Abstract
Objective
Gabapentin and its prodrug are candidate therapeutic agents for akathisia. An open-label pilot study was conducted to investigate the therapeutic potential of gabapentin enacarbil (GE) for akathisia.
Methods
In an open-labeled investigator-initiated clinical trial, nine outpatients with antipsychotics-induced akathisia were administered GE (300 or 600 mg/day) over 2 weeks. The BARS global akathisia score was used to assess akathisia. The BPRS was used to assess psychiatric symptoms. The subjects were also systematically questioned regarding the adverse events described in an interview form following GE treatment. An intension-to-treat analysis including all patients enrolled in the present study was completed.
Results
One patient declined to participate further in the study on the third day after the start of treatment. Eight patients thus completed the entire trial (male: 2, female: 6, age [mean±SD]: 38.8±11.6 years). The average dosage of GE was 567 mg/day (300 mg/day [n=1], 600 mg/day [n=8]). The BARS global akathisia score significantly decreased after 1 and 2 weeks of treatment when compared to baseline (P=0.01 and P=0.01, respectively). There were no significant differences in BPRS score 1 or 2 weeks after the start of treatment. No serious adverse events occurred.
Conclusion
GE has therapeutic potential for antipsychotics-induced akathisia. No additional risk of GE use for the management of akathisia was indicated.
Introduction
Akathisia, one of the major side effects of antipsychotics,Citation1 should be treated early, as it is associated with suicidality,Citation2 exacerbation of psychotic symptoms,Citation3 poorer treatment outcome,Citation4 non-compliance with antipsychotic treatment,Citation5 and reduced well-being in patients with schizophrenia.Citation6 The Maudsley Prescribing Guidelines recommend the reduction/cessation of drugs causing akathisia as a first step and the use of antipsychotic drugs that are less likely to cause akathisia, such as quetiapine and clozapine (CLZ),Citation7 as a second step. However, in practice, it is often difficult to reduce the dose of or change the type of antipsychotics in patients with schizophrenia, as indicated in the guideline. Although propranolol is the first-line drug in the guidelines, the response rate of this drug is as low as 30.0% and the discontinuation rate is as high as 16.7%.Citation8 Asthma as a contraindication of the drug is also a problem in clinical practice because its prevalence in schizophrenia patients is up to 10%.Citation9 Therefore, new akathisia drug therapies are required.
Gabapentin and its prodrug are candidate therapeutic agents for akathisia.Citation10–Citation12 The case of a single patient whose akathisia symptoms decreased after gabapentin use was first reported by Pfeffer et al.Citation10 Another successful case of gabapentin use for the treatment of akathisia was reported by Sullivan and Wilbur.Citation11 In a previous case study, the authors also reported the therapeutic potential of gabapentin enacarbil (GE), a prodrug of gabapentin, for akathisia.Citation12 Although gabapentin and its prodrug have therapeutic potential, all previous reports of its use for the treatment of akathisia describe studies with one or two cases. In addition, reduced central γ-aminobutyric acid (GABA)-ergic activity is suggested as a pathogenic mechanism for akathisia.Citation13 Because gabapentin increases GABA activity,Citation14,Citation15 this drug and its prodrug have a potential to attenuate akathisia symptoms. The authors have, therefore, conducted an open-label pilot study to investigate the effectiveness of GE for akathisia.
Patients and methods
Subjects
This study was a 2-week long, open-labeled, prospective pilot trial. The subjects were nine outpatients at the Departments of Neuropsychiatry at Akita University Hospital and Yuri Kumiai General Hospital with antipsychotics-induced akathisia. Akathisia was diagnosed based on the DSM-5 criteria.Citation16 This study was conducted from September 2016 to July 2018. The exclusion criteria were 1) age <20 years; 2) pregnancy or lactation; 3) additional use of other drugs with therapeutic potential for akathisia (β-blockers, benzodiazepines, anticholinergic agents, gabapentin, or GE) within 2 weeks of starting this study; 4) having suicidal ideas; 5) severe renal failure; 6) having a past history of restless leg syndrome; and 7) severe akathisia with the maximum BARS score.Citation17 These criteria were applied at the time of research recruitment.
