Abstract
Objective
To study the differences in immunopathogenesis based on chemokine profile in neuromyelitis optica patients positive for AQP4 antibodies or MOG antibodies.
Patients and methods
We measured 52 cytokines/chemokines using ELISA in 59 serum samples, which were divided into three groups according to CBA results: HCs (n=16), AQP4+ (n=20) and MOG+ (n=23). The regression equation (R2>0.98) of the standard curve was calculated according to the standard concentration and the corresponding A value. And then the corresponding sample concentration was calculated according to the A value of the sample.
Results
Eleven of 52 measured serum cytokine/chemokines (CCL22/MDC, CCL13/MCP-4, CCL21/6Ckine, CCL27/CTACK, CCL8/MCP-2, CXCL14/BRAK, Contactin-1, Kallilrein 6/Neurosin, Midkine, VCAM-1 and Fas) were significantly different between MOG+ group and controls. Ten of 52 measured serum cytokine/chemokines (CCL1/I-309, CCL22/MDC, CCL28, CCL17/TARC, CCL27/CTACK, CXCL2/GRO beta, Contactin-1, Midkine, Chemerin and Synuclein-alpha) were significantly different between AQP4+ group and controls. There was no difference between serum AQP4+ and MOG+ groups for CC chemokines. All measured chemokines CXC except CXCL6/GCP-2 showed no significant differences in serum AQP4+ group compared to MOG+ group. However, there was significant difference between serum AQP4+ and MOG+ groups for C5/C5a and Midkine. C5/C5a and Midkine were significantly higher in AQP4+ group compared to MOG+ group (P<0.05).
Conclusion
Our findings suggest that the differences of mean concentration in CXCL6/GCP-2, Midkine and C5/C5a probably reveal different immunologic mechanism between AQP4+ NMO and MOG+ NMO. This cytokine/chemokine profiling provides new insight into NMO pathogenesis associated with MOG antibody seropositivity and provides guidance to monitor inflammation and response to treatment in a way.
Introduction
Demyelinating disease of the CNS is closely related to optica neuritis (ON), an inflammatory demyelinating disease involving optic nerve and causing axonal damage.Citation1,Citation2 ON is characterized by sudden monocular visual loss and eye pain in young adults, more commonly in women. ON is regarded as a common initial manifestation of MS.Citation3 In addition, a small number of ON patients with positive autoimmunity antibody like ANAs probably undergo optic neuropathy, which was considered as inflammatory optic neuropathy or autoimmune optic neuropathy.Citation4 In western countries, ON is mostly defined as IDON, which is closely related to MS.Citation5 And NMO, a severe autoimmune and inflammatory disease of the CNS, is characterized by recurrent ON and LETM.Citation6 NMO is one of the most common causes of non-traumatic disability in middle-aged and youth, the incidence of which is second only to MS. In 2004, a specific antibody NMO-immunoglobulin G (IgG) was found in the serum of NMO patients and the target antigen of NMO-IgG is AQP4, the detection of NMO-IgG/AQP4 antibodies in NMO serum has high sensitivity and specificity, up to 73% and 91%, respectively.Citation7 According to the 2006 revised diagnostic criteria for NMO, seropositivity for IgG antibodies that bind to AQP4 on astrocytes is one of three supporting conditions, thus there is no doubt that AQP4-Ab is of great importance in the diagnosis of NMO.
