responder rate, reduction in seizure frequency, neuropsychological findings, congenital malformation, serious AEs, total AEs, single AEs and other outcomes (). Among the included RCTs, 12 compared LEV with placebo,Citation52,Citation55,Citation56,Citation58,Citation60–Citation63,Citation65,Citation66,Citation68,Citation78 9 compared LEV with CBZ,Citation53,Citation69,Citation70,Citation73,Citation74,Citation79–Citation82 4 compared LEV with lamotrigine (LTG),Citation57,Citation64,Citation71,Citation81 3 compared LEV with phenobarbital (PB),Citation64,Citation75,Citation85 3 compared LEV with VPA,Citation70,Citation74,Citation82 2 compared LEV with oxcarbazepine (OXC),Citation54,Citation83 2 compared LEV with sulthiame,Citation72,Citation84 1 compared LEV with pregabalin,Citation77 1 compared LEV with phenytoinCitation59 and 1 compared LEV with topiramate.Citation67 Outcome measures included seizure freedom, 50% responder rate, reduction in seizure frequency, QoL, serious AEs, total AEs, single AEs and other outcomes ().
Figure 2 Evidence mapping of included systematic reviews (A) and randomized controlled trials (B).
The two economic studies were from Canada and Korea, both of which focus on add-on therapy for refractory epilepsy.Citation162,Citation163 The two studies used a decision-tree model from the social perspective and payer perspective, respectively.
Study characteristics of the included observational studies and case reports are shown in and , respectively.
In general, the quality of included SRs and economic studies was good. The included RCTs were generally of low risk of bias. Sixteen RCTs used the double-blind design and 24 adopted the intention-to-treat principle to analyze data ().
Efficacy
Seizure freedom
Thirteen SRs evaluated rates of seizure freedomCitation23,Citation26,Citation31,Citation37,Citation40,Citation41,Citation43–Citation46,Citation49–Citation51 () and indicated that LEV increased the rates of seizure freedom compared with placebo,Citation23,Citation26,Citation31,Citation40,Citation44,Citation46,Citation49,Citation50 but there was no difference when LEV was compared with OXC,Citation41,Citation49 LTGCitation23,Citation37,Citation45,Citation51 and brivaracetam.Citation40
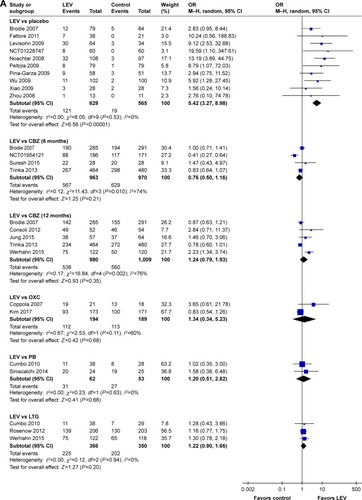
Meta-analysis of newly included RCTs indicated that LEV increased the rates of seizure freedom compared with placebo (19.2% [121/629] vs 3.4% [19/565], OR=5.42, 95% CI: 3.27–8.98). Meta-analyses of newly included RCTs showed that there was no difference when LEV was compared with CBZ (treatment for 6 months: 58.9% [567/963] vs 64.8% [629/970], OR=0.76, 95% CI: 0.50–1.16; treatment for 12 months: 54.9% [538/980] vs 55.5% [560/1,009], OR=1.24, 95% CI: 0.79–1.93), OXC (57.7% [112/194] vs 59.8% [113/189], OR=1.34, 95% CI: 0.34–5.23), PB (50.0% [31/62] vs 50.9% [27/53], OR=1.20, 95% CI: 0.51–2.82) and LTG (61.5% [225/366] vs 57.7% [202/350], OR=1.22, 95% CI: 0.90–1.66). We observed significant heterogeneity across included studies in the subgroup of CBZ (I2=74% for 6 months treatment and I2=76% for 12 months treatment), as shown in .
Figure 3 Rate of seizure freedom of included randomized controlled trials (A) and ≥50% responder rates of included randomized controlled trials (B).
≥50% responder rates
Sixteen SRs evaluated ≥50% responder ratesCitation23,Citation24,Citation26,Citation27,Citation29–Citation31,Citation36,Citation40–Citation43,Citation46,Citation49–Citation51 () and 12 SRs indicated that LEV increased the rates of ≥50% responder rates compared with placebo,Citation23,Citation24,Citation26,Citation27,Citation29–Citation31,Citation36,Citation40,Citation42,Citation46,Citation49 but there was no difference when LEV was compared with brivaracetam.Citation40
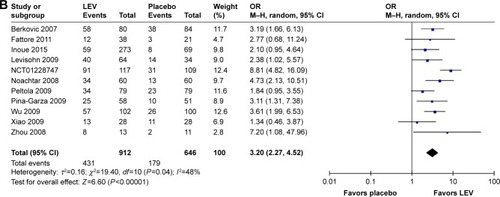
Meta-analysis of newly included RCTs indicated that LEV increased the rates of ≥50% responder rates compared with placebo (n=1,558, 47.3% [431/912] vs 27.7% [179/646], OR=3.20, 95% CI: 2.27–4.52), as shown in .
Improvement of QoL
One SR suggested that LEV had a positive effect on some aspects of QoL in adults.Citation27
Meta-analysis of newly included RCTs showed that there was no difference between LEV and placebo in improvement of QoL (n=224, OR=2.76, 95% CI: 0.85–8.94). We observed significant heterogeneity (I2=72%) across included studies.
Safety
Discontinuation due to AEs
SRs indicated that there was no difference in risk of discontinuation due to AEs when LEV was compared with placebo.Citation24
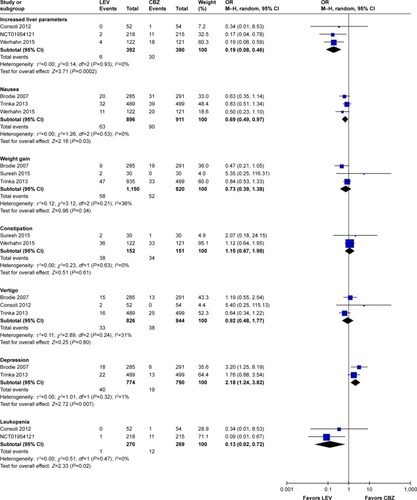
Meta-analysis of newly included RCTs indicated that LEV decreased discontinuation due to AEs compared with CBZ (OR=0.52, 95% CI: 0.41–0.65), while there was no difference when LEV was compared with placebo (OR=1.16, 95% CI: 0.92–1.46) and LTG (OR=1.24, 95% CI: 0.55–2.83). We observed significant heterogeneity (I2=74%) across included studies in the subgroup of LTG.
