Abstract
Some of trauma-exposed individuals develop posttraumatic stress disorder (PTSD), an incapacitating psychiatric disorder that is characterized by intrusion, avoidance, negative changes in mood and cognition, and hyperarousal. A number of other trauma-related conditions are very frequently found in individuals with PTSD. Traumatic brain injury (TBI) is one of the most frequently observed trauma-related conditions that trauma-exposed individuals with PTSD may experience. TBI refers to transient or permanent brain dysfunction that results in a wide range of neurological, cognitive, and psychiatric symptoms. These trauma-related conditions significantly affect one’s quality of life, leading to substantial disability and socioeconomic burden. As the prevalence of PTSD with comorbid TBI is increasing in the general population along with the rates of crimes and accidents, effective prevention and intervention strategies are necessitated. However, a definitive treatment for PTSD with comorbid TBI is still lacking, resulting in high rates of treatment resistance and chronicity. It is essential to investigate the neurobiological mechanisms and potential therapeutics of PTSD with comorbid TBI. Yet, a few repetitive transcranial magnetic stimulation (rTMS) studies have recently investigated therapeutic efficacy in treatment-resistant patients with PTSD and/or TBI. Thus, this article reviews rTMS studies in trauma-related conditions, mainly focusing on PTSD and PTSD with TBI as one of the comorbidities. The review focuses on the applications of rTMS in reducing PTSD symptoms with and without comorbidities based on differential parameters and effects of rTMS as well as concomitant clinical conditions. The section on PTSD with comorbidities focuses on TBI with neurological, cognitive, and psychiatric symptoms. Although there were some inconsistencies in the clinical outcomes and optimized parameters of rTMS applied in PTSD and TBI, low frequency stimulation over the hyperactive frontal regions and/or high frequency stimulation over the hypoactive frontal regions generally improved the clinical symptoms of PTSD and TBI. Lastly, the limitations of the rTMS studies in PTSD and TBI as well as potential directions for future research are discussed.
Introduction
Some of trauma-exposed individuals experience posttraumatic stress disorder (PTSD), an incapacitating psychiatric disorder that is characterized by intrusion, avoidance, negative changes in mood and cognition, and hyperarousal after a traumatic experience.Citation1 A number of other trauma-related conditions are increasingly found in individuals with PTSD that include neuropsychiatric disorders such as traumatic brain injury (TBI), major depression, and alcohol use disorders (AUDs).Citation1–Citation3 One of the most frequently observed comorbidities in PTSD is TBI. TBI refers to transient or permanent brain dysfunction that results in a wide range of neurological, cognitive, and psychiatric symptoms after a head trauma and categorized as “mild” or “moderate to severe” depending on its severity.Citation2 The prevalence of PTSD with TBI in the general population has been increasing along with the growing rates of crimes and accidents, necessitating effective interventions.Citation3 Both PTSD and TBI, as posttraumatic conditions, highly overlap and co-occur, suggesting shared etiology and pathophysiology between these two.Citation3 The co-occurrence of PTSD and TBI has fewer treatment options available and increases prognostic factors for chronicity, leading to substantial disability and socioeconomic burden.Citation3
Among the PTSD patients who received standard pharmacotherapy (anticonvulsants, antipsychotics, and antidepressants) and psychotherapy (cognitive–behavioral treatment and eye movement desensitization, and reprocessing), one-third of them remain diagnosed.Citation4,Citation5 Moreover, TBI and related clinical symptoms often show low response and compliance to standard pharmacological and non-pharmacological interventions (exercise and rehabilitation) potentially due to its complicated pathophysiology.Citation6 As a definitive treatment for PTSD with multiple comorbidities is still lacking, treatment of chronic PTSD with comorbid TBI as shown in veterans is particularly challenging.
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive neuromodulation treatment by rapidly and repetitively changing electromagnetic fields through the coil applied over the scalp.Citation7 It has been increasingly applied in various clinical fields including psychiatry and neurology. Depending on clinical applications, differential rTMS parameters such as target brain region and frequency have been selected for optimized usage. It has been believed that low frequency (LF) rTMS (≤1 Hz) reduces and high frequency (HF) rTMS (>1 Hz) enhances cortical excitability.Citation7,Citation8 The dorsolateral prefrontal cortex (DLPFC) has been a promising target brain region as a hub mainly involved in emotion and cognitive control.Citation9 It has been suggested that differential clinical effects may occur depending on the hemisphere that is targeted by rTMS.Citation10–Citation12 Considering the previously suggested antidepressant effects of rTMS, a growing number of rTMS studies have recently investigated therapeutic efficacy in treatment-resistant PTSD patients with multiple comorbidities.Citation13 As trauma-exposed individuals with PTSD commonly experience TBI, the neurobiological mechanisms of rTMS applications in PTSD with comorbid TBI needs to be investigated. An integrated information on optimized rTMS protocol for PTSD with comorbid TBI may provide a therapeutic benefit in actual clinical settings.