The discovery of an NMO-specific auto-antibody to the AQP4 water channel has improved knowledge of NMO pathogenesis. However, through the clinical practice, it is found that negative AQP4 Ab was presented in about 10%–20% NMO patients.Citation7,Citation8 The AQP4 Ab-negative NMO patients may have a completely different pathogenesis from the AQP4 Ab positive NMO. In 2011, it was first reported that auto-antibodies against oligodendrocyte glycoprotein are present in the NMO patients, namely MOG, which is often detected in the serum of AQP4-negative NMO patients.Citation9 MOG is a myelin antigen located at the outer surface of the CNS myelin sheath and is a target for autoimmune responses that results in CNS inflammation and demyelination. There is emerging evidence that MOG antibodies are pathogenic in human demyelinating diseases. In vitro experiments have shown that patient serum containing MOG antibodies can induce complement, natural killer cell and antibody dependent cell-mediated toxicity, and disrupt oligodendrocyte cytoskeleton.Citation9–Citation13 NMO associated with AQP-4 antibodies is an astrocytopathy, and there are a large number of studies demonstrating a complex immunopathology involving B cells, eosinophils, neutrophils and Th17 cell mechanisms.Citation14–Citation16 By contrast, other than autoantibody-associated mechanisms, there is limited information available on the immune-pathogenesis of MOG antibody-mediated inflammation. Likewise, although the presence of different CSF cytokines and chemokines has previously been investigated in NMO,Citation17 Uzawa et al proposed that NMO patients have elevated CSF cyto/chemokines associated with Th17 (IL-6, IL-8 and G-CSF), Th2 (IL-1ra, IL-10 and IL-13) and B cell axes (CXCL13, BAFF, APRIL and IL-21), which contrasts with MS which is primarily a Th1-dominant disease.Citation17
Previous study also points that increased concentrations of BAFF, IL-17, IL-6 and CXCL13 have been proposed to be key factors that induce the formation of NMO lesions, mainly by promoting the infiltration of neutrophils or plasma cells,Citation17–Citation21 and these molecules have been shown to correlate with disease severity and expanded disability status scale score.Citation22,Citation23 However, serum cytokine studies in MOG-associated demyelination are lacking.
In view of emerging evidence of pathogenicity, we hypothesize that AQP4 Ab POS NMO has different pathogenicity of MOG Ab POS NMO. In this study, we investigated and analyzed a broad array of serum cytokine/chemokine levels in AQP4+ group and MOG+ group and their association, to identify whether some cytokine/chemokine can be biomarkers to identify the immunologic mechanism of MOG+ NMO.
Patients and methods
Subjects
We studied 43 patients with the following criteria were selected: 1) initial diagnosis of NMO. All patients fulfilled the 2006 diagnostic criteria for NMO, revised by Wingerchuk et al, 2) in the acute phase with the first attack and 3) participants had not been treated with large dose of corticosteroid and not used immunosuppressive agents and never received immunomodulatory treatments.
Ethics statment
This work was approved by the People’s Liberation Army General Hospital Research Ethics Committee (No S2015-029-01) and was conducted according to the Declaration of Helsinki in its latest applicable version.
Plasma sample analysis
Patients donated 10 mL of blood between January 2016 and January 2018 and serum was collected in heparinized tubes. Blood was diluted 1:1 with 0.9% NaCl and overlaid onto lymphocytes separation medium (PAA Laboratories GmbH, les Mureaux, France). After centrifugation, the plasma was collected and stored at -80°C.
CBA for detection of AQP4 and MOG IgG status
HEK293T cells, polyethylenimine transfected with human M23-AQP4 and FL-MOG were used as the substrate for live CBAs, which were performed as described elsewhere.Citation7–Citation9 Patient sera were tested at 1:100 dilution. The Alexa Fluor 488 goat anti-human IgG (H + L) (cat. no ab201540; Abcam, Cambridge, UK) was used at 1:250 dilution. The AQP4 and MOG IgG status of NMO patients was then determined; AQP4 and MOG are transmembrane glycoproteins, and NMO associated IgGs are marked by green fluorescence in the cell membrane. A positive and negative control was set up in the experiment as follows: rabbit anti-human MOG (1 µg/mL; cat. no M0695) and AQP4 (1 µg/mL) antibody (cat. no G9626), both Sigma-Aldrich; Merck KGaA, were added to the serum obtained from a healthy individual as the positive control at room temperature overnight, while serum obtained from a healthy individual was used as a negative control.