Serious AEs
Meta-analysis of newly included RCTs showed that there was no difference when LEV was compared with placebo (OR=1.10, 95% CI: 0.59–2.05), CBZ (OR=0.83, 95% CI: 0.35–1.95) and LTG (OR=1.40, 95% CI: 0.74–2.62) in the rates of serious AEs.
Total AEs
SRs indicated that AEs were not significantly different between the LEV group and the placebo group.Citation31
Meta-analysis of newly included RCTs showed that there was no difference when LEV was compared with placebo (OR=1.16, 95% CI: 0.92–1.46) and OXC (OR=0.73, 95% CI: 0.47–1.15) in the rates of total AEs.
Single AEs
Malformations and prenatal outcomes
Two SRs reported the safety of AEDs during pregnancy, both of which indicated that LEV was not associated with a higher risk compared to control (RR=0.32, 95% CI: 0.10–1.07 and OR=0.72, 95% CI: 0.43–1.16, respectively).Citation39,Citation47
Two observational studies used data from deliveries recorded in the compulsory Medical Birth Registry of Norway 1999–2011 and International Registry of Antiepileptic Drugs and Pregnancy (EURAP) registry, respectively.Citation91,Citation95 While data in the Norway registry showed LEV had a low malformation rate for pregnant women (OR=0.63, 95% CI: 0.16–2.55 for monotherapy and OR=1.08, 95% CI: 0.27–4.43 for polytherapy), data in the EURAP registry indicated low intrauterine death rates (8.6%, 95% CI: 5.8%–12.3%).
Neurological development
One SR showed that LEV did not increase the risk for delayed development of children (cognitive development delay: OR=3.42, 95% Credible Interval: 0.65–16.40; psychomotor development delay: OR=0.27, 95% Credible Interval: 0.00–4.65).Citation48
An observational study by Javed et alCitation93 indicated a low risk of cognitive side effects of LEV (OR=0.68, 95% CI: 0.48–0.99 in patients newly started on polypharmacy).
Psychiatric and behavioral side effects (PBSEs)
One SR showed from various types of studies that LEV administration was associated primarily with adverse psychotropic effects including anxiety, irritability and depression.Citation28 One SRCitation32 indicated that LEV increased the risk of developing several behavioral side effects (RR=2.18, 95% CI: 1.42–3.37) such as aggression, hostility and nervousness, while the other SR reported lower rates of behavioral effects.Citation33 Another SR indicated that LEV may have a relationship with suicidality in epilepsy ().Citation34
Meta-analysis of newly included RCTs indicated that LEV increased the risk of irritability compared with placebo (n=328, OR=11.55, 95% CI: 2.12–62.90; ) and the risk of depression compared with CBZ (n=1,564, OR=2.18, 95% CI: 1.24–3.82; ). But no difference was found in the risk of depression when LEV was compared with LTG (n=673, OR=1.80, 95% CI: 0.82–3.97).
Figure 4 Risk of single adverse events (LEV vs placebo, A; LEV vs CBZ, B).
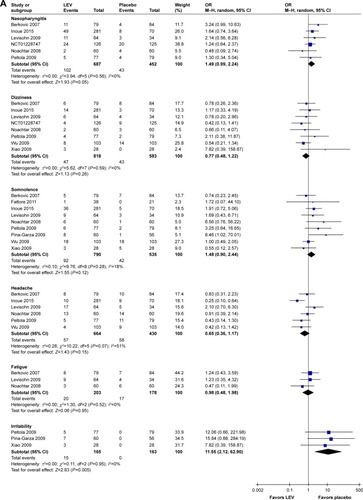
For observational studies, Bootsma et alCitation86 indicated the most prevalent AEs for LEV were activating mood disorders (8.1% for 6 months, 5.2% for 12 months and 10.6% for 18 months), Arif et alCitation88 indicated psychiatric AEs were the most common adverse effects leading to intolerability and Andersohn et alCitation87 indicated LEV was associated with an increased risk of self-harm or suicidal behavior. Chen et alCitation97 indicated that LEV had the greatest PBSE rate in adults with epilepsy. However, Bektaş et alCitation96 indicated that psychosocial and behavioral side effects of LEV treatment are not frequent and they do not emerge in most of the children at lower doses, and Stephen et alCitation103 indicated a lower rate of psychiatric side effects for LEV than sodium channel blocking AEDs.
Among the 58 case reports, 17 reported PBSEs, including depression, suicidality and hypersexuality.
Other AEs
SRs indicated that LEV did not increase the risk of imbalance,Citation22 but increased the risk of diplopia ().Citation25
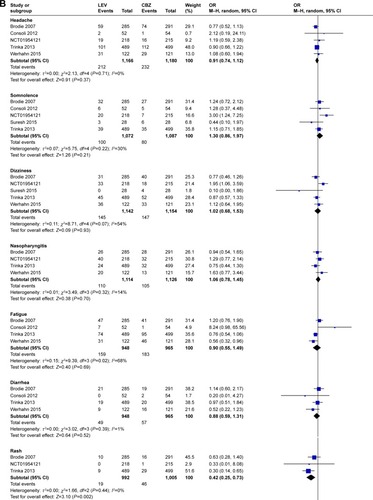
Meta-analysis of newly included RCTs indicated LEV had a lower risk of leukopenia (OR=0.13, 95% CI: 0.02–0.72), rash (OR=0.42, 95% CI: 0.25–0.73), increased liver parameters (OR=0.19, 95% CI: 0.08–0.46) and nausea (OR=0.69, 95% CI: 0.49–0.97) compared with CBZ (). LEV had a lower risk of nausea (OR=0.62, 95% CI: 0.39–0.98) and a higher risk of fatigue (OR=1.87, 95% CI: 1.26–2.77) compared with LTG. Meta-analyses of newly included RCTs showed that there was no difference when LEV was compared with placebo, CBZ, LTG and OXC in headache (). No difference was found in somnolence and dizziness when LEV was compared with placebo, CBZ and LTG ().
