Abstract
Background
Certain psychotropics and a number of other medications used to treat medical conditions in psychiatric patients can increase the risk of prolonging the corrected QT (QTc) interval on the electrocardiogram, which puts patients at risk of life-threatening ventricular arrhythmias such as torsades de pointes. Pharmacists are often consulted about medications which are known to prolong the QTc interval. Although this information is often accessible, advising how to identify, assess, manage, and refer psychiatric patients at risk for drug-induced QTc prolongation is more challenging.
Objectives
The objective of this project was first to review the literature, which describes guidelines and recommendations for the assessment and management of drug-induced QTc prolongation, and then to design an algorithm to be used by pharmacists working closely with mental health professionals or who provide care to psychiatric patients.
Methods
A review of the literature was undertaken. Predefined keywords were used to perform the database search in MEDLINE, EMBASE, and International Pharmaceutical Abstracts to identify reviews, reports and guidelines on the assessment, prevention and monitoring of drug-induced QTc prolongation with an emphasis on psychotropic medications and management in the psychiatric population.
Results
The electronic database search retrieved 637 relevant citations. These were initially screened by title and all duplicates were removed. The abstracts were then reviewed for relevancy based on the inclusion/exclusion criteria. Additional citations were retrieved from the bibliography of the articles identified in the initial search. A total of 79 articles describing QTc prolongation in the psychiatric population were thoroughly examined, but only 31 articles were selected to guide the development of the algorithm.
Conclusion
The literature-based algorithm developed provides a stepped-based approach for the assessment, monitoring, and management of drug-induced QTc prolongation in the psychiatric population. The algorithm may assist mental health clinicians in the decision-making process when psychiatric patients are prescribed medications known to increase the QTc interval.
Background
Prolongation of the QT interval is a concern for all clinicians as it can lead to fatal consequences, such as sudden cardiac death (SCD).Citation1 Among psychiatric patients, SCD is one of the major causes of premature mortality.Citation2–Citation5 Psychotropic medications have been implicated in the increased risk of SCD among psychiatric patients, primarily in view of their potential for prolonging the QT interval.Citation3–Citation6 Although relatively rare, prolongation of the QT interval can be followed by a life-threatening polymorphic ventricular tachyarrhythmia called torsades de pointes (TdP).Citation7,Citation8 The mortality risk associated with TdP is high, estimated to be approximately 10%.Citation8
TdP is commonly associated with hereditary or acquired forms of prolonged QT interval (also known as long QT syndrome [LQTS]).Citation1 Mutations of the human hERG protein ion channels (potassium, calcium, and sodium), which contribute to the electrical activity of the heart, result in congenital LQTS (cLQTS).Citation9 Acquired LQTS is almost always due to blockage or inhibition of the inward potassium rectifier (IKr) channel, which is critical in the phase 3 repolarization of the cardiac action potential.Citation10 As with cLQTS, most cases of drug-induced long QT and TdP result from an action of the drugs on the ion channel proteins encoded by the hERG gene that is responsible for the IKr repolarizing current.Citation11 A recent study by Itoh et al reported that up to 28% of patients with drug-induced QT prolongation carry mutations causing the LQTS.Citation12 The concept of repolarization reserve has been employed to account for individual differences in the susceptibility to develop TdP with drugs or conditions known to affect the heart’s potassium currents.Citation11
The potential risk of TdP raises concerns among health care providers when prescribing medications known to inhibit hERG IKr channel, particularly in patients who may be predisposed.Citation13 In addition, various psychotropic drugs have also been shown to inhibit inward ionic currents mediated by sodium and calcium.Citation14,Citation15 Besides psychotropic medications, other medications commonly used by psychiatric patients have also been implicated in prolonging the QT interval such as antiarrhythmic medications, antibiotics, antifungals, and antiemetic medications, some of which were removed from the market due to associated TdP.Citation13
Studies have demonstrated that clinicians are often unable to identify TdP risk factors or medications that can prolong the QT interval.Citation16,Citation17 Fongemie et al reported discordance among cardiologists in the decision-making process when corrected QT (QTc)-prolonging medications were to be used.Citation17 Furthermore, in daily practice, because of an ever-increasing number of medications available and other nondrug factors that must be accounted for during a risk assessment, clinicians may face difficulties on how to assess, manage, monitor and refer patients at risk of QTc prolongation.