Treatment protocol
Eligible subjects were administered GE (300 or 600 mg/day) for 2 weeks while continuing to use the antipsychotics that caused akathisia. During the study period, no akathisia medication (β-blockers, benzodiazepines, or anticholinergic drugs) was used in addition to GE. The primary endpoint was the BARS global akathisia score (range: 0–5). The subjects were assessed for the severity of akathisia at baseline and after 1 and 2 weeks of treatment. Patients whose BARS global akathisia scores were higher at week 1 than at baseline were dropped from the study at that time. The BARS Objective akathisia score, BARS Subjective akathisia score (Awareness of restlessness and Distress related to restlessness), and BPRS scores were examined at baseline and after 1 and 2 weeks of treatment as secondary measures.Citation18 The subjects were also systematically questioned regarding the following adverse events in an interview after GE treatment:Citation19 somnolence, sedation, abnormal dreams, insomnia, irritability, depressed mood, anxiety, illusion, disorientation, disturbance in attention, decreased libido, nausea, constipation, diarrhea, weight gain, weight loss, abdominal pain, vomiting, abdominal discomfort, dyspepsia, gastroesophageal reflux disease, flatulence, increased appetite, tremor, balance disorder, ataxia, dysarthria, dizziness, vertigo, fatigue, headache, palpitation, dry mouth, dysgeusia, paresthesia, feeling abnormal, feeling drunk, pain in extremity, myalgia, arthralgia, muscle spasm, peripheral edema, malaise, asthenia, back pain, rash, pruritus, and blurred vision. During the study period, clinical evaluation of the participants was performed by a single evaluator.
Ethics approval and informed consent
This study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Ethical Committees for Human Research of Akita University and Yuri Kumiai General Hospital. The fact that reduction in dose or switching of antipsychotic drugs is a principal management strategy for patients with acute antipsychotic-induced akathisia was explained to all participants during recruitment. Some of the participants were participating in this study after failure of such principal management strategies. Written informed consent was obtained from all subjects, and the research protocol was explained to all participants.
Statistical analyses
All statistical analyses were performed using PAWS statistics 11.5 (SPSS Japan; Tokyo, Japan). Data are expressed as means±SDs. An intention-to-treat analysis including all patients enrolled in the present study was completed. Friedman tests with post hoc analyses were used to examine differences in BARS global akathisia scores and BPRS scores among the different time points. The Wilcoxon single-rank test was used to perform post hoc analysis for the Friedman test, and statistical significance was set at P<0.016 (0.05/number of comparisons=0.016) based on the Bonferroni correction.
Results
Samples
and show the demographic data of the nine subjects. All patients had schizophrenia as defined in the DSM-5. One patient declined to continue this study on the third day after the start of the study because the patient wanted to be treated using some other strategy. Therefore, eight patients thus completed the study (male: 2, female 6, age: 38.8±11.6 years). The average dosage of GE was 567 mg/day (300 mg/day [n=1], 600 mg/day [n=8]). The drugs that caused akathisia were aripiprazole (n=5), risperidone (n=1), paliperidone (n=1), CLZ (n=1), and perospirone (n=1). From the onset of akathisia to the end of the study, the patients did not take any of the following drugs, which may be considered therapeutic agents for akathisia: propranolol,Citation20,Citation21 mirtazapine,Citation22,Citation23 mianserin,Citation22 trazodone,Citation24 cyproheptadine,Citation25,Citation26 zolmitriptan,Citation27 clonidine,Citation28 dopamine agonists,Citation28 and vitamin B6 supplement.Citation29 MT evaluated all the patients.