NMO grouping
AQP4 antibody and MOG IgG in patients meeting the diagnostic criteria for NMO were detected via the CBA at the General Hospital of the People’s Liberation Army (PLAGH). According to the CBA results, serum samples were divided into MOG IgG (+) AQP4 IgG (-) group (MOG+ group) and MOG IgG (-) AQP4 IgG (+) group (AQP4 IgG+ group). None of the patients were positive and negative for both MOG-Abs and AQP4-Abs. A total of 20 patients with AQP4 Ab POS (1 male, 19 females; age mean ± SEM: 39.65±3.105; range =13–62) and 23 patients with MOG Ab POS (11 females, 12 males; age mean ± SEM: 32.18±2.927; range =10–62) demyelination were included. And 16 HCs were also selected in the control group. Clinical characteristics in AQP4 Ab POS and MOG Ab POS demyelination syndromes are shown in . All serum samples were collected during routine diagnostic work-up, prior to commencing treatment, and samples were frozen and stored at -80°C until analysis for cytokines and chemokines. As part of the ethically approved protocol, we have got written consent from all involved patients and participants for this study. And this study was also approved by the People’s Liberation Army General Hospital Research Ethics Committee (No S2015-029-01) and was conducted according to the Declaration of Helsinki in its latest applicable version.
Table 1 Clinical characteristics in AQP4 Ab POS and MOG Ab POS demyelination syndromes
Cytokine and chemokine assay
Fifty-two cytokines (IL-28A, IL-17E/IL-25, IL-16, IL-31, IL-21), a series of chemokines (CCL1/I-309, CCL2/MCP-1, CCL24/Eotaxin-2, CCL22/MDC, CCL4/MIP-1 beta, CCL13/MCP-4, CCL13/MIP-3 beta, CCL28, CCL26/Eotaxin-3, CCL25/TECK, CCL11/Eotaxin, CCL17/TARC, CCL21/6Ckine, CCL27/CTACK, CCL20/MIP-3, CCL8/MCP-2, CXCL10/IP-10, CXCL2/GRO beta, CXCL13/BLC/BCA-1, CXCL1/GRO alpha, CXCL5/ENA-78, CXCL16, CXCL14/BRAK, CXCL6/GCP-2, CX3CL/Fractalkine, CXCL9/MIG and CXCL11/ITAC-1) and others (C5/C5a, APP, Granzyme A, Contactin-1, Kallilrein 6/Neurosin, Granzyme B, Synuclein-alpha, Midkine, Chemerin, BMP-9, PARK5/UCH-L1, GDNF, NCAM-1/CD56, S100B, Fas Ligand, LT-alpha/TNF-beta, Enolase 2/NSE, VCAM-1, ICAM-1 and Fas) were measured by means of ELISA. The regression equation of the standard curve (R2>0.98) was calculated according to the standard concentration and the corresponding A value. And the corresponding sample concentration was calculated according to the A value of the sample.
Statistical analysis
Statistical analysis was performed using R statistical programme and graphs were composed using Graph Pad Prism software version 5.0. The serum cytokine/chemokine data were analyzed to check for a normal distribution. As data did not show Gaussian distribution, statistical analyses of cytokine/chemokine levels were performed using Kruskal–Wallis test for multiple groups and Mann–Whitney test for two groups and two-tailed P-value was calculated. This study was largely exploratory with an intention to study the immunopathological mechanisms based on serum cytokines/chemokines. The P-values from these analyses should be viewed as a measure of the strength of evidence for each association. To reduce type I errors, Bonferroni correction had been applied on the computed P-values. In the correlation analysis, P-values <0.05 were considered to indicate statistical significance.
Results
Comparison of serum cytokine/chemokine levels between AQP4+ group, MOG+ NMO group and controls
presents the mean concentration for all serum cyto-kine/chemokines and statistical comparisons, presented according to CC, CXC, interleukins and other cytokines/chemokines.
Table 2 Serum cytokine/chemokine concentrations in AQP4+, MOG+ group and controls according to chemokines CC, CXC, IL and other subsets
Eleven of 52 measured serum cytokine/chemokines (CCL22/MDC, CCL13/MCP-4, CCL21/6Ckine, CCL27/CTACK, CCL8/MCP-2, CXCL14/BRAK, Contactin-1, Kallilrein 6/Neurosin, Midkine, VCAM-1 and Fas) were significantly different between MOG+ group and controls.