Among the observational studies, Merrell et al indicated LEV had fewer side effects than phenytoin.Citation89 Rauchenzauner et al indicated LEV did not seem to induce changes in reproductive endocrine functions and clinically relevant endocrine side effects in prepubertal children.Citation90 Tinchon et al indicated LEV has no additional impact on medium-term hematological toxicity in glioblastoma multiforme patients.Citation94 Xiao et al reported all AEs of LEV were either mild or transient and thus did not lead to withdrawal from drug treatment.Citation92
Other case reports were related to side effects in the hematological system, skin, kidney, liver and other systems ().
Table 1 The characteristics of included observational studies
Table 2 The characteristics of included case reports
Table 3 Risk of bias of included randomized controlled trials
Cost-effectiveness
Two cost-effectiveness evaluations for refractory epilepsy with the decision-tree model were conducted in Canada and Korea, respectively.
The Canadian study showed the incremental cost- effectiveness ratio (ICER) was US$ 76.18 per seizure-free day (SFD) gained for the base-case scenario; when the cost of surgical investigation and surgery was included in the model, the ICERs decreased to US$ 39.18, which was the most cost-effective situation.Citation162
The Korean study showed that LEV add-on therapy gained 18.3 SFDs per patient per year and the ICERs were US$ 44 per SFD per patient and US$ 11,084 per quality-adjusted life year gained from the third-party payer perspective.Citation163
Discussion
In our evidence map, the included SRs and newly conducted meta-analyses showed consistent results regarding clinical benefits and potential harms of LEV. Our evidence map indicated that LEV had similar efficacy in seizure freedom compared with conventional AEDs and was superior to placebo in seizure freedom and ≥50% responder rates. What is more, LEV had a lower risk of discontinuation due to AEs compared with CBZ and did not increase the risk of malformations and prenatal outcomes as well as neurological development. Limited evidence suggested it was cost-effective in certain settings.
LEV has been classified by the US Food and Drug Administration as a category C drug, with the caution that it should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. A Cochrane review included in our study analyzed the incidence of congenital malformations in pregnant women during AED treatment and reported that LEV and LTG exposure carried the lowest risk of overall malformation.Citation39 A recently published prospective cohort study based on the EURAP international registry reported the lowest prevalence of major congenital malformations of LEV (2.8%, 17/599 pregnancies) compared with other seven commonly used AEDs.Citation164 Two observational studiesCitation91,Citation95 included in this evidence map drew similar conclusions. A published study found that compared with VPA, LEV did not cause apoptosis in immature rat brain neurons, which may be the reason of its safety for pregnant women.Citation165 Neurologists are also concerned with the effect of AEDs on cognitive function, which significantly affects the QoL of patients, especially children and the elderly. No AEs of LEV on cognitive function were found in our study, which was consistent with the guidelines. However, there are some RCTs, observational studies and case reports indicating the AEs of mood disorders of LEV. We should monitor these AEs during the course of medication.
A number of guidelines included LEV as a main drug for antiepileptic treatment. The National Institute for Health and Care Excellence (NICE; 2017) recommended that LEV could be used as a monotherapy and in the adjunctive treatment of focal epilepsy (with or without secondary generalization) and adjunctive therapy of myoclonic seizures in patients with juvenile myoclonic epilepsy and generalized tonic clonic seizures.Citation7 The Scottish Intercollegiate Guidelines Network gave a similar recommendation and further suggested that LEV or LTG may be a reasonable alternative for women of childbearing age. Moreover, the guideline also suggested that LEV was better tolerated than sustained-release CBZ in poststroke seizures and produced fewer cognitive AEs than LTG or PB in the elderly with epilepsy and Alzheimer disease.Citation166 The Biopharmaceutics Drug Disposition Classification System predicted that the risk of skin rash by LEV is not as high as by CBZ or LTG,Citation167 and that human leukocyte antigen testing is not necessary. With the increasing number of studies on LEV, guideline recommendations need to update the evidence for LEV.Citation168 Our research provides supplements for evidence update in future guidelines.
The economic evaluation of LEV showed that LEV appeared to be cost-effective when the costs of surgical investigation were discounted. Besides, when LEV is added to the usual treatment of patients with refractory epilepsy, the increase in drug costs may at least be partially offset by savings in other medical costs due to an increase in SFDs and improvement of QoL.Citation169 But until now, the NICE guideline still has suggested LEV monotherapy as a second-line drug and LEV is considered when the standard first-line drugs such as CBZ and LTG are unsuitable or develop intolerance in the newly diagnosed focal seizure. The economic profiles of our research can help with the cost-effectiveness decision making in certain conditions.
To the best of our knowledge, this study is the most comprehensive evidence of LEV in the following aspects. First, we included various types of studies, such as high-quality RCTs, cohort studies, observational studies, case reports and economic studies. The literature included was comprehensive and involved a large number of patients. Second, we evaluated the clinical application of LEV from three dimensions: efficacy, safety and economy, while the three aspects were studied respectively or the evaluation of LEV was among the overall evaluation of a variety of AEDs in the previous published studies.Citation30,Citation36,Citation163,Citation170 Thus, our study can provide comprehensive evidence of LEV for physicians or policymakers.
Our study still had some limitations. First, only English language studies were included. We tried to include important conference abstracts found in the databases, but failed to find relevant studies. Moreover, the literature included in this study was published after 2007, although previously published studies were included in the SRs of the evidence map. Third, some special types of seizures such as status epilepticus (SE) were excluded and data of LEV in special populations were not assessed separately. Fourth, no subgroup analysis of different types of seizures and/or epilepsy syndromes was conducted.
The NICE guideline suggested that LEV is potentially as effective as PB and safer for SE. Currently available intravenous AEDs are limited, and intravenous LEV may have advantages for patients who cannot be administered orally with SE or in the perioperative period.Citation171,Citation172 A chart review in Germany showed LEV was the first choice for intravenous treatment of SE compared with valproate, phenytoin and lacosamide.Citation173 We can evaluate the role of LEV for SE in future studies.
Conclusion
LEV has been applied for diverse epilepsies, and the evidence map shows that it increases the rates of seizure freedom and ≥50% responder rates compared with placebo, has similar efficacy with CBZ, OXC, PB and LTG, and also has an advantage for pregnant women as well as in cognitive functions. LEV does not increase the risks of serious AEs and discontinuation from studies due to AEs. Limited evidence supports its cost-effectiveness.