Multimodal neuroimaging has been used to investigate the pathophysiological correlates of PTSD with comorbid TBI, potentially providing a rationale for optimized rTMS parameters (target site and frequency) for effective treatment. Previous functional neuroimaging studies of PTSD have suggested dysfunctional top-down control (characterized by hypoactive prefrontal and hyperactive limbic regions) as the pathophysiological underpinnings.Citation14–Citation16 Functional and structural neuroimaging studies of TBI revealed altered resting functional connectivity in the frontal region as well as microstructural and macrostructural white matter changes that correlate with clinical dysfunction.Citation17–Citation20 The prefrontal cortex including the DLPFC is suggested as the pathophysiological brain correlate of PTSD with comorbid TBI. PTSD and TBI may be explained by dysfunctional cognitive and emotional control of psychological distress and neurological symptoms following trauma exposure.
The aim of the present study was, thus, to critically review the clinical studies of rTMS in trauma-related conditions. The current review is divided into two main sections of PTSD and PTSD with comorbidities mainly focusing on TBI. The section on PTSD is further divided based on the rTMS parameters, concomitant psychotherapy, main clinical effects, and multiple comorbidities. The section on PTSD with comorbidities focuses on TBI with neurological, cognitive, and psychiatric symptoms, considering that these highly co-occurring trauma-related conditions share pathophysiology and clinical manifestations.
Methods
A literature search was conducted in Google Scholar, PubMed/MEDLINE, and Web of Science using the terms that include “rTMS,” “repetitive transcranial magnetic stimulation,” “trauma,” “posttraumatic stress disorder,” “PTSD,” “TBI,” “traumatic brain injury,” “brain injuries,” and “concussion.” The bibliographies of review articles on rTMS in PTSD and TBI published to date were ensured to be included. Two authors (EN and MK) independently performed the search. The third author (SY) discussed the disagreements and led to consensus.
A total of 30 articles published from 2002 to 2018 were initially searched and 15 of them were excluded due to the following reasons: full-text unavailability, duplication, language written, animal studies, poster presentations, and study design (open-label studies in PTSD patients using rTMS, case studies in PTSD patients using rTMS, and case studies in TBI patients using rTMS). The current review includes a total of 15 studies on rTMS applications in PTSD and TBI: 12 randomized-controlled trials (RCTs) that are sham controlled; one case study; and two open-label studies.
rTMS in PTSD
The potential therapeutic efficacy of rTMS in PTSD has been investigated through a series of pilot studies, case studies, and randomized-controlled trials. All rTMS studies of PTSD reviewed in this review are presented in . The target brain regions and frequency of rTMS applied in the studies are schematically presented in . Considering the heterogeneous nature of treatment-resistant PTSD, the first section on rTMS in PTSD is divided into three sections based on concomitant psychotherapy, main clinical effects, and multiple comorbidities. The rTMS on PTSD with multiple comorbidities main focused on TBI with neurological, cognitive, and psychiatric symptoms given the high co-occurrence of PTSD and TBI.
Figure 1 Schematic representation of the target brain regions according to frequency of rTMS among trauma-exposed individuals in the review: (A) PTSD patients and (B) TBI patients.
Abbreviations: DLPFC, dorsolateral prefrontal cortex; HF, high frequency; L, left; LF, low frequency; PMC, primary motor cortex; PTSD, posttraumatic stress disorder; R, right; rTMS, repetitive transcranial magnetic stimulation; TBI, traumatic brain injury.