Ten of 52 measured serum cytokine/chemokines (CCL1/I-309, CCL22/MDC, CCL28, CCL17/TARC, CCL27/CTACK, CXCL2/GRO beta, Contactin-1, Midkine, Chemerin and Synuclein-alpha) were significantly different between AQP4+ group and controls.
Comparison of serum cytokine/chemokine levels between AQP4+ and MOG+ groups
Chemokines CC
There was no difference between serum AQP4+ and MOG+ groups for CC chemokines.
Chemokines CXC and IL
All measured chemokines CXC except CXCL6/GCP-2 showed no significant differences in serum AQP4+ group compared to MOG+ group.
Other cytokines
There was significant difference between serum AQP4+ and MOG+ groups for C5/C5a and Midkine. C5/C5a and Midkine were significantly higher in AQP4+ group compared to MOG+ group (P<0.05).
Discussion
NMO, characterized by ON and LETM, is an inflammatory demyelination disease of the CNS. AQP4-IgG is highly expressed in NMO and detected in the serum of 75% NMO patients.Citation24 Also previous study reported that about 10%–20% NMO patients shown negative expression to AQP4,Citation7 these patients often miss early treatment and thus suffer from condition aggravation.Citation8 With the technology improvement of AQP4 antibody detection, increase AQP4 seroprevalence and reverse results from negative expression to positive expression of AQP4,Citation25,Citation26 there is emerging voice of doubt about NMO with negative expression of AQP4.Citation27 Some argued that the false negative of AQP4 is caused by methodological differences among different AQP4 antibody detection methods,Citation8,Citation28 and relatively low AQP4 antibody titer,Citation27 which cannot be accurately detected. However, others insisted that NMO patients with true negative to AQP4 indeed exist, because still a large number of NMO patients with negative expression of AQP4 remained unchanged after antibody detection review. It is also suggested that NMO patients with positive AQP4 and negative AQp4 are different in the aspect of etiology, epidemiology and clinical presentation.Citation29,Citation30 Astrocyte destruction peculiar to AQP4+ NMO patients does not appear in AQP4- NMO patients, implying that the pathogenesis of AQP4+ NMO and AQP4- NMO are different.Citation31–Citation33
Previous study reported that MOG is often present in NMO patients with negative expression of AQP4.Citation9 Different from AQP4 antibody specifically binding astrocytes with the participation of complements, MOG antibody specifically bind oligodendrocyte without a large amount of complement involvement, thus inflammatory response is not strong. Therefore, AQP4- NMO is a disease characterized by other autoantibodies such as MOG seroprevalence.Citation34 All in all, AQP4+ NMO and MOG+ NMO have different pathogenesis mechanism. In this study, from the aspect of cytokines and chemokines, ELISA was used to screen panels of cytokines and chemokines in NMO, and the comparison between experimental group and HC was made, as shown in . It is found that CXCL6/GCP-2, Midkine and C5a probably may be key factors to distinguish the immunopathogenesis of AQP4+ NMO and MOG+ NMO.
Figure 1 Serum chemokine concentrations that were found to be significantly modified between AQP4+ group and MOG+ group.
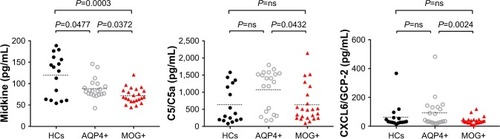
The MK, a secretory heparin binding growth factor, is a low-molecular weight protein. For adults, MK is restricted to a certain organization. However, the expression of MK significantly increased in the process of tumorigenesis, inflammation and tissue cell repair.Citation35 MK was higher in AQP4+ group compared to MOG+ group, there were significant differences between the serum AQP4+ group and MOG+ group, which probably reveal the poor repair of cells in the inflammation process. However, this conclusion needs to be further verified because there is little research on the mean concentration level of midkine in NMO at present.
C5a is an activated component of the complement system, which in addition to playing an important role in the defense system, contributes to the amplification of inflammation. C5a was significantly higher in AQP4+ group compared to MOG+ group, indicating that C5a was involved in the immune response mechanism of NMO patients with positivity to AQP4 Ab. In another word, the immune reaction mechanism of AQP4+ NMO is different from MOG+ NMO.