Acknowledgments
We extend special thanks to Wei Huang from Zhejiang Province Chinese Medical Hospital, Le Gao, Ji-Chun Yang and Yang Xu from Peking University Health Science Center as well as Jun-Wen Zhou from the Public Health Department, and Aix-Marseille University for their contribution in conducting the literature review and drafting figures for this manuscript. We would like to thank Li Wang from Peking University First Hospital and Yuan Zhang from McMaster University for expert consultation. This study was funded by UCB China Inc. At no point did UCB China Inc. attempt to influence the manuscript. The abstract of this paper was presented as a poster in the ISPE’s 11th Asian Conference on Pharmacoepidemiology with interim findings.
Disclosure
The authors report no conflicts of interest in this work.
References
- BeghiEAddressing the burden of epilepsy: many unmet needsPharmacol Res2016107798426952026
- FiestKMSauroKMWiebeSPrevalence and incidence of epilepsy: a systematic review and meta-analysis of international studiesNeurology201788329630327986877
- OlesenJGustavssonASvenssonMThe economic cost of brain disorders in EuropeEur J Neurol201219115516222175760
- BegleyCEDurginTLThe direct cost of epilepsy in the United States: a systematic review of estimatesEpilepsia20155691376138726216617
- KortlandLMAlfterABährOCosts and cost-driving factors for acute treatment of adults with status epilepticus: a multicenter cohort study from GermanyEpilepsia201657122056206627753082
- WillemsLMRichterSWatermannNTrends in resource utilization and prescription of anticonvulsants for patients with active epilepsy in Germany from 2003 to 2013 – a ten-year overviewEpilepsy Behav201883283529649671
- Epilepsies: diagnosis and management (clinical guideline [CG137]) Available from: https://www.nice.org.uk/guidance/cg137Accessed July 18, 2017
- ZhuFLangSYWangXQLong-term effectiveness of antiepileptic drug monotherapy in partial epileptic patients: a 7-year study in an epilepsy center in ChinaChin Med J2015128223015302226608980
- MatagneAMargineanuDGKendaBMichelPKlitgaardHAnti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2ABr J Pharmacol200815481662167118500360
- RigoJMHansGNguyenLThe anti-epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA- and glycine-gated currentsBr J Pharmacol2002136565967212086975
- DeshpandeLSDelorenzoRJMechanisms of levetiracetam in the control of status epilepticus and epilepsyFront Neurol201451124550884
- EmswilerMPCumpstonKLSecond generation anticonvulsants: gabapentin, lamotrigine, levetiracetam, and topiramate Available from: https://www.researchgate.net/publication/312660054_Second_Generation_Anticonvulsants_Gabapentin_Lamotrigine_Levetiracetam_and_Topiramate?ev=auth_pubAccessed July 18, 2017
- FrenchJEdrichPCramerJAA systematic review of the safety profile of levetiracetam: a new antiepileptic drugEpilepsy Res2001471–2779011673023
- SpencerDLevetiracetam in men with epilepsy: testosterone is left alone but sperm count is paramountEpilepsy Curr20171729910028490999
- FaganAFuldJSoonELevetiracetam-induced eosinophilic pneumoniaBMJ Case Rep20172017bcr2016219121
- KimJShinJWLevetiracetam-induced thrombocytopenia in a patient with status epilepticusEpileptic Disord201719110410828202425
- di LorenzoRLiYRhabdomyolysis associated with levetiracetam administrationMuscle Nerve2017561E1E228039868
- SheaBJGrimshawJMWellsGADevelopment of AMSTAR: a measurement tool to assess the methodological quality of systematic reviewsBMC Med Res Methodol2007711017302989
- HigginsJPAltmanDGGøtzschePCThe Cochrane Collaboration’s tool for assessing risk of bias in randomised trialsBMJ2011343d592822008217
- WellsGASheaBO’ConnellDThe Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspAccessed July 18, 2017
- HusereauDDrummondMPetrouSConsolidated Health Economic Evaluation Reporting Standards (CHEERS) statementValue Health2013162e1e523538200
- SirvenJIFifeTDWingerchukDMDrazkowskiJFSecond-generation antiepileptic drugs’ impact on balance: a meta-analysisMayo Clin Proc2007821404717285784
- CostaJFareleiraFAscençãoRBorgesMSampaioCVaz-CarneiroAClinical comparability of the new antiepileptic drugs in refractory partial epilepsy: a systematic review and meta-analysisEpilepsia20115271280129121729036
- LoBWKyuHHJichiciDUptonAMAklEAMeadeMOMeta-analysis of randomized trials on first line and adjunctive levetiracetamCan J Neurol Sci201138347548621515509
- HanHQuWKangHEffect of second-generation antiepileptic drugs on diplopia: a meta-analysis of placebo-controlled studiesJ Huazhong Univ Sci Technolog Med Sci201232455756222886970
- MaguireMMarsonAGRamaratnamSEpilepsy (generalized)BMJ Clin Evid201220121201
- MbizvoGKDixonPHuttonJLMarsonAGLevetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane ReviewCochrane Database Syst Rev201299CD001901
- PiedadJRickardsHBesagFMCavannaAEBeneficial and adverse psychotropic effects of antiepileptic drugs in patients with epilepsy: a summary of prevalence, underlying mechanisms and data limitationsCNS Drugs201226431933522393904
- AryaRGlauserTAPharmacotherapy of focal epilepsy in children: a systematic review of approved agentsCNS Drugs201327427328623515971
- BodaliaPNGrossoAMSofatRComparative efficacy and tolerability of anti-epileptic drugs for refractory focal epilepsy: systematic review and network meta-analysis reveals the need for long term comparator trialsBr J Clin Pharmacol201376564966723351090
- FangYWuXXuLRandomized-controlled trials of levetiracetam as an adjunctive therapy in epilepsy of multiple seizure typesJ Clin Neurosci2014211556224231559
- HalmaEde LouwAJKlinkenbergSAldenkampAPIjffDMMajoieMBehavioral side-effects of levetiracetam in children with epilepsy: a systematic reviewSeizure201423968569124981629