Pharmacists are often consulted about medications that are known to prolong the QTc interval. To be meaningful, advice provided should extend beyond simple information about the potential for QT interval prolongation with a particular medication. However, this information may be more challenging for pharmacists to retrieve and difficult to interpret, particularly when caring for mental health patients who often have complex treatment regimens and who are often on poly-pharmacy. As such, the purpose of this paper was to summarize the literature describing guidelines and recommendations for the assessment and management of drug-induced QTc prolongation in psychiatric patients, and to develop an algorithm to guide all clinicians in the decision-making process when psychiatric patients are prescribed medications with the potential of prolonging the QTc interval.
Methods
A literature search was conducted to identify pertinent published literature. Three databases were searched: MEDLINE (Ovid), from 1946 to November 2017, EMBASE, from 1974 to November 2017, and International Pharmaceutical Abstracts, from 1970 to November 2017. The following search strategy was used to identify relevant literature: (“guideline”[All Fields] OR “guidelines as topic”[MeSH Terms] OR “guidelines”[All Fields]) AND (“organization and administration”[MeSH Terms] OR (“organization”[All Fields] AND “administration”[All Fields]) OR “organization and administration”[All Fields] OR “management”[All Fields] OR “disease management”[MeSH Terms] OR (“disease”[All Fields] AND “management”[All Fields]) OR “disease management”[All Fields]) AND QTc[All Fields] AND interval[All Fields] AND prolongation[All Fields] AND (“patients”[MeSH Terms] OR “patients”[All Fields]) AND (“mental disorders”[MeSH Terms] OR (“mental”[All Fields] AND “disorders”[All Fields]) OR “mental disorders”[All Fields] OR (“mental”[All Fields] AND “illness”[All Fields]) OR “mental illness”[All Fields]) AND (“psychiatry”[MeSH Terms] OR “psychiatry”[All Fields] OR “psychiatric”[All Fields]) AND (“pharmaceutical preparations”[MeSH Terms] OR (“pharmaceutical”[All Fields] AND “preparations”[All Fields]) OR “pharmaceutical preparations”[All Fields] OR “medications”[All Fields]).
The initial screening consisted of reviewing the relevance of the article titles and then reviewing the abstracts of the articles that passed the initial title screening. Articles describing individual studies or systematic reviews discussing assessment, risk stratification, monitoring, and prevention of QTc prolongation in the psychiatric population were included. Additional citations were retrieved from the bibliography of the articles identified in the initial search. Articles written in languages other than English, for which full-text articles could not be obtained or published before 2010, were excluded. This timeline restriction ensured that only the most updated research findings were used in guiding the development of the algorithm.
Results
The electronic database search retrieved 637 relevant citations. These were initially screened by title and all duplicates were removed. A total of 79 articles describing QTc prolongation in the psychiatric population were thoroughly examined, but only 31 articles were selected to guide the development of the algorithm. Based on the inclusion/exclusion criteria described previously, the articles were grouped into the following categories:
Articles describing individual studies discussing risk factor assessment, risk stratification, and/or monitoring of the QTc interval in the psychiatric population. A total of 14 articles were included, a summary of the findings of these studies is presented in .Citation18–Citation31
Table 1 Articles reviewing QTc prolongation assessment and risk stratification among psychiatric patients
Articles describing guidelines or protocols for preventing, monitoring or managing drug-induced QTc prolongation in the psychiatric population. A total of nine articles were included and a summary of the recommendations provided in these articles is presented in .Citation32–Citation40
Table 2 Articles describing guidelines and protocols for assessing, monitoring, preventing or managing drug-induced QTc prolongation in the psychiatric population
Review articles about drug-induced QTc prolongation risk stratification in nonpsychiatric patients from which relevant recommendations were extrapolated to support development of the algorithm. A total of eight review articles were included and these are summarized in .Citation41–Citation48
Table 3 Review articles about QTc prolongation (not specifically in the psychiatric population) included in the development of the algorithm
As illustrated in , the literature-based algorithm developed provides a stepped-based approach for the assessment, management, and monitoring of psychotropic-induced QTc prolongation in the psychiatric population. The algorithm includes a set of questions which facilitate the decision-making process and the evidence that supports the decision points.