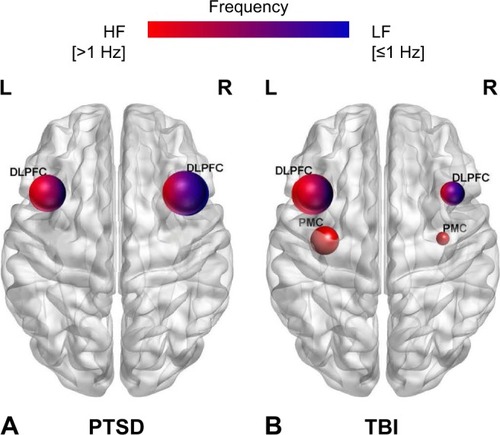
Table 1 rTMS studies in patients with PTSD
Previous functional neuroimaging studies of PTSD have suggested dysfunctional top-down control as the pathophysiological underpinning, providing a rationale for optimized rTMS parameters.Citation14–Citation16 Heightened resting connectivity in the salience network (SN) and default mode network (DMN) has been suggested to underlie compromised threat detection system and emotional dysregulation in PTSD.Citation21 This was further supported by lower gray matter density and white matter integrity in the frontal region as indicated by structural neuroimaging in PTSD.Citation14,Citation22 Diffusion tensor imaging reported microstructural and macrostructural abnormalities, suggesting pathological sensory gating in PTSD.Citation23 Interestingly, preferential right hemispheric involvement in adverse emotion and left hemispheric predominance in episodic memory retrieval have been implicated based on the task-dependent hemispheric activation of previous functional neuroimaging studies in PTSD.Citation24–Citation27
For the treatment of PTSD,Citation9,Citation11,Citation28–Citation35 differential combinations of rTMS parameters have been used to augment psychotherapyCitation29–Citation31 and examine therapeutic effects on anxiety vs depressionCitation32,Citation33 as well as on PTSD with multiple comorbidities.Citation34,Citation35 Most of the rTMS studies of PTSD have suggested LF stimulation over the right DLPFC as an effective treatment for PTSD,Citation28–Citation30,Citation33,Citation35 particularly in treating anxiety-related hyperarousal.Citation30 HF rTMS, mostly applied over the left DLPFC, showed comparable improvement in PTSD and major depressive disorder (MDD) symptoms.Citation32,Citation34 HF rTMS applied to the right frontal regionsCitation32,Citation33 or bilateral frontal regionsCitation31 also improved PTSD core symptoms. Generally, the rTMS treatment for PTSD was well-tolerated without serious adverse events.Citation28,Citation29,Citation32 Mild and transient adverse effects included headache, vertigo, uneasiness, and sleep problem.Citation28,Citation29,Citation32
The right DLPFC, primarily involved in the regulation of negative emotion, has been a promising target region for HF and LF rTMS in PTSD.Citation28,Citation33 LF (≤1 Hz) rTMS treatment over the right DLPFC revealed alleviation in reexperiencing and overall severity of PTSD symptoms.Citation28
Chronic PTSD patients tend to be highly unresponsive to standard pharmacotherapy and psychotherapy, possibly due to high comorbidity and heterogeneity. Therefore, PTSD patients who are chronic and treatment-refractory with high comorbidity have been extensively studied in relation to rTMS. This is particularly true for veterans who are repetitively exposed to traumatic experiences.
It has been reported that more than 50% of the PTSD patients develop MDD, suggesting a high comorbidity rate and shared neurobiological mechanisms between these two common psychiatric disorders.Citation36,Citation37 Since PTSD patients with comorbid MDD show relatively poorer outcomes to standard treatment,Citation38–Citation40 rTMS studies have investigated clinical improvement in treatment-resistant PTSD patients with comorbid MDD.
rTMS for the treatment of PTSD with concomitant psychotherapy
rTMS has been applied to augment standard psychotherapy in treatment-refractory and chronic PTSD patients. Specifically, evidence-based psychotherapies such as cognitive processing therapy (CPT) and prolonged exposure therapy have been recommended in longstanding patients with combat-related PTSD. However, two-thirds of them did not fully remit, necessitating additional rTMS to augment standard psychotherapy.Citation41 It has been believed that standard psychotherapy followed by rTMS treatment elicits traumatic experiences and emotions that are targeted by rTMS.
Two LF (≤1 Hz) rTMS studies have been performed on the right DLPFC to augment psychotherapy in chronic PTSD patients.Citation29,Citation30 These results further provided evidence in support of LF rTMS as an effective treatment for PTSD-related adverse emotions.Citation11
Veterans with chronic combat-related PTSD received 12–15 sessions of LF (≤1 Hz) rTMS to the right DLPFC prior to weekly CPT. CPT aims to alleviate negative emotions during elicitation of traumatic experiences. The active group (rTMS + CPT) revealed greater PTSD symptom reductions across CPT sessions and at 6-month posttreatment assessment as compared to the sham group (sham + CPT).Citation29
In line with this, nine treatment-resistant PTSD patients had 20 sessions of LF (≤1 Hz) rTMS to the right DLPFC combined with exposure therapy in a placebo-controlled crossover study. Each session consisted of a 30-minute active or sham rTMS treatment, an imaginal exposure therapy of discussing personally distressing topics, and a 20-minute active or sham rTMS treatment. The active group with concomitant exposure therapy showed greater improvement in the hyperarousal symptom, higher 24-hour urinary norepinephrine, higher serum thyroxin, and lower serum prolactin relative to the sham group with concomitant exposure therapy. This finding indicates physiological and symptomatic treatment effects of active rTMS combined with exposure therapy.Citation30