- MbizvoGKDixonPHuttonJLMarsonAGThe adverse effects profile of levetiracetam in epilepsy: a more detailed lookInt J Neurosci2014124962763424256446
- FountoulakisKNGondaXBaghaiTCReport of the WPA section of pharmacopsychiatry on the relationship of antiepileptic drugs with suicidality in epilepsyInt J Psychiatry Clin Pract201519315816725547437
- VerrottiAPreziosoGdi SabatinoFFrancoVChiarelliFZaccaraGThe adverse event profile of levetiracetam: a meta-analysis on children and adultsSeizure201531495526362377
- WeijenbergABrouwerOFCallenbachPMLevetiracetam monotherapy in children with epilepsy: a systematic reviewCNS Drugs201529537138226013703
- CamposMSAyresLRMoreloMRMarquesFAPereiraLREfficacy and tolerability of antiepileptic drugs in patients with focal epilepsy: systematic review and network meta-analysesPharmacotherapy201636121255127127779771
- EgunsolaOChoonaraISammonsHMSafety of levetiracetam in paediatrics: a systematic reviewPLoS One2016113e014968626930201
- WestonJBromleyRJacksonCFMonotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the childCochrane Database Syst Rev201611CD01022427819746
- ZhangLLiSLiHZouXLevetiracetam vs. brivaracetam for adults with refractory focal seizures: a meta-analysis and indirect comparisonSeizure201639283327236448
- GengHWangCEfficacy and safety of oxcarbazepine in the treatment of children with epilepsy: a meta-analysis of randomized controlled trialsNeuropsychiatr Dis Treat20171368569528293110
- SongJMHahnJKimSHChangMJEfficacy of treatments for infantile spasms: a systematic reviewClin Neuropharmacol2017402638428288483
- MchughDCLancasterSManganasLNA systematic review of the efficacy of levetiracetam in neonatal seizuresNeuropediatrics2018491012017
- Mohd-TahirNALiSCMeta-analyses of newer antiepileptic drugs as adjunct for treatment of focal epilepsy in childrenEpilepsy Res201813911312229220742
- NevittSJSudellMWestonJTudur SmithCMarsonAGAnti-epileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant dataCochrane Database Syst Rev20176CD01141228661008
- RosatiAIlventoLLucenteforteEComparative efficacy of antiepileptic drugs in children and adolescents: a network meta-analysisEpilepsia201859229731429270989
- VeronikiAACogoERiosPComparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomesBMC Med20171519528472982
- VeronikiAARiosPCogoEComparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: a systematic review and network meta-analysisBMJ Open201777e017248
- ZhangLWangCLiWA meta-analysis of randomized controlled trials on levetiracetam in the treatment of pediatric patients with epilepsyNeuropsychiatr Dis Treat20181476977929559784
- ZhaoTFengXLiuJGaoJZhouCEvaluate the efficacy and safety of anti-epileptic medications for partial seizures of epilepsy: a network meta-analysisJ Cell Biochem201711892850286428214290
- ZhuLNChenDXuDTanGWangHJLiuLNewer antiepi-leptic drugs compared to levetiracetam as adjunctive treatments for uncontrolled focal epilepsy: an indirect comparisonSeizure20175112113228854405
- BerkovicSFKnowltonRCLeroyRFSchiemannJFalterULevetiracetam N01057 Study GroupPlacebo-controlled study of levetiracetam in idiopathic generalized epilepsyNeurology200769181751176017625106
- BrodieMJPeruccaERyvlinPBen-MenachemEMeenckeHJLevetiracetam Monotherapy Study GroupComparison of levetiracetam and controlled-release carbamazepine in newly diagnosed epilepsyNeurology200768640240817283312
- CoppolaGFranzoniEVerrottiALevetiracetam or oxcarbazepine as monotherapy in newly diagnosed benign epilepsy of childhood with centrotemporal spikes (BECTS): an open-label, parallel group trialBrain Dev200729528128417055681
- NoachtarSAndermannEMeyvischPLevetiracetam for the treatment of idiopathic generalized epilepsy with myoclonic seizuresNeurology200870860761618285535
- ZhouBZhangQTianLXiaoJStefanHZhouDEffects of levetiracetam as an add-on therapy on cognitive function and quality of life in patients with refractory partial seizuresEpilepsy Behav200812230531018024209
- LabinerDMEttingerABFakhouryTAEffects of lamotrigine compared with levetiracetam on anger, hostility, and total mood in patients with partial epilepsyEpilepsia200950343444219016830
- LevisohnPMMintzMHunterSJYangHJonesJN01103 Levetiracetam Study GroupNeurocognitive effects of adjunctive levetiracetam in children with partial-onset seizures: a randomized, double-blind, placebo-controlled, noninferiority trialEpilepsia200950112377238919702752
- LimDATaraporePChangESafety and feasibility of switching from phenytoin to levetiracetam monotherapy for glioma-related seizure control following craniotomy: a randomized Phase II pilot studyJ Neurooncol200993334935419169651
- PeltolaJCoetzeeCJiménezFOnce-daily extended-release levetiracetam as adjunctive treatment of partial-onset seizures in patients with epilepsy: a double-blind, randomized, placebo-controlled trialEpilepsia200950340641419317886
- Piña-GarzaJENordliDRRatingDAdjunctive levetiracetam in infants and young children with refractory partial-onset seizuresEpilepsia20095051141114919243423
- WuXYHongZWuXMulticenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in Chinese patients with refractory partial-onset seizuresEpilepsia200950339840518657175
- XiaoZLiJMWangXFEfficacy and safety of levetiracetam (3,000 mg/day) as an adjunctive therapy in Chinese patients with refractory partial seizuresEur Neurol200961423323919176965
- CumboELigoriLDLevetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s diseaseEpilepsy Behav201017446146620188634
- de La LogeCHunterSJSchiemannJYangHAssessment of behavioral and emotional functioning using standardized instruments in children and adolescents with partial-onset seizures treated with adjunctive levetiracetam in a randomized, placebo-controlled trialEpilepsy Behav201018329129820547106