Figure 1 A literature-based algorithm for the assessment, management and monitoring of drug-induced QTc prolongation.
Abbreviations: cLQTS, congenital long QT syndrome; ECG, electrocardiogram; EF, ejection fraction; QTc, corrected QT; TdP, torsades de pointes.
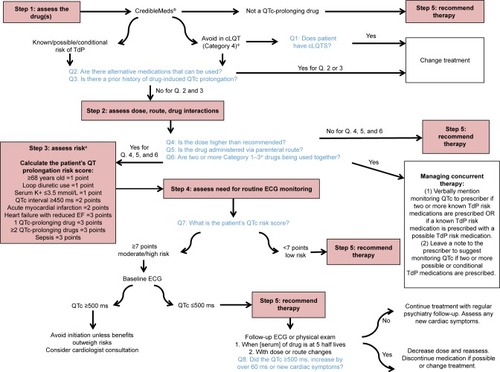
Step 1: assessing the medication risk
The starting point is assessing the medication prescribed. When prescribing a psychotropic agent or any other medication for psychiatric patients who are taking psychotropic medications, particularly antipsychotics or antidepressants, the first step consists of assessing the prescribed drug.Citation18,Citation20,Citation22,Citation29,Citation30,Citation34,Citation38,Citation42,Citation47 The most comprehensive and easily accessible reference source for medications known or suspected to cause QT prolongation is CredibleMeds® accessible through the world wide web (WWW) at www.crediblemeds.org.Citation49 This website is maintained by AZCERT (The Arizona Center for Education and Research on Therapeutics), a nonprofit organization, that uses a rigorous, systematic risk stratification process to determine a medication’s likelihood to cause QT prolongation or TdP.Citation50 The evidence on this website is monitored closely and updated frequently. Clinicians can register and subscribe to updates from the website. CredibleMeds® is also available through a mobile application which can easily be accessed by clinicians on their cell phones, iPads or other pocket devices.
CredibleMeds® classifies medications into four categories: Category 1 Drugs, those with known risk of TdP; these drugs prolong the QT interval and have an associated known risk of TdP even when taken as recommended. Category 2 Drugs, those with possible risk of TdP; these drugs have the potential to cause QT prolongation, but there is a lack of evidence for TdP risk. Category 3 Drugs, those with conditional risk of TdP; these drugs are associated with TdP but only under certain circumstances or in patients with certain “at-risk” characteristics. Category 4 Drugs, those to be avoided in patients with cLQTS.Citation50 Currently, 57 medications are on the list of drugs known to cause TdP. Another 92 are on the list with “possible risk of TdP” and 43 are on the “conditional risk” list. Two hundred and twenty-four medications are on the list of “drugs to avoid” for patients with cLQTS.Citation50
Step 2: assessing the prescribed medication dose, route of administration, and drug interactions
For the majority of QT-prolonging drugs, risk increases as a function of dose and plasma drug concentration.Citation19,Citation21,Citation22,Citation25,Citation30,Citation37,Citation38,Citation42,Citation43,Citation48 It has been recognized that drug plasma concentrations could be affected by multiple factors including dose, formulation form, route, frequency, administration time, drug–drug interaction, food–drug interaction, genetics, sex, age, body weight, pregnancy, circadian rhythms, comorbidities, pathophysiology status, and smoking.Citation51 As such, depending on the type of medication used, weight-based dosing should be considered particularly for populations who are more vulnerable to increased plasma levels if not dosed according to weight (eg, elderly).Citation52
The route of administration can also have an effect on the QT-prolonging extent of a drug, as medications administered intravenously tend to have higher potential of causing QT prolongation as opposed to oral administration.Citation30,Citation32,Citation35 Intravenous administration can be associated with higher drug concentrations and thus greater cardiac exposure than corresponding oral dosing.Citation47 Finally, rapid infusion of a QT-prolonging medication is also a risk factor for QT prolongation.Citation30,Citation42,Citation43 Conditions that lead to elevated plasma concentrations of QTc interval-prolonging drugs increase the risk of drug-induced TdP, including pharmacokinetic drug interactions and inadequate dose adjustment of renally eliminated QTc interval-prolonging drugs in patients with acute kidney injury or chronic kidney disease.Citation42,Citation47 In addition, competitive protein binding can potentially cause increased serum levels of medications that prolong QTc when combined with seemingly benign medications that are highly protein bound, such as acetylsalicylic acid and sodium valproate, and some herbal preparations.Citation30 A comprehensive and easily accessed reference source in the WWW for pharmacokinetic interactions is available from Medscape® at https://reference.medscape.com/drug-interactionchecker. CredibleMeds® also lists medication combinations which should be used with caution by identifying medications that could cause additive pharmacodynamic interactions with psychotropic medications that prolong QTc.Citation49,Citation50