Isserles et alCitation31 suggested that effectiveness of deep bilateral HF (20 Hz) rTMS preceded by a script-driven exposure therapy depends on the inclusion of traumatic script. Interestingly, lower intrusion symptom and heart rate response were observed only when traumatic script-driven imagery preceded active rTMS treatment. This finding suggests a need for elicitation booktraumatic memory followed by rTMS treatment in alleviating intrusion symptoms. Treatment-resistant PTSD patients were divided into three treatment groups, and they received 12 sessions of deep HF (20 Hz) repeated TMS (DTMS): group A receiving DTMS after traumatic script-driven imagery, group B receiving DTMS after positive experience-driven imagery, and group C receiving sham DTMS after traumatic script-driven imagery. Group A demonstrated post-rTMS improvement in intrusion symptoms relative to groups B and C. In accordance with this finding, increasingly lower heart rate during cued traumatic re-experiencing was observed only in group A. This was further supported by a strong post-rTMS association between heart rate response and PTSD severity scores.Citation31 One patient in the group A had a self-limited tonic-clonic generalized seizure that was transient and did not require any treatment. Taken together, LF rTMS to the right DLPFC with concomitant psychotherapy has improved hyperarousal and overall PTSD symptoms;Citation29,Citation30 HF rTMS to the bilateral medial prefrontal cortex augmented the preceded exposure therapy that used traumatic script-driven imagery, leading to improvement in intrusion symptoms.Citation31
rTMS for the treatment of PTSD with main clinical effects
Two double-blind placebo-controlled trials compared clinical efficacy of rTMS depending on differential rTMS parameters (target sites – the right DLPFC vs left DLPFC and frequency – HF vs LF). Notably, PTSD-related anxiety symptoms improved with HF rTMS over the right DLPFC. PTSD-related depressive symptoms improved with HF rTMS over the left DLPFC and LF rTMS over the right DLPFC.Citation32,Citation33
A double-blind placebo-controlled trial compared therapeutic efficacy of rTMS depending on two different target sites – the right DLPFC vs left DLPFC. Thirty PTSD patients were divided into three groups – the active right DLPFC group, active left DLPFC group, and sham group – and received 10 sessions of HF (20 Hz) rTMS. The right DLPFC group (48.6%) showed clinical efficacy in overall PTSD symptoms more so than the left DLPFC group (22.8%) at the posttreatment assessment. Verbal fluency also improved after the TMS treatment only in the right DLPFC group. Notably, anxiety symptoms improved particularly in the right DLPFC group, while depressive symptoms demonstrated meaningful improvement only in the left DLPFC group, suggesting differential hemispheric involvement in anxiety vs depression related to PTSD.Citation32
Another double-blind placebo-controlled trial compared high (10 Hz) vs low (≤1 Hz) frequency rTMS treatment over the right DLPFC in 24 PTSD patients. It suggested therapeutic efficacy of HF rTMS in alleviating PTSD core symptoms and anxiety symptoms and LF rTMS in improving depressive symptoms. The HF group demonstrated post-rTMS improvement in hyperarousal, reexperiencing, and avoidance symptoms relative to the LF and sham groups. Scores in the PTSD checklist decreased in the high (29.3%) vs low (10.4%) frequency group at posttreatment. Interestingly, the HF group revealed marked improvement in anxiety, but not in depression, as compared to the LF and sham groups. One patient in LF group and one in HF group reported a transient manic episode after receiving rTMS. Other adverse events included muscular discomfort (n=2), transient ear discomfort, dizziness (n=1), and a mild rage attack (n=1) that did not require additional treatments.Citation33 Taken together, HF rTMS to the right DLPFC improved anxiety and overall PTSD symptoms of PTSD, along with verbal fluency, while LF rTMS to the right DLPFC and HF rTMS to the left DLPFC improved depressive symptoms of PTSD.Citation32,Citation33
rTMS for the treatment of PTSD with multiple comorbidities
Due to the heterogeneous nature of psychiatric disorders, PTSD patients with comorbid MDD also had mild TBI (mTBI) and other psychiatric comorbidities including panic disorder (PD), AUD, obsessive-compulsive disorder (OCD), borderline personality disorder (BPD), social anxiety disorder (SAD), and eating disorder. Considering a suggested relationship between comorbidity and treatment resistance in PTSD, rTMS studies have also examined PTSD patients with multiple comorbidities that include depression.Citation34,Citation35
Patients with PTSD, mTBI, or both who had a depressive episode received nine sessions of HF (10 Hz) rTMS over the left DLPFC. The active group transiently showed lower suicidal ideation relative to the sham group only at post-rTMS day 1 vs baseline. One patient discontinued the treatment due to erythema and second-degree burn at the coil stimulation site, but fully recovered with the appropriate treatment.Citation34 PTSD patients with comorbid eating disorder also had other psychiatric disorders including MDD, BPD, PD, and SAD. PTSD patients with comorbid psychiatric disorders including MDD, PD, and OCD demonstrated improvement in PTSD and MDD symptoms after active LF (≤1 Hz) rTMS to the right DLPFC.Citation35 Overall, PTSD patients with multiple comorbidities including depression were treated using various combinations of rTMS parameters.