- UCB Japan Co LtdA double-blind, placebo-controlled study of levetiracetam in epilepsy patients with generalized tonic-clonic seizures (except partial seizures evolving to secondarily generalized seizures) Available from: https://ClinicalTrials.gov/show/NCT01228747Accessed July 18, 2017
- UCB Korea Co., LtdLevetiracetam versus topiramate as adjunctive therapy to evaluate efficacy and safety in subjects with refractory partial onset seizures Available from: https://ClinicalTrials.gov/show/NCT01229735Accessed July 18, 2017
- FattoreCBoniverCCapovillaGA multicenter, randomized, placebo-controlled trial of levetiracetam in children and adolescents with newly diagnosed absence epilepsyEpilepsia201152480280921320119
- ConsoliDBoscoDPostorinoPLevetiracetam versus carbamazepine in patients with late poststroke seizures: a multicenter prospective randomized open-label study (EpIC Project)Cerebrovasc Dis201234428228923128439
- HakamiTTodaroMPetrovskiSSubstitution monotherapy with levetiracetam vs older antiepileptic drugs: a randomized comparative trialArch Neurol201269121563157122945760
- RosenowFSchade-BrittingerCBurchardiNThe LaLiMo Trial: lamotrigine compared with levetiracetam in the initial 26 weeks of monotherapy for focal and generalised epilepsy – an open-label, prospective, randomised controlled multicenter studyJ Neurol Neurosurg Psychiatry201283111093109822595362
- BorggraefeIBonfertMBastTLevetiracetam vs. sulthiame in benign epilepsy with centrotemporal spikes in childhood: a double-blinded, randomized, controlled trial (German HEAD Study)Eur J Paediatr Neurol201317550751423642492
- UCB Pharma SAOpen-label, randomized, active-controlled study of LEV used as monotherapy in patients with partial-onset seizures Available from: https://ClinicalTrials.gov/show/NCT01954121Accessed July 18, 2017
- TrinkaEMarsonAGvan PaesschenWKOMET: an unblinded, randomised, two parallel-group, stratified trial comparing the effectiveness of levetiracetam with controlled-release carbamazepine and extended-release sodium valproate as monotherapy in patients with newly diagnosed epilepsyJ Neurol Neurosurg Psychiatry201384101138114722933814
- University of RochesterA safety and feasibility study of enteral LVT vs. standard of care for seizure control in pediatric CM (LVT2) Available from: https://ClinicalTrials.gov/show/NCT01982812Accessed July 18, 2017
- RossettiAOJeckelmannSNovyJRothPWellerMStuppRLevetiracetam and pregabalin for antiepileptic monotherapy in patients with primary brain tumors. A Phase II randomized studyNeuro Oncol201416458458824311644
- ZaccaraGAlmasMPitmanVKnappLPosnerHEfficacy and safety of pregabalin versus levetiracetam as adjunctive therapy in patients with partial seizures: a randomized, double-blind, noninferiority trialEpilepsia20145571048105724902473
- InoueYYagiKIkedaAEfficacy and tolerability of levetiracetam as adjunctive therapy in Japanese patients with uncontrolled partial-onset seizuresPsychiatry Clin Neurosci2015691064064825854635
- JungDEYuRYoonJRNeuropsychological effects of levetiracetam and carbamazepine in children with focal epilepsyNeurology201584232312231925948717
- SureshSHChakrabortyAVirupakshaiahAKumarNEfficacy and safety of levetiracetam and carbamazepine as monotherapy in partial seizuresEpilepsy Res Treat20152015416
- WerhahnKJTrinkaEDobesbergerJA randomized, double-blind comparison of antiepileptic drug treatment in the elderly with new-onset focal epilepsyEpilepsia201556345045925684224
- HakamiTO’BrienTJPettySJMonotherapy with levetiracetam versus older AEDs: a randomized comparative trial of effects on bone healthCalcif Tissue Int201698655656526842957
- KimJHLeeSKLoeschCComparison of levetiracetam and oxcarbazepine monotherapy among Korean patients with newly diagnosed focal epilepsy: a long-term, randomized, open-label trialEpilepsia2017584e70e7428395124
- TackeMGerstlLHeinenFEffect of anticonvulsive treatment on neuropsychological performance in children with BECTSEur J Paediatr Neurol201620687487927553576
- SiniscalchiAScaglioneFSanzaroEEffects of phenobarbital and levetiracetam on PR and QTc intervals in patients with post-stroke seizureClin Drug Investig20143412879886
- BootsmaHPRickerLDiepmanLLong-term effects of levetiracetam and topiramate in clinical practice: a head-to-head comparisonSeizure2008171192617618131
- AndersohnFSchadeRWillichSNGarbeEUse of antiepileptic drugs in epilepsy and the risk of self-harm or suicidal behaviorNeurology201075433534020660863
- ArifHBuchsbaumRPierroJComparative effectiveness of 10 antiepileptic drugs in older adults with epilepsyArch Neurol201067440841520385905
- MerrellRTAndersonSKMeyerFBLachanceDHSeizures in patients with glioma treated with phenytoin and levetiracetamJ Neurosurg201011361176118120560728
- RauchenzaunerMBitscheGSvalheimSEffects of levetiracetam and valproic acid monotherapy on sex-steroid hormones in prepubertal children – results from a pilot studyEpilepsy Res2010882–326426820015617
- VeibyGDaltveitAKEngelsenBAGilhusNEFetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancyJ Neurol2014261357958824449062
- XiaoFAnDDengHChenSRenJZhouDEvaluation of levetiracetam and valproic acid as low-dose monotherapies for children with typical benign childhood epilepsy with centrotemporal spikes (BECTS)Seizure201423975676124998415
- JavedACohenBDetynieckiKRates and predictors of patient-reported cognitive side effects of antiepileptic drugs: an extended follow-upSeizure201529344026076842
- TinchonAOberndorferSMarosiCHaematological toxicity of valproic acid compared to levetiracetam in patients with glioblastoma multiforme undergoing concomitant radio-chemotherapy: a retrospective cohort studyJ Neurol2015262117918625359262
- TomsonTBattinoDBonizzoniEAntiepileptic drugs and intrauterine death: a prospective observational study from EURAPNeurology201585758058826187231