Step 3: assessing the patient’s risk of QTc prolongation and TdP
Electrolyte abnormalities, particularly hypokalemia, hypomagnesemia, and hypocalcemia, are known risk factors for QT prolongation.Citation27,Citation30,Citation34,Citation40–Citation43,Citation47,Citation48 Drug-induced QTc prolongation also occurs more commonly in females than in males, and the effects are more pronounced in older patients above the age of 65 years.Citation20,Citation22,Citation23,Citation30,Citation31,Citation34,Citation42,Citation43,Citation47 Patients with an underlying heart disease including cLQTS, myocardial ischemia, cardiomyopathy, cardiac arrhythmias, bradycardia, or congestive heart failure are also predisposed to QT prolongation in comparison to healthy counterparts.Citation22,Citation26,Citation27,Citation30,Citation31,Citation34,Citation37,Citation38,Citation42,Citation43,Citation48 Because many hospitalized patients often have other risk factors for a proarrhythmic response, such as older age with an underlying heart disease who may also have renal or hepatic dysfunction, electrolyte abnormalities, or bradycardia and to whom drugs may be administered rapidly via the intravenous route, administration of a QT-prolonging drug to a hospitalized population may be more likely to cause TdP than administration of the same drug to an outpatient population.Citation32,Citation35,Citation43,Citation48,Citation49
There are three manual stratification tools published in the literature that clinicians can use to determine individual QTc-prolongation risk.Citation45,Citation46,Citation49 Of these, only the one developed by Tisdale et al has been validated.Citation46 This risk scoring tool allows quantification of a patient’s risk based on the presence or absence of a total of ten possible risk factors, with a maximum possible score of 21. This scoring system classifies patients as low, medium or highrisk (QTc score ≤7, 8–10 or ≥11, respectively). In a subsequent study, the same authors were able to demonstrate that incorporating this validated risk scoring tool into a decision support tool system influenced the prescribing of QT-prolonging drugs and reduced the risk of QTc interval prolongation in hospitalized patients with TdP risk factors.Citation44
Step 4: determining the need for measuring the QT interval
Although monitoring of the QT interval by electrocardiogram (ECG) prior to treatment with QT-prolonging drugs is often recommended by the drug manufacturers, indications for obtaining a baseline ECG and/or routine ECG monitoring are mixed in the literature.Citation23,Citation26,Citation28,Citation34,Citation36,Citation47,Citation53 Although it may be easier in an inpatient setting, a wearable remote monitoring system has been recommended for the identification of subjects with cLQTS or at risk for drug-induced LQTS in outpatient settings.Citation54 De Hert et al conducted a systematic review of various guidelines for cardiovascular risk with antipsychotic medications and found that 50% of guidelines support ECG monitoring for psychotropic agents with the potential to prolong the QTc interval.Citation36 Based on the risk assessment, patients with a QTc risk score of 7 when using the validated risk scoring tool developed by Tisdale et al, or those taking high-risk drug combinations, should have an ECG when the plasma concentration of a new QT-prolonging agent reaches steady state (ie, five half-lives following initiation).Citation42
Magnesium and potassium levels should also be checked with ECG.Citation46 When following-up on an ECG done at steady state, drug-induced QT prolongations of <25 ms are considered insignificant by some clinicians, though the increase of 60 ms or QTc intervals of over 500 ms are a cause for concern and more frequent monitoring or the cessation of therapy is recommended in such cases.Citation24,Citation42
Other situations that warrant ECG monitoring include overdoses of medication with QT-prolonging potential, when more than one QTc-prolonging medication is prescribed in combination, when a patient experiences cardiovascular symptoms, in patients with cardiovascular disease, and in cases of electrolyte derangements.Citation26,Citation34,Citation42 Some medications have specific ECG recommendations available. For instance, the Center for Substance Abuse and Mental Health Services Administration (CSAT), an independent multidisciplinary expert panel, developed guidelines on cardiac safety recommendations for methadone prescribers.Citation28 The guidelines recommend a pretreatment ECG for all patients prior to initiating methadone, a follow-up ECG within 30 days, and then annually thereafter or sooner if there is a change in dosage. Increased monitoring is recommended if a patient has a QT interval over 450 ms. If the QT interval exceeds 500 ms, discontinuation of methadone should be considered.