rTMS in TBI
As the prevalence of PTSD with comorbid TBI is increasing in the general population along with the rates of crimes and accidents, effective prevention and intervention strategies are necessitated. However, the neurobiological underpinnings for PTSD with comorbid TBI have not yet been clearly investigated, resulting in high rates of treatment resistance and chronicity. As the need to deepen the understanding of neurobiological mechanisms about the therapeutic efficacy of rTMS is of utmost importance in treatment-resistant patients with PTSD and/or TBI, we focused on rTMS applications of TBI among PTSD-related comorbidities.
The potential therapeutic efficacy of rTMS in TBI has been investigated through a series of pilot studies, case studies, and randomized-controlled trials. All rTMS studies of TBI reviewed in this review are presented in . The target brain regions and frequency of the stimulation applied in the studies are schematically presented in . The rTMS studies of TBI presented in this review mainly focused on the three most common clinical symptoms following TBI, which are neurological, cognitive, and psychiatric symptoms, in consideration of the complicated pathophysiology of TBI. The section on the TBI-related neurological symptoms are further divided into subsections of headache, central pain, altered consciousness, and tinnitus subsequent to mild or severe TBI, given the high prevalence and multiple subtypes of TBI-related neurological symptoms.
Table 2 rTMS studies in patients with TBI
Altered resting functional connectivity in the frontal region as well as microstructural and macrostructural white matter changes that correlate with clinical dysfunction underlying TBI may provide insights regarding the optimized rTMS parameters in TBI-related conditions.Citation17–Citation20 Altered functional connectivity in the DMN and lower integrity in the corpus callosum were reported in previous neuroimaging studies of mTBI.Citation17,Citation18 Moderate to severe TBI patients have shown lower resting functional connectivity in the SN and frontoparietal network, indicating dysfunctions in self-awareness and attention, as well as widespread white matter loss.Citation19,Citation20
Most rTMS studies of TBI have focused on the DLPFC as the target brain region as well as HF to facilitate clinical dysfunction. With regard to neurological symptoms, HF rTMS applied over the left DLPFC or left primary motor cortex (PMC) alleviated mTBI-related headache.Citation42–Citation45 HF rTMS also improved central pain related to mTBICitation46 as well as altered consciousness related to severe TBI.Citation47 HF rTMS over the left hemisphere improved cognitive dysfunction in mTBICitation45 and severe TBI patients.Citation48 HF rTMS to the left DLPFC improved depression in mTBI.Citation43,Citation44 The rTMS treatment for TBI was generally well-tolerated without serious adverse events; mild and transient adverse events included headache, dizziness, eye twitch, and muscular discomfort.Citation46
rTMS for the treatment of TBI-related neurological symptoms
rTMS has been increasingly applied in TBI-related headache that often becomes treatment-refractory to standard treatment despite its high prevalence. In four recent studies, an active HF (10 Hz) rTMS treatment over the left PMC and/or left DLPFC showed therapeutic efficacy in relieving mTBI-related headache.Citation42–Citation45
A case study reported lower intensity, duration, and frequency of mild TBI headache (mTBI-HA) after HF (10 Hz) rTMS over the left PMC and left DLPFC. Six patients who completed rTMS treatment reported 53.1% decrease in headache intensity and 79% decrease in headache exacerbation frequency. Among the completers, four patients with persistent headache exacerbations reported 50% and 31% reduction in duration and intensity of the exacerbation, respectively. Complete cessation of severe headache episodes was reported by two patients.Citation42 Although HF rTMS to the left PMC and left DLPFC both showed therapeutic efficacy on mTBI-related headache, a different brain pathway was suggested to be involved for each target region.Citation44
To confirm the potential therapeutic effects in the case study, Leung et alCitation43,Citation44 performed two other randomized controlled-trials in veterans with chronic MTBI-HA. The patients received active vs sham HF (10 Hz) rTMS over the left PMC. The active group (58.3%) reported at least 50% lower intensity of headache compared to the sham group (16.6%) at 1-week follow-up assessment. The active group also demonstrated lower scores of functionally debilitating headache relative to the sham group at 4-week follow-up assessment, suggesting long-lasting effects of the rTMS treatment in relieving mTBI-related headache.Citation43
In a most recent study, veterans with MTBI-HA were randomized into the active vs sham group and received HF (10 Hz) rTMS over the left DLPFC. The active group reported 50% and 57% reduction in the prevalence of persistent headache at 1 week and at 2 weeks following the rTMS completion, respectively. In contrast, the sham group reported 7% and 20% reduction at 1-week and 2-week follow-up assessments, respectively.Citation44
mTBI patients with post-concussive symptoms received 20 sessions of HF (10 Hz) rTMS to the left DLPFC in an open-label study. The post-concussive symptoms including headache, depression, sleep deprivation, and cognitive impairment were examined at pre-rTMS, post-rTMS, and post 3-month follow-up assessment. Those who completed the treatment showed improvement in sleep functioning, mental focus, and particularly headache. The adverse events reported included increased sleep problems (n=3), anxiety about the treatment (n=1), an episode of vertigo (n=1), and headache (n=3).Citation45 HF (10 Hz) rTMS to the left PMC and/or left DLPFC have improved mTBI-related headache.Citation42–Citation45