Table 1 Clinical and demographic characteristics
Table 2 Changes in symptom evaluation scores during the study
Primary outcome
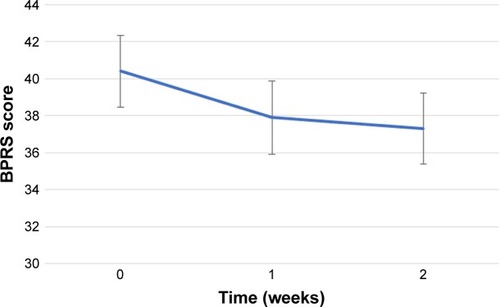
shows the changes in the BARS global akathisia score. The mean±SD of BARS global scores at baseline and the endpoint is 3.3±0.5 and 1.1±1.3, respectively. The result of the Friedman’s test was highly significant (P=0.0005). Therefore, post hoc analyses were performed using the Wilcoxon signed-rank test. The BARS global akathisia score was significantly decreased after 1 and 2 weeks of treatment compared with baseline (P=0.01 and P=0.01, respectively). There was no significant difference in the BARS global akathisia score between week 1 and week 2.
Figure 1 Changes in the BARS global akathisia score.
Abbreviation: BARS, Barnes Akathisia Rating Scale.
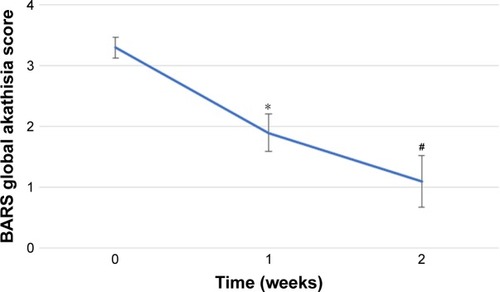
Secondary outcome
illustrates the change in the BPRS score. The result of the Friedman’s test was significant (P=0.0058). Therefore, post hoc analyses were performed. There were no significant differences in BPRS scores in the post hoc analyses. The mean±SD of the BARS Objective akathisia score was 1.9±0.8 at week 0, 0.3±1.0 at week 1, and 0.3±1.0 at week 2. The mean±SD of the BARS Subjective akathisia score (Awareness of restlessness) was 2.6±0.5 at week 0, 1.8±0.7 at week 1, and 1.0±1.0 at week 2. The mean±SD of the BARS Subjective akathisia score (Distress related to restlessness) was 2.3±0.5 at week 0, 1.3±0.7 at week 1, and 0.9±0.9 at week 2.
Adverse events
No serious adverse events due to GE administration occurred. Mild somnolence was observed in one patient and dry mouth in another.
Discussion
This is the first study to investigate the effectiveness of GE for akathisia. The present findings suggest that GE significantly improves the severity of akathisia within 2 weeks. Based on the results, GE may be a candidate therapeutic agent for akathisia. The akathisia treatment described in Maudsley’s guideline does not improve all cases of akathisia.Citation7 It is, therefore, difficult to state that akathisia treatment is well established. GE may be a new treatment option for patients with akathisia, particularly cases wherein it is difficult to simply reduce the dosage of antipsychotics or withdraw antipsychotic treatment.
The mechanism by which GE improves akathisia is still unclear. Reduced central GABA-ergic activity is suggested as a pathogenic mechanism for akathisia.Citation13 In fact, gabapentin has a structure similar to that of the neurotransmitter GABA and increases GABA activity by inhibiting certain calcium channels.Citation14,Citation15 Because GE is a prodrug of gabapentin, it may have exerted the effects observed here through the GABA neurotransmitter system.