Step 5: recommending strategies to minimize the likelihood of drug-induced TdP
If cardiac risks are identified, the cardiac risk factors should be optimized and/or a drug with a more favorable risk profile should be chosen if possible. In case of structural heart disease, QT prolongation, electrolyte disturbances, or cardiac symptoms, referral to a cardiologist should be considered.Citation34 Re-evaluation of the ECG and symptoms should take place within 1 or 2 weeks or at steady state (five times the drug half-life) after initiation of treatment with class 1 drugs or with class 3/4 drugs if used in combination.Citation34 Similarly, a significant increase in dose of these drugs necessitates re-evaluating symptoms and a new ECG.Citation34
A QTc interval above 500 ms or an increment above 60 ms when compared with baseline is generally associated with a definite increased risk of TdP and should in most cases lead to discontinuation of the drug.Citation34,Citation47 It is suggested to consult a cardiologist if the QTc stays prolonged after discontinuing medication, if cardiovascular symptoms are present, or in case of pre-existing cardiac disease or when a strong family history exists. Several other QTc scenarios occurring during treatment also merit specialist consultation, such as in pediatric patients, in the elderly, and in frail (ie, hospitalized) individuals.Citation48
Discussion
The literature review undertaken provides a comprehensive list of studies, reports, and guidelines for the assessment, monitoring, and management of drug-induced QTc prolongation in the psychiatric population. In order to facilitate the translation of evidence-based recommendations into practice, a stepped-care approach was used in the development of the algorithm to assist mental health clinicians in making decisions when medications known to increase the QTc interval are used.
In a similar algorithm developed by Fanoe et al, the risk of arrhythmia associated with various classes of psychotropic medications was described.Citation34 We believe that our algorithm adds value to the one developed by Fanoe et al as it provides a stepped-care approach with a set of clinical questions that are intended to help in rationalizing the decision-making process. Fanoe et al did not recommend the use of a specific QTc risk scoring tool, and rather suggested seeking a cardiologist consultation when patients present with any positive risk factors for prolonged QTc. Our algorithm still supports cardiology consultation, especially in those whose risk score is >7 points. In 2002, the Cardiac Safety in Schizophrenia Group released a series of consensus statements on how to minimize the risks associated with significant QTc prolongation in people with schizophrenia.Citation55 These, however, have not been updated since and consequently have no information on newer medications or on medications other than those used for the management of schizophrenia; thus, our algorithm is more inclusive of other psychiatric diagnoses and provides a more updated resource for clinicians.Citation53
Common findings in the literature review which guided the development of this algorithm include the use of CredibleMeds® as an important evidence-based resource, as well as the importance of cardiac risk stratification in the assessment and management of drug-induced QTc prolongation. Studies have shown that prescribers do not routinely screen for cardiac risk factors in psychiatric patients.Citation53,Citation56,Citation57
In addition, because ECG monitoring may not be possible or readily accessible in both outpatient and inpatient settings, or in all psychiatric facilities, the algorithm developed highlights the importance of assessing the associated risk factors as early as possible before making recommendations on the use of medications with QTc-prolonging risk, primarily those listed as Category 1–3 by CredibleMeds® or those that are known inhibitors of the hERG IKr channel.Citation11,Citation49,Citation53,Citation56–Citation58 Current research initiatives, such as the Comprehensive in vitro Proarrhythmia Assay (CiPA), are developing and validating a model to test the proarrhythmic risk of drugs by assessing the effect of drugs on multiple ion channels and integrating ECG analysis in early phase I clinical trials to better predict proarrhythmic risk.Citation59 If successful, CiPA will 1) create a pathway for drugs with hERG block/QT prolongation to advance without intensive ECG monitoring in phase III trials if they have low proarrhythmic risk and 2) enable updating of drug labels to be more informative about proarrhythmic risk, not just QT prolongation.Citation59
Despite the robustness of our literature review, the algorithm developed is not without limitations. The algorithm incorporates a tool to predict the risk of QTc prolongation that was developed by Tisdale et al and validated only in nonpsychiatric populations.Citation42,Citation44 Thus, it is uncertain how the risk scoring tool will perform in patients with psychiatric disorders who are known to be at higher risk of cardiac events regardless of the medications they are on.Citation60 Future testing of this stepped-based algorithm with regard to its usefulness in guiding pharmacists and other clinicians in the decision-making process when medications are prescribed to mental health patients is necessary.