A pilot study suggested that HF (10 Hz) rTMS over the PMC of the affected hemisphere may alleviate mTBI-related central pain, thus improving quality of life of those affected. Twelve mTBI patients with central pain revealed partial injury in the spinothalamocortical tracts. They received either 10 sessions of rTMS treatment or sham rTMS treatment over the PMC of the affected hemisphere. The active group reported lower intensity of central pain and greater improvement in physical and mental health status during and after the rTMS treatment sessions when compared to the sham group.Citation46
Three patients with minimally conscious state (MCS) and other three with vegetative state received a single HF (20 Hz) rTMS session to the bilateral PMC. After the rTMS treatment, only one patient with MCS showed long-lasting improvement in consciousness and motor excitability as manifested by EEG. All other patients showed greater motor excitability, lower motor threshold, as well as a trend toward improvement in consciousness. This finding suggests that a single HF rTMS session may not be enough to restore consciousness in severe TBI.Citation47 Whether severe TBI patients in minimally conscious or vegetative state may restore unconsciousness after HF rTMS remains debated.Citation47
rTMS for the treatment of TBI-related cognitive symptoms
rTMS, mostly HF stimulation over the left hemisphere improved cognitive dysfunction in severe TBI patients.Citation48 Yet, some of the mTBI patients did not show cognitive enhancement after HF rTMS over the left hemisphere.Citation43,Citation44
mTBI patients with somatic, psychiatric, and cognitive impairments received HF (10 Hz) rTMS over the left DLPFC and reported cognitive enhancement in working memory, executive function, processing speed, and attention. Moreover, a task-related functional magnetic resonance imaging showed that this cognitive improvement was related to stronger activation in the DLPFC and deactivation in the anterior cingulate cortex (ACC) during a verbal and nonverbal working memory task at post-rTMS vs pre-rTMS.Citation45
mTBI patients with headache did not report cognitive enhancement after the HF (10 Hz) rTMS treatment to the left DLPFC.Citation44 In line with this, attention preservation transiently improved at 1 week after completing HF (10 Hz) rTMS over the left PMC, but this cognitive enhancement did not last long.Citation43
HF rTMS to the left DLPFC may improve cognitive dysfunction related to severe TBI. Severe TBI patients with diffuse axonal injury underwent 10 sessions of HF (10 Hz) rTMS over the left DLPFC. The completers demonstrated improvement in mood, cortical excitability, and cognition such as executive function and non-verbal memory.Citation48 These results are consistent with the previous HF rTMS studies that have shown cognitive enhancement through stimulation of the hypoactive brain regions.Citation49,Citation50 Overall, HF rTMS to the left frontal regions has improved cognitive dysfunction related to mTBICitation45 and severe TBI.Citation48
rTMS for the treatment of TBI-related psychiatric symptoms
Three rTMS studies suggested that LF rTMS to the right DLPFC, HF rTMS to the left DLPFC, or both may improve depression in mTBI.Citation34,Citation43,Citation44 These results are in line with the pathophysiological correlates that previous rTMS studies of MDD have suggested.Citation51,Citation52
Leung et alCitation44 suggested transient antidepressant effects of HF (10 Hz) rTMS treatment to the left DLPFC in mTBI patients with headache. The active group showed lower depressive symptoms than the sham group at 1-week follow-up. A trend toward lower depressive symptoms was observed in the active group than in the sham group at 2-week follow-up, suggesting decreasing antidepressant effects over time. However, the two groups did not show differences in PTSD severity at pre-rTMS vs post-rTMS.Citation44 These findings consistent with the HF (10 Hz) rTMS treatment may stimulate and normalize the hypoactive left DLPFC responsible for emotional dysregulation in MDD.Citation53–Citation55 This finding contrasts with the finding that HF (10 Hz) rTMS therapy over the left PMC did not improve depression in mTBI patients with headache.Citation43 These contrasting results of the two studies further add evidence in support of the left DLPFC as the neural correlate underlying depression.Citation43
Yet, suicidal ideation was not improved in mTBI patients with PTSD and a depressive episode, who received HF rTMS to the left DLPFC. Lower suicidal ideation was reported only at day 1 after completing nine sessions of HF (10 Hz) rTMS treatment to the left DLPFC. At 6-month follow-up assessment, no one committed a suicide. The reported adverse events included erythema (n=1) that was appropriately treated and recovered as well as temporary eye pain (n=1) and jaw pain (n=1).Citation34
Conclusion
The present study reviews the applications of rTMS in trauma-related conditions divided into PTSD and PTSD with comorbidities. Among PTSD-related comorbidities, TBI mainly co-occurs with PTSD and may increase prognostic factors for chronicity, leading to substantial disability and socioeconomic burden. This review provides a clue regarding the optimized rTMS parameters for the trauma-exposed individuals with comorbid PTSD and TBI that share common etiology and pathophysiology. It is generally agreed that LF rTMS to the right DLPFC effectively improves PTSD-related anxiety symptoms and HF rTMS to the left DLPFC improves headache, cognitive dysfunction, and depression related to TBI. Thus, the present review suggests the DLPFC to be a promising target brain region for rTMS interventions on trauma-related conditions. The current review supports that the DLPFC plays a pivotal role in emotion and cognitive control of psychological distress and neurological symptoms following trauma exposures.