- BektaşGTekinUÖzkanMUThe influence of levetiracetam on psychosocial and behavioral functioning in children: a case–control and follow-up studyEpilepsy Behav201772394228575765
- ChenBChoiHHirschLJPsychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsyEpilepsy Behav201776243128931473
- EgunsolaOChoonaraISammonsHMWhitehouseWPSafety of antiepileptic drugs in children and young people: a prospective cohort studySeizure201856202529427834
- FreyNBodmerMBircherAThe risk of Stevens–Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptic drugsEpilepsia201758122178218529027197
- LeeTWarrickBJSarangarmPLevetiracetam in toxic seizuresClin Toxicol (Phila)201856317518128753046
- MaschioMZarablaAMaialettiAQuality of life, mood and seizure control in patients with brain tumor related epilepsy treated with lacosamide as add-on therapy: a prospective explorative study with a historical control groupEpilepsy Behav201773838928623754
- ShihFYChuangYCChuangMJEffects of antiepileptic drugs on thyroid hormone function in epilepsy patientsSeizure20174871028364656
- StephenLJWishartABrodieMJPsychiatric side effects and anti-epileptic drugs: observations from prospective auditsEpilepsy Behav201771Pt A737828551500
- NewsomeSDXueLYJenningsTCastanedaGYLevetiracetam-induced diffuse interstitial lung diseaseJ Child Neurol200722562863017690072
- GalleraniMMariEBoariBCarlettiRMarraACavalloMPancytopenia associated with levetiracetam treatmentClin Drug Investig20092911747751
- HacquardMRichardSLacourJCLecompteTVespignaniHLevetiracetam-induced platelet dysfunctionEpilepsy Res2009861949619447009
- HurwitzKAIngulliEGKrousHFLevetiracetam induced interstitial nephritis and renal failurePediatr Neurol2009411575819520278
- Peer MohamedBMohamedBPPrabhakarPThrombocytopenia as an adverse effect of levetiracetam therapy in a childNeuropediatrics200940524324420221962
- TamarelleCPanditFMazaratiARiquetAValléeLAuvinSLevetiracetam-induced depression in a 5-year-old child with partial epilepsySeizure200918323523618848469
- vande GriendJPLinneburSABainbridgeJLProbable levetiracetam-associated depression in the elderly: two case reportsAm J Geriatr Pharmacother20097528128419948304
- BroliMProviniFNaldiIUnexpected gamma glutamyltransferase rise increase during levetiracetam monotherapyEpileptic Disord2010121818220159672
- CaraballoRHCersósimoRde Los SantosCLevetiracetam-induced seizure aggravation associated with continuous spikes and waves during slow sleep in children with refractory epilepsiesEpileptic Disord201012214615020483715
- OghlakianRNockCKoubeissiMA case of levetiracetam-induced thrombocytopeniaEpileptic Disord201012433533721078583
- SahayaKGoyalMKSarwalASinghNNLevetiracetam-induced thrombocytopenia among inpatients: a retrospective studyEpilepsia201051122492249521204814
- BachmannTBertheussenKHSvalheimSHaematological side effects of antiepileptic drug treatment in patients with epilepsyActa Neurol Scand Suppl20111911912327
- GivonLPorterSPadmanabhanBGorenJCohenPALevetiracetam, seizures, and suicidalityHarv Rev Psychiatry2011191475521250896
- AlkhotaniAMclachlanRSLevetiracetam induced angioedema in a patient with previous anticonvulsant hypersensitivity reaction to phenytoin and lamotrigineSeizure201221540740822524985
- BabtainFALevetiracetam may worsen myoclonus in patients with juvenile myoclonic epilepsy: case reportsClin Neuropharmacol201235420120222805231
- Bishop-FreemanSCKornegayNCWineckerREPostmortem levetiracetam (Keppra®) data from North CarolinaJ Anal Toxicol201236642242822635608
- CalabròRSItalianoDMilitiDBramantiPLevetiracetam-associated loss of libido and anhedoniaEpilepsy Behav201224228328422560189
- CamachoAEspínJCNuñezNSimónRLevetiracetam-induced reversible autistic regressionPediatr Neurol2012471656722704022
- ChauKYongJIsmailKGriffithNLiuMMakrisALevetiracetam-induced severe acute granulomatous interstitial nephritisClin Kidney J20125323423626069773
- Gómez-ZorrillaSFerrazAVPedrósCLemusMPeñaCLevetiracetam-induced drug reaction with eosinophilia and systemic symptoms syndromeAnn Pharmacother2012467–8e2022764327
- XiongNHouLLuNMohamedAAWangTHuangYProbable levetiracetam-related serum alkaline phosphatase elevationBMC Neurol2012129722994584
- ZouLPDingCHSongZJLiXFStevens–Johnson syndrome induced by levetiracetamSeizure2012211082382523036769
- HommetCBeaufilsERoubeauVEncephalopathy induced by levetiracetam in an elderly womanAging Clin Exp Res201325111111323740641
- KaradagASBilgiliSGCalkaOOnderSKosemMBurakgazi-DalkilicEA case of levetiracetam induced bullous pemphigoidCutan Ocul Toxicol201332217617823030621
- KaufmanKRBisenVZimmermanATobiaAManiRWongSApparent dose-dependent levetiracetam-induced de novo major depression with suicidal behaviorEpilepsy Behav Case Rep2013111011225667841
- MetinSZOzmenMOzkaraCOzmenEHypersexuality in a patient with epilepsy during treatment of levetiracetamSeizure201322215115223200413
- SethiNKSethiPKTorgovnickJArsuraECukierwarFAsymptomatic elevation of liver enzymes due to levetiracetam: a case reportDrug Metabol Drug Interact201328212312423420283
- AkiyamaHHagaYSasakiNYanagisawaTHasegawaYA case of rhabdomyolysis in which levetiracetam was suspected as the causeEpilepsy Behav Case Rep2014215215525667895
- AksoyDCevikBKurtSPekdasESolmazVHypokalemia and hypomagnesaemia related to levetiracetam useJ Clin Neurosci201421111989199024906211
- AzarNJAunePAcute pancreatitis and elevated liver transaminases after rapid titration of oral levetiracetamJ Clin Neurosci20142161053105424291473
- BuiMBasletGWeisholtzDMcelrathTLevetiracetam-induced psychosis in a pregnant woman with prior substance abuseHarv Rev Psychiatry201422319320024802726
- HwangESSiemianowskiLASenSPatelRLevetiracetam: an unusual cause of deliriumAm J Ther2014216e225e22823782757