CXCL6/GCP-2 is an important member of ELR + CXC chemokines family and has effects on the recruitment of cells such as neutrophils, hyaline leukocyte, macrophage and lymphocyte to site of inflammation contributing to inflammatory reaction. CXCL6/GCP-2 was significantly lower in AQP4+ group compared to MOG+ group, probably indicating that the inflammatory response is obviously relieved for AQP4+ NMO patients compared to MOG+ group. It shown that the immune reaction mechanism of NMO is different between patients with positivity to AQP4 Ab and with positivity to MOG Ab.
The limitations of our study are as following: 1) our AQP4 Ab POS and MOG Ab POS groups were not identical in clinical and demographic parameters. 2) The sample size was small in each group. Our control group aimed to generate normative data by using non-inflammatory neurological controls. And long-term storage of samples is probably result in theoretical degradation of cytokines/chemokines, although it was not clearly observe and identified. 3) Lacking of available stored frozen CSF samples, we could not perform comparisons between serum and CSF samples, thus it is not clear that whether the cytokine/chemokines are predominantly produced intrathecally or peripherally.
Conclusion
In summary, our study has roughly discussed the mean concentration change of chemokines and cytokine based on the serum AQP4+ and MOG+. The differences of mean concentration in CXCL6/GCP-2, Midkine and C5a probably reveal different immunologic mechanism between AQP4+ NMO and MOG+ NMO. In another word, CXCL6/GCP-2, Midkine and C5a may be influencing factors for NMO pathogenesis and provide guidance to monitor inflammation and response to treatment.
Data sharing statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
| ANA | = | antinuclear antibody |
| AQP4 | = | aquaporin-4 |
| AQP4+ | = | patients seropositive for AQP4 antibodies |
| CBA | = | cell-based assay |
| CCR10 | = | CC chemokine receptor-10 |
| CNS | = | central nervous system |
| CTACK | = | cutaneous T cell-attracting chemokine |
| HCs | = | healthy controls |
| IDON | = | idiopathic demyelinating optic neuritis |
| LETM | = | longitudinally extensive transverse myelitis |
| MK | = | midkine |
| MOG | = | myelin oligodendrocyte glycoprotein antibody |
| MOG+ | = | patients seropositive for MOG antibodies |
| MS | = | multiple sclerosis |
| NMO | = | neuromyelitis optica |
| ON | = | neuritis optica |
| VCAM-1 | = | vascular cell adhesion molecule 1 |
Acknowledgments
This work was supported by the 863 Plan Biological and Medical Technology project “Development of equipments in diagnosis and visual function evaluation for optic neuritis”, China (No 2015AA020511).
Disclosure
The authors report no conflicts of interest in this work.
References
- HickmanSJKoMChaudhryFJayWMPlantGTOptic neuritis: an update typical and atypical optic neuritisNeuroophthalmology2008325237248
- ShamsPNPlantGTOptic neuritis: a reviewInt MS J2009163828919878630
- InnisMDLandyPJOptic neuritis and multiple sclerosisLancet1979314813252
- MinLXin-YueQThe clinical application of a variety of methods to detect AQP4-Ab in serum and cerebrospinal fluid of neuromyelitis optica patientsChin J Lab Diagn2015812611263
- TingLDaqiZChunshengYHuiZThe significance of serum aquaporin 4 antibody in the spectrum of optic neuromyelitisShandong Med J2015175253
- WingerchukDMLennonVAPittockSJLucchinettiCFWeinshenkerBGRevised diagnostic criteria for neuromyelitis opticaDigest World Core Med J200666101485
- JariusSWildemannBAquaporin-4 antibodies (NMO-IgG) as a serological marker of neuromyelitis optica: a critical review of the literatureBrain Pathol201323666168324118483
- JariusSPaulFFechnerKAquaporin-4 antibody testing: direct comparison of M1-AQP4-DNA-transfected cells with leaky scanning versus M23-AQP4-DNA-transfected cells as antigenic substrateJ Neuroinflammation201411112925074611