Conclusion
The algorithm developed provides a stepped approach for assessing and managing the risk of QTc prolongation when prescribing medications for patients with psychiatric disorders. The stepped approach described may also be appropriate when assessing drug-induced QTc prolongation risk in individuals without psychiatric disorders. Further investigations dedicated to the validation of this stepped-based algorithm are required, including face validation and the feasibility for its routine use by clinicians, including an evaluation of the outcome of its use over time.
Acknowledgments
An earlier version of this research was presented as a poster at the Canadaian Society of Hospital Pharmacists Annual Professional Practice Conference, in Toronto, Canada, from February 3–8, 2018. The abstract of this poster was published as part of the conference proceedings as follows: Zolezzi M, Chelung L. A literature-based algorithm for the assessment, management and monitoring of drug-induced QTc prolongation in the psychiatric population. Can J Hosp Pharm. 2018;71(1):66.
Disclosure
The authors report no conflicts of interest in this work.
References
- SchwartzPJWoosleyRLPredicting the unpredictable: drug-induced QT prolongation and torsades de pointesJ Am Coll Cardiol201667131639165027150690
- IfteniPCorrellCUBurteaVKaneJMManuPSudden unexpected death in schizophrenia: autopsy findings in psychiatric inpatientsSchizophr Res20141551–3727624704220
- RayWAChungCPMurrayKTHallKSteinCMAtypical antipsychotic drugs and the risk of sudden cardiac deathN Engl J Med2009360322523519144938
- GirardinFSztajzelJCardiac adverse reactions associated with psychotropic drugsDialogues Clin Neurosci2007919295
- BuckleyNASandersPCardiovascular adverse effects of antipsychotic drugsDrug Saf200023321522811005704
- BeachSRCelanoCMNoseworthyPAJanuzziJLHuffmanJCQTc prolongation, torsades de pointes, and psychotropic medicationsPsychosomatics201354111323295003
- MalikMCammAJEvaluation of drug-induced QT interval prolongation: implications for drug approval and labellingDrug Saf200124532335111419561
- DarpoBSpectrum of drugs prolonging QT interval and the incidence of torsades de pointesEur Heart J20013Suppl KK70K80
- ChingCKTanECCongenital long QT syndromes: clinical features, molecular genetics and genetic testingExpert Rev Mol Diagn20066336537416706739
- RodenDMViswanathanPCGenetics of acquired long QT syndromeJ Clin Invest200511582025203216075043
- CubedduLXIatrogenic QT Abnormalities and fatal arrhythmias: mechanisms and clinical significanceCurr Cardiol Rev20095316617620676275
- ItohHCrottiLAibaTThe genetics underlying acquired long QT syndrome: impact for genetic screeningEur Heart J201637181456146426715165
- TrinkleyKEPageRLLienHYamanouyeKTisdaleJEQT interval prolongation and the risk of torsades de pointes: essentials for cliniciansCurr Med Res Opin201329121719172624020938
- WitchelHJHancoxJCNuttDJPsychotropic drugs, cardiac arrhythmia, and sudden deathJ Clin Psychopharmacol2003231587712544377
- SilvestreJSO’NeillMFProusJREvidence for a crucial modulating role of the sodium channel in the QTc prolongation related to antipsychoticsJ Psychopharmacol201428432934024327451
- Al-KhatibSMAllen LapointeNMKramerJMA survey of health care practitioners’ knowledge of the QT intervalJ Gen Intern Med200520539239615963159
- FongemieJMAl-QadheebNSEstesNAAgreement between ICU clinicians and electrophysiology cardiologists on the decision to initiate a QTc-interval prolonging medication in critically ill patients with potential risk factors for torsade de pointes: a comparative, case-based evaluationPharmacotherapy201333658959723529904