The present critical review on rTMS applied in trauma-related conditions provides insights into protective and risk factors for the development of PTSD and/or TBI in trauma-exposed individuals. Taken together, rTMS studies reviewed in this article generally demonstrated therapeutic efficacy on PTSD symptoms with multiple comorbidities as well as neurological, cognitive, and psychiatric symptoms related to TBI without serious adverse events. Although there is some inconsistency depending on the characteristics and main symptoms of the patients, rTMS has been mostly applied to normalize the frontal dysfunction related to PTSD and TBI through HF over the hypo-active or LF over the hyper-active brain regions. This may provide a clue regarding the optimized rTMS parameters for the trauma-exposed individuals with comorbid PTSD and TBI.
Clinical outcomes and applied parameters of rTMS have rather been inconsistent, in part, because the pathophysiology underlying PTSD and TBI is multifaceted and complex. Furthermore, differences in sample size, experimental paradigms, comorbidity, concomitant therapy, as well as duration, type, and/or severity of illnesses may potentially explain these discrepancies. Alternatively, future larger longitudinal studies with optimized experimental designs are needed to control these factors. Moreover, an integration of multimodal neuroimaging with rTMS may offer an expansive view of neurobiological mechanisms underlying PTSD and TBI, potentially providing an important insight into the early prevention and intervention. Future systematic review and meta-analysis are warranted to investigate clinical efficacy of rTMS in trauma-related conditions with confidence interval and odds ratio of randomized-controlled trials that used neuroimaging.
Acknowledgments
This research was supported by the Brain Research Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (2015M3C7A1028373), from the ICT R&D program of Institute for Information & Communications Technology Promotion (B0132-15-1001), by the Field-oriented Support of Fire Fighting Technology Research and Development Program funded by the National Fire Agency (MPSS-Fire Fighting Safety-2016-86), and by the National Institute on Drug Abuse (R01DA024070).
Disclosure
The authors report no conflicts of interest in this work.
References
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders (DSM-5 TM)5th edArlingtonAmerican Psychiatric Publishing2013
- American Congress of Rehabilitation MedicinesDefinition of mild traumatic brain injuryJ Head Trauma Rehabil199388687
- VanderploegRDBelangerHGCurtissGMild traumatic brain injury and posttraumatic stress disorder and their associations with health symptomsArch Phys Med Rehabil20099071084109319577020
- KesslerRCSonnegaABrometEHughesMNelsonCBPosttraumatic stress disorder in the National comorbidity surveyArch Gen Psychiatry19955212104810607492257
- BissonJAndrewMPsychological treatment of post-traumatic stress disorder (PTSD)Cochrane Database Syst Rev20073CD003388
- RetiIMSchwarzNBowerATibbsMRaoVTranscranial magnetic stimulation: a potential new treatment for depression associated with traumatic brain injuryBrain Inj2015297–878979725950260
- GeorgeMSRamanRBenedekDMA two-site pilot randomized 3 day trial of high dose left prefrontal repetitive transcranial magnetic stimulation (rTMS) for suicidal inpatientsBrain Stimul20147342143124731434
- RosaMALisanbySHSomatic treatments for mood disordersNeuropsychopharmacology201237110211621976043
- PhilipNSBarredoJvan’t Wout-FrankMTyrkaARPriceLHCarpenterLLNetwork mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorderBiol Psychiatry201883326327228886760
- IannoneACruzApdemBrasil-NetoJPBoechat-BarrosRTranscranial magnetic stimulation and transcranial direct current stimulation appear to be safe neuromodulatory techniques useful in the treatment of anxiety disorders and other neuropsychiatric disordersArq Neuropsiquiatr2016741082983527759809
- TillmanGDKimbrellTACalleyCSKrautMAFreemanTWHartJJrRepetitive transcranial magnetic stimulation and threat memory: selective reduction of combat threat memory p300 response after right frontal-lobe stimulationJ Neuropsychiatry Clin Neurosci2011231404721304137