- IsaacsonJEChoeDJDohertyMJCreatine phosphokinase elevation exacerbated by levetiracetam therapyEpilepsy Behav Case Rep2014218919125667904
- KokluEArigulogluEAKokluSLevetiracetam-induced anaphylaxis in a neonatePediatr Neurol201450219219424262344
- KumarNSwaroopHSChakrabortyAChandranSLevetiracetam induced acute reversible psychosis in a patient with uncontrolled seizuresIndian J Pharmacol201446556056125298593
- ParkEMHolmesJAReeder-HayesKEAcute mania associated with levetiracetam treatmentPsychosomatics20145519810023932538
- SpenglerDCMontourisGDHohlerADLevetiracetam as a possible contributor to acute kidney injuryClin Ther201436813031306
- ZakiSAGuptaSLevetiracetam-induced acute psychosis in a childIndian J Pharmacol201446334134224987186
- ZouXHongZZhouDHair loss with levetiracetam in five patients with epilepsySeizure201423215816024315496
- ArıHKahramanFAcabanMBThe first case of levetiracetam-induced and tolvaptan-resistant hyponatremiaTurk Kardiyol Dern Ars201543328428725906002
- EleniKDress syndrome induced by levetiracetamJ Eur Acad Dermatol Venereol201529237737824397826
- FlanneryAHWilleyMDThompson BastinMLBuchKPBensadounESEosinophilia and fever with levetiracetam: a case reportPharmacotherapy2015358e131e13526235978
- FujikawaMKishimotoYKakisakaYObsessive–compulsive behavior induced by levetiracetamJ Child Neurol201530794294425008911
- GenclerOSGenclerBAltunelCTArslanNLevetiracetam induced psoriasiform drug eruption: a rare case reportSaudi Pharm J201523672072226702269
- KawakamiYOkazakiTTakaseMFujinoOItohYA girl with idiopathic epilepsy showing forced normalization after levetiracetam administrationJ Nippon Med Sch201582525025326568392
- MakkeYHmaimessGNasreddineWFawazABeydounAParadoxical exacerbation of myoclonic-astatic seizures by levetiracetam in myoclonic astatic epilepsyBMC Pediatr201515625884503
- MolokwuOAEzeala-AdikaibeBAOnwuekweIOLevetiracetam-induced rage and suicidality: two case reports and review of literatureEpilepsy Behav Case Rep20154798126543810
- PeyrlAWeichertNKühlJSEbellWHernáiz DrieverPLevetiracetam as a possible cause of secondary graft failure after allogenic hematopoietic stem cell transplantationEur J Paediatr Neurol2015191757725468262
- Taberner BonastreMTPeralta MuñozSBozaFMGumàIPadróJNeutropenia secondary to exposure to levetiracetamTumori20151015145146
- BayramAKCanpolatMÇınarSLDrug reaction with eosinophilia and systemic symptoms syndrome induced by levetiracetam in a pediatric patientJ Emerg Med2016502e61e6626597350
- DarWRSofiNLatiefMDarIAKasanaBALevetiracetam induced drug reaction with eosinophilia and systemic symptom syndromeIndian J Dermatol2016612235
- García CarreteroRRomero BrugeraMOlid-VelillaMSalamanca-RamirezIPancytopenia associated with levetiracetam in an epileptic womanBMJ Case Rep20162016bcr2016217407
- JonesRTEvansWMersfelderTLKavanaughKRare red rashes: a case report of levetiracetam-induced cutaneous reaction and review of the literatureAm J Ther2016233e944e94625259954
- JuJZouLPShiXYHuLYPangLYLevetiracetam: probably associated diurnal frequent urinationAm J Ther2016232e624e62726938751
- TuratiMGlardYAfonsoDGriffetJBigoniMOsteochondral alteration in a child treated with levetiracetam: a rare case of juvenile osteochondritis dissecans of the talar headJ Pediatr Orthop B201726218919227341121
- KubotaKYamamotoTKawamotoMLevetiracetam-induced rhabdomyolysis: a case report and literature reviewNeurol Asia201722275278
- OzdemirHSumerSKarabagliHB cell aplasia and hypogammaglobulinemia associated with levetiracetamAnn Saudi Med2018381545548
- SereflicanBKarapinarTDuzcuSETurkogluŞADisseminated eruptive granuloma annulare induced by levetiracetamCutan Ocul Toxicol201736330030128095713
- BeghiEAtzeniLGarattiniLEconomic analysis of newer antiepileptic drugsCNS Drugs2008221086187518788837
- SuhGHLeeSKEconomic evaluation of add-on levetiracetam for the treatment of refractory partial epilepsy in KoreaPsychiatry Investig200963185193
- TomsonTBattinoDBonizzoniEComparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registryLancet Neurol201817653053829680205
- KimJKondratyevAGaleKAntiepileptic drug-induced neuronal cell death in the immature brain: effects of carbamazepine, topiramate, and levetiracetam as monotherapy versus polytherapyJ Pharmacol Exp Ther2007323116517317636003
- CumboELigoriLDLevetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s diseaseEpilepsy Behav201017446146620188634
- ChanRWeiCYChenYTBenetLZUse of the Biopharmaceutics Drug Disposition Classification System (BDDCS) to help predict the occurrence of idiosyncratic cutaneous adverse drug reactions associated with antiepileptic drug usageAAPS J201618375776626951484
- FongJKChanELLeungHAn update of the Hong Kong Epilepsy Guideline: consensus statement on the use of antiepileptic drugs in Hong KongHong Kong Med J2017231748828184017
- WijnenBFMvan MastrigtGEversSA systematic review of economic evaluations of treatments for patients with epilepsyEpilepsia201758570672628098939
- KazerooniRBounthavongMCost-effectiveness analysis of intravenous levetiracetam versus intravenous phenytoin for early onset seizure prophylaxis after neurosurgery and traumatic brain injuryClinicoecon Outcomes Res20102152321935311
- LyttleMDGambleCMessahelSEmergency treatment with levetiracetam or phenytoin in status epilepticus in children – the EcLiPSE study: study protocol for a randomised controlled trialTrials201718128328629473
- BährOHermissonMRonaSIntravenous and oral levetiracetam in patients with a suspected primary brain tumor and symptomatic seizures undergoing neurosurgery: the HELLO trialActa Neurochir (Wien)2012154222923521909835
- KellinghausCStögbauerFTreatment of status epilepticus in a large community hospitalEpilepsy Behav201223323524022341964