- Ojero-SenardABeneventJBondon-GuittonEA comparative study of QT prolongation with serotonin reuptake inhibitorsPsychopharmacology2017234203075308128770276
- BarbuiCBighelliICarràGAntipsychotic Dose Mediates the Association between Polypharmacy and Corrected QT IntervalPLoS One2016112e014821226840602
- DanielssonBCollinJJonasdottir BergmanGBorgNSalmiPFastbomJAntidepressants and antipsychotics classified with torsades de pointes arrhythmia risk and mortality in older adults – a Swedish nationwide studyBr J Clin Pharmacol201681477378326574175
- NosèMBighelliICastellazziMPrevalence and correlates of QTc prolongation in Italian psychiatric care: cross-sectional multicentre studyEpidemiol Psychiatr Sci201625653254026467074
- SchächteleSTümenaTGaßmannKGFrommMFMaasRCo-prescription of QT-interval prolonging drugs: an analysis in a large cohort of geriatric patientsPLoS One2016115e015564927192430
- PoncetAGencerBBlondonMElectrocardiographic screening for prolonged QT interval to reduce sudden cardiac death in psychiatric patients: a cost-effectiveness analysisPLoS One2015106e012721326070071
- RabkinSWImpact of age and sex on QT prolongation in patients receiving psychotropicsCan J Psychiatry201560520621426174524
- TakeuchiHSuzukiTRemingtonGUchidaHAntipsychotic polypharmacy and corrected QT interval: a systematic reviewCan J Psychiatry201560521522226174525
- ShahAAAftabACoverdaleJQTc prolongation with antipsychotics: is routine ECG monitoring recommended?J Psychiatr Pract201420319620624847993
- GirardinFRGex-FabryMBerneyPShahDGaspozJMDayerPDrug-induced long QT in adult psychiatric inpatients: the 5-year cross-sectional ECG Screening Outcome in Psychiatry studyAm J Psychiatry2013170121468147624306340
- KatzDFSunJKhatriVQTc interval screening in an opioid treatment programAm J Cardiol201311271013101823820570
- TranEDishmanBCitalopram-induced QTc prolongation: a brief review of the dataMent Health Clin201226139141
- Wenzel-SeifertKWittmannMHaenEQTc prolongation by psychotropic drugs and the risk of Torsade de PointesDtsch Arztebl Int20111084168769322114630
- YangFDWangXQLiuXPSex difference in QTc prolongation in chronic institutionalized patients with schizophrenia on long-term treatment with typical and atypical antipsychoticsPsychopharmacology2011216191621301815
- WahidiNJohnsonKMBrenzelAde LeonJTwo sudden and unexpected deaths of patients with schizophrenia associated with intramuscular injections of antipsychotics and practice guidelines to limit the use of high doses of intramuscular antipsychoticsCase Rep Psychiatry2016201694068131427597919
- ChouRCrucianiRAFiellinDAMethadone safety: a clinical practice guideline from the American Pain Society and College on Problems of Drug Dependence, in collaboration with the Heart Rhythm SocietyJ Pain201415432133724685458
- FanoeSKristensenDFink-JensenARisk of arrhythmia induced by psychotropic medications: a proposal for clinical managementEur Heart J201435201306131524644307
- PacciardiBMauriMCargioliCIssues in the management of acute agitation: how much current guidelines consider safety?Front Psychiatry201342623675355
- de HertMVancampfortDCorrellCUGuidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluationBr J Psychiatry201119929910521804146
- MartinJACampbellAKillipTQT interval screening in methadone maintenance treatment: report of a SAMHSA expert panelJ Addict Dis201130428330622026519
- NielsenJGraffCKantersJKToftETaylorDMeyerJMAssessing QT interval prolongation and its associated risks with antipsychoticsCNS Drugs201125647349021649448
- FishmanGIChughSSDimarcoJPSudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society WorkshopCirculation2010122222335234821147730