Safety is important in medicinal therapies for akathisia. The only contraindications for GE are advanced renal dysfunction and a history of anaphylaxis due to GE. GE has been reported to be safe even after long-term administration.Citation30,Citation31 Propranolol, which is widely used for the treatment of akathisia, sometimes cannot be used in patients with conditions, such as asthma, bradycardia, and hypotension.Citation8 While mirtazapine and mianserin are therapeutic agents for akathisia, they may cause akathisia on their own, and care must be taken when treating patients with akathisia using such medications.Citation32,Citation33 In some cases, anticholinergic drugs cannot be used for treatment of akathisia due to side effects such as ileus, urinary disorders, and elevated intraocular pressure. In addition, anticholinergic drugs lead to tardive dyskinesia after long-term administration.Citation34
This study has some limitations. First, a blinded, placebo control group was not examined. It was thus unclear whether the improvement in the BARS global akathisia score was a consequence of GE administration. Second, the sample size was small. Because this study began as a pilot study, we minimized recruitment for this study. Third, gender bias may also be considered one of limitation. The subject of this study was a group with a strong gender bias. However, no research has been published to indicate sex differences in the action of gabapentin. Therefore, the possibility that the gender bias in this study population affected the research results would be minimal. Fourth, the study period was probably short. Fifth, a concomitant drug with therapeutic potential for akathisia used in some participants might have influenced the study findings. These limitations should be considered in further research involving more time and a large sample size.
Conclusion
GE has a therapeutic potential for antipsychotics-induced akathisia. Future trials with well-established study designs are warranted to examine the effectiveness of GE for akathisia.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Abbreviations
| BARS | = | Barnes Akathisia Rating Scale |
| BPRS | = | Brief Psychiatric Rating Scale |
| CLZ | = | clozapine |
| DSM-5 | = | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| GABA | = | γ-aminobutyric acid |
| GE | = | Gabapentin enacarbil |
Acknowledgments
We thank Editage (www.editage.jp) for English language editing.
Disclosure
MT has received speaker’s honoraria from Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical, Sumitomo Dainippon Pharma, Daiichi Sankyo Company, and Eisai. TK has received speaker’s honoraria from Otsuka Pharmaceutical, MSD, and Eisai. TS has received research grants from Eisai and MSD, and speaker’s honoraria from MSD, Takeda Pharmaceuticals, Pfizer, and Yoshitomi Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
- PoyurovskyMAcute antipsychotic-induced akathisia revisitedBr J Psychiatry20101962899120118449
- Cem AtbaşogluESchultzSKAndreasenNCThe relationship of akathisia with suicidality and depersonalization among patients with schizophreniaJ Neuropsychiatry Clin Neurosci200113333634111514639
- DuncanEJAdlerLAStephanidesMSanfilipoMAngristBAkathisia and exacerbation of psychopathology: a preliminary reportClin Neuropharmacol200023316917310895402
- PraMvan PuttenTYaleCPredicting individual responses to drug treatment in schizophreniaJ Nerv Ment Dis19761253177183
- van PuttenTWhy do schizophrenic patients refuse to take their drugs?Arch Gen Psychiatry197431167724151750
- LeeYWKimJHAnnJHSubjective well-being in patients with schizophrenia treated with atypical antipsychotics: the impact of psychopathology and adverse drug effectsClin Psychopharmacol Neurosci201083149155
- TaylorDPatonCKapurSThe Maudsley Prescribing Guidelines in Psychiatry12th edWest SussexJohn Willey & Sons20158889
- PoyurovskyMPashinianAWeizmanRFuchsCWeizmanALow-dose mirtazapine: a new option in the treatment of antipsychotic-induced akathisia. A randomized, double-blind, placebo- and propranolol-controlled trialBiol Psychiatry200659111071107716497273