- MaderSGredlerVSchandaKComplement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disordersJ Neuroinflammation20118118422204662
- MartaCBTaylorCMCoetzeeTAntibody cross-linking of myelin oligodendrocyte glycoprotein leads to its rapid repartitioning into detergent-insoluble fractions, and altered protein phosphorylation and cell morphologyJ Neurosci200323135461547112843245
- DaleRCTantsisEMMerhebVAntibodies to MOG have a demyelination phenotype and affect oligodendrocyte cytoskeletonNeurol Neuroimmunol Neuroinflamm201411e1225340056
- PiddlesdenSJLassmannHZimprichFMorganBPLiningtonCThe demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complementAm J Pathol1991352555
- MayerMCMeinlEGlycoproteins as targets of autoantibodies in CNS inflammation: MOG and moreTher Adv Neurol Disord20125314715922590479
- JariusSWildemannBPaulFNeuromyelitis optica: clinical features, immunopathogenesis and treatmentClin Exp Immunol2014176214916424666204
- LucchinettiCFMandlerRNMcGavernDA role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis opticaBrain2002125Pt 71450146112076996
- MitsdoerfferMKuchrooVKornTImmunology of neuromyelitis optica: a T cell-B cell collaborationAnn N Y Acad Sci201312831576623617588
- UzawaAMoriMAraiKCytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6Mult Scler201016121443145220739337
- MatsushitaTTateishiTIsobeNCharacteristic cerebrospinal fluid cytokine/chemokine profiles in neuromyelitis optica, relapsing remitting or primary progressive multiple sclerosisPLoS One201384e6183523637915
- TanakaMMatsushitaTTateishiTDistinct CSF cytokine/chemokine profiles in atopic myelitis and other causes of myelitisNeurology2008711397498118809833
- UzawaAMoriMItoMMarkedly increased CSF interleukin-6 levels in neuromyelitis optica, but not in multiple sclerosisJ Neurol2009256122082208419655191
- XiaonanZHonghaoWYongqiangDCerebrospinal fluid levels of CXCL13 are elevated in neuromyelitis opticaJ Neuroimmunol2011240–241104108
- AlvarezEPiccioLMikesellRJCXCL13 is a biomarker of inflammation in multiple sclerosis, neuromyelitis optica, and other neurological conditionsMult Scler20131991204120823322500
- WangHWangKZhongXCerebrospinal fluid BAFF and APRIL levels in neuromyelitis optica and multiple sclerosis patients during relapseJ Clin Immunol20123251007101122644841
- BoveRElsoneLAlvarezEFemale hormonal exposures and neuromyelitis optica symptom onset in a multicenter studyNeurol Neuroimmunol Neuroinflamm201743e33928382320
- MarignierRBernard-ValnetRGiraudonPAquaporin-4 antibody-negative neuromyelitis optica: distinct assay sensitivity-dependent entityNeurology201380242194220023658379
- JiaoYFryerJPLennonVAUpdated estimate of AQP4-IgG serostatus and disability outcome in neuromyelitis opticaNeurology201381141197120423997151
- LevyMDoes aquaporin-4-seronegative neuromyelitis optica exist?JAMA Neurol201471327127224424919
- WatersPJMcKeonALeiteMISerologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assaysNeurology201278966567122302543
- JariusSPaulFFranciottaDNeuromyelitis optica spectrum disorders in patients with myasthenia gravis: ten new aquaporin-4 antibody positive cases and a review of the literatureMult Scler20121881135114322183934
- JariusSWildemannBThe history of neuromyelitis opticaJ Neuroinflammation201310111223282009
- BradlMMisuTTakahashiTNeuromyelitis optica: pathogenicity of patient immunoglobulin in vivoAnn Neurol200966563064319937948
- FujiharaKLeiteMISeronegative NMO: a sensitive AQP4 antibody test clarifies clinical features and next challengesNeurology201380242176217723658387
- FujiharaKMisuTAQP4 in biopsied demyelinating lesions as a diagnostic clue to NMOSD and MS: final answer?Neurology201584211011125503619
- JariusSRuprechtKKleiterIMOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and originJ Neuroinflammation201613127927788675
- YaoHPengYTongJClinical significance of midkine expression in gestational trophoblastic diseaseJ Med Res2014438127130