- WassermannEMLisanbySHTherapeutic application of repetitive transcranial magnetic stimulation: a reviewClin Neurophysiol200111281367137711459676
- HerroldAAKletzelSLHartonBCChambersRAJordanNPapeTLTranscranial magnetic stimulation: potential treatment for co-occurring alcohol, traumatic brain injury and posttraumatic stress disordersNeural Regen Res20149191712173025422632
- RauchSLShinLMPhelpsEANeurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research-past, present, and futureBiol Psychiatry200660437638216919525
- BrewinCRWhat is it that a neurobiological model of PTSD must explain?Prog Brain Res200816721722818037017
- KoenigsMHueyEDRaymontVFocal brain damage protects against post-traumatic stress disorder in combat veteransNat Neurosci200811223223718157125
- JohnsonBZhangKGayMAlteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI studyNeuroimage201259151151821846504
- ZhouYMilhamMPLuiYWDefault-mode network disruption in mild traumatic brain injuryRadiology2012265388289223175546
- HamTEBonnelleVHellyerPThe neural basis of impaired self-awareness after traumatic brain injuryBrain2014137258659724371217
- SharpDJBeckmannCFGreenwoodRDefault mode network functional and structural connectivity after traumatic brain injuryBrain201113482233224721841202
- KochSBvan ZuidenMNawijnLFrijlingJLVeltmanDJOlffMAberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic reviewDepress Anxiety201633759260526918313
- KimMJLyooIKKimSJDisrupted white matter tract integrity of anterior cingulate in trauma survivorsNeuroreport200516101049105315973146
- OlsonEACuiJFukunagaRNickersonLDRauchSLRossoIMDisruption of white matter structural integrity and connectivity in posttraumatic stress disorder: a TBSS and tractography studyDepress Anxiety201734543744528294462
- RauchSLvan der KolkBAFislerREA symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imageryArch Gen Psychiatry19965353803878624181
- SimmonsAMatthewsSCSteinMBPaulusMPAnticipation of emotionally aversive visual stimuli activates right insulaNeuroreport200415142261226515371746
- WhalleyMGKroesMCHuntleyZRuggMDDavisSWBrewinCRAn fMRI investigation of posttraumatic flashbacksBrain Cogn201381115115923207576
- NardoDHögbergGFlumeriFSelf-rating scales assessing subjective well-being and distress correlate with rCBF in PTSD-sensitive regionsPsychol Med201141122549256121672299
- NamDHPaeCUChaeJHLow-frequency, repetitive transcranial magnetic stimulation for the treatment of patients with posttraumatic stress disorder: a double-blind, sham-controlled studyClin Psychopharmacol Neurosci20131129610224023554
- KozelFAMotesMADidehbaniNRepetitive TMS to augment cognitive processing therapy in combat veterans of recent conflicts with PTSD: a randomized clinical trialJ Affect Disord201822950651429351885
- OsuchEABensonBELuckenbaughDAGeraciMPostRMMccannURepetitive TMS combined with exposure therapy for PTSD: a preliminary studyJ Anxiety Disord2009231545918455908
- IsserlesMShalevAYRothYEffectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder-a pilot studyBrain Stimul20136337738322921765
- BoggioPSRochaMOliveiraMONoninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorderJ Clin Psychiatry201071899299920051219
- CohenHKaplanZKotlerMKoupermanIMoisaRGrisaruNRepetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled studyAm J Psychiatry2004161351552414992978
- GeorgeMSRamanRBenedekDMA two-site pilot randomized 3 day trial of high dose left prefrontal repetitive transcranial magnetic stimulation (rTMS) for suicidal inpatientsBrain Stimul20147342143124731434
- WattsBVLandonBGroftAYoung-XuYA sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorderBrain Stimul201251384322264669
- RytwinskiNKScurMDFeenyNCYoungstromEAThe co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysisJ Trauma Stress201326329930923696449
- FloryJDYehudaRComorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerationsDialogues Clin Neurosci201517214115026246789
- HoltzheimerPERussoJZatzickDBundyCRoy-ByrnePPThe impact of comorbid posttraumatic stress disorder on short-term clinical outcome in hospitalized patients with depressionAm J Psychiatry2005162597097615863800
- CampbellDGFelkerBLLiuCFPrevalence of