- ChenYHLeeHCLinHCPrevalence and risk of atopic disorders among schizophrenia patients: a nationwide population based studySchizophr Res20091081–319119619171465
- PfefferGChouinardGMargoleseHCGabapentin in the treatment of antipsychotic-induced akathisia in schizophreniaInt Clin Psychopharmacol200520317918115812271
- SullivanMAWilburRGabapentin pharmacotherapy for antipsychotic-induced akathisia: single-patient experiment and case reportTher Adv Psychopharmacol20144210010224688760
- TakeshimaMIshikawaHKikuchiYKanbayashiTShimizuTSuccessful management of clozapine-induced akathisia with gabapentin enacarbil: a case reportClin Psychopharmacol Neurosci201816334634830121987
- LimaARSoares-WeiserKBacaltchukJBarnesTRBenzodiazepines for neuroleptic-induced acute akathisiaCochrane Database Syst Rev20021CD001950
- Garcia-BorregueroDLarrosaOde La LlaveYVergerKMas-ramonXHernandezGTreatment of restless legs syndrome with gabapentin: a double-blind, cross-over studyNeurology200259101573157912451200
- ManeufYPGonzalezMISuttonKSChungFZPinnockRDLeeKCellular and molecular action of the putative GABA-mimetic, gabapentinCell Mol Life Sci200360474275012785720
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders5th edVirginiaAmerican Psychiatric Publishing2013
- BarnesTRA rating scale for drug-induced akathisiaBr J Psychiatry19891546726762574607
- OverallJEGormanDRThe Brief Psychiatric Rating ScalePsychol Rep1962103799812
- Astellas Pharma IncRegnite® Tablets 300 mg Interview Form, 7th versionTokyo2018 Available from: https://amn.astellas.jp/jp/di/list/reg/pi_reg.pdfAccessed November 14, 2018 Japanese
- AdlerLAngristBPeselowECorwinJMaslanskyRRotrosenJA controlled assessment of propranolol in the treatment of neuroleptic-induced akathisiaBr J Psychiatry198614942452877708
- KramerMSGorkinRADijohnsonCShevesPPropranolol in the treatment of neuroleptic-induced akathisia (NIA) in schizophrenics: a double-blind, placebo-controlled studyBiol Psychiatry19882478238272906547
- LaoutidisZGLuckhausC5-HT2A receptor antagonists for the treatment of neuroleptic-induced akathisia: a systematic review and meta-analysisInt J Neuropsychopharmacol201417582383224286228
- PoyurovskyMWeizmanRWeizmanAMirtazapine – a multifunctional drug: low dose for akathisiaCNS Spectr20111626322789775
- StryjerRRosenzcwaigSBarFUlmanAMWeizmanASpivakBTrazodone for the treatment of neuroleptic-induced acute akathisia: a placebo-controlled, double-blind, crossover studyClin Neuropharmacol201033521922220838215
- WeissDAizenbergDHermeshHCyproheptadine treatment in neuroleptic-induced akathisiaBr J Psychiatry199516744834868829717
- FischelTHermeshHAizenbergDCyproheptadine versus propranolol for the treatment of acute neuroleptic-induced akathisia: a comparative double-blind studyJ Clin Psychopharmacol200121661261511763011
- AvitalAGross-IsseroffRStryjerRHermeshHWeizmanAShilohRZolmitriptan compared to propranolol in the treatment of acute neuroleptic-induced akathisia: a comparative double-blind studyEur Neuropsychopharmacol200919747648219342206
- MillerCHFleischhackerWWManaging antipsychotic-induced acute and chronic akathisiaDrug Saf2000221738110647977
- MiodownikCLernerVStatsenkoNVitamin B6 versus mianserin and placebo in acute neuroleptic-induced akathisia: a random-ized, double-blind, controlled studyClin Neuropharmacol2006292687216614537
- InoueYUchimuraNKurodaKHirataKHattoriNLong-term efficacy and safety of gabapentin enacarbil in Japanese restless legs syndrome patientsProg Neuropsychopharmacol Biol Psychiatry201236225125722036917
- EllenbogenALTheinSGWinslowDHA 52-week study of gabapentin enacarbil in restless legs syndromeClin Neuropharmacol201134181621242741
- GulsunMDorukAMirtazapine-induced akathisiaJ Clin Psychopharmacol2008284467
- AggarwalAKhandelwalAGargAJilohaRCAkathisia associated with mianserinJ Clin Psychopharmacol201030333833920473077
- OginoSMiyamotoSMiyakeNYamaguchiNBenefits and limits of anticholinergic use in schizophrenia: focusing on its effect on cognitive functionPsychiatry Clin Neurosci2014681374924102938