- Modesto-LoweVBrooksDPetryNMethadone deaths: risk factors in pain and addicted populationsJ Gen Intern Med201025430530920087676
- Institute for Safe Medication Practices Canada®Pharmacist Evaluation of QT Prolongation Risk and Recommendation. [Internet]2017 Available from: https://www.ismp-canada.org/ToolQit_QTprolongation/Accessed September 03, 2018
- TisdaleJEDrug-induced QT interval prolongation and torsades de pointes: Role of the pharmacist in risk assessment, prevention and managementCan Pharm J20161493139152
- JardinCGPutneyDMichaudSAssessment of drug-induced torsade de pointes risk for hospitalized high-risk patients receiving QT-prolonging agentsAnn Pharmacother201448219620224301687
- TisdaleJEJaynesHAKingeryJREffectiveness of a clinical decision support system for reducing the risk of QT interval prolongation in hospitalized patientsCirc Cardiovasc Qual Outcomes20147338139024803473
- HaugaaKHBosJMTarrellRFMorlanBWCaraballoPJAckermanMJInstitution-wide QT alert system identifies patients with a high risk of mortalityMayo Clin Proc201388431532523541006
- TisdaleJEJaynesHAKingeryJRDevelopment and validation of a risk score to predict QT interval prolongation in hospitalized patientsCirc Cardiovasc Qual Outcomes20136447948723716032
- NachimuthuSAssarMDSchusslerJMDrug-induced QT interval prolongation: mechanisms and clinical managementTher Adv Drug Saf20123524125325083239
- DrewBJAckermanMJFunkMPrevention of Torsade de Pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation, endorsed by the American Association of Critical-Care Nurses and the International Society for Computerized ElectrocardiologyCirculation201012181047106020142454
- CredibleMeds® [homepage on the Internet]FAQs on QTdrugs lists, 2013 Available from: https://www.crediblemeds.org/everyone/articlesbrochures-library/consumerfaqAccessed September 3, 2018
- WoosleyRLBlackKHeiseCWRomeroKCredibleMeds.org: what does it offer?Trends Cardiovasc Med2018282949928801207
- PanSDZhuLLChenMXiaPZhouQWeight-based dosing in medication use: what should we know?Patient Prefer Adherence20161054956027110105
- LuscombeDKFactors influencing plasma drug concentrationsJ Int Med Res197751 Suppl8297863093
- BoszkoMStanciuCNSurvey of EKG monitoring practices: a necessity or prolonged nuisance?Am J Psychiatry Residents’ Journal201711369
- CastellettiSDagradiFGouleneKA wearable remote monitoring system for the identification of subjects with a prolonged QT interval or at risk for drug-induced long QT syndromeInt J Cardiol2018266899429887480
- AmesDCammJCookPMinimizing the risks associated with QTc prolongation in people with schizophrenia. A consensus statement by the Cardiac Safety in Schizophrenia GroupEncephale2002286 Pt 1115124
- GirgisSJMaroneyMELiuMTEvaluation of the use of electrocardiogram monitoring in patients on psychotropic medications that have a risk of QT prolongationMent Health Clin20166417117729955466
- WarnierMJRuttenFHSouvereinPCde BoerAHoesAWde BruinMLAre ECG monitoring recommendations before prescription of QT-prolonging drugs applied in daily practice? The example of haloperidolPharmacoepidemiol Drug Saf201524770170826013175
- VandaelEFoulonVDrug-induced QTc-prolongation: risk management in a community pharmacyJournal of the Malta College of Pharmacy Practice2017233712
- VicenteJZusterzeelRJohannesenLMechanistic model-informed proarrhythmic risk assessment of drugs: review of the “CiPA” initiative and design of a prospective clinical validation studyClin Pharmacol Ther20181031546628986934
- ManuPKaneJMCorrellCUSudden deaths in psychiatric patientsJ Clin Psychiatry201172793694121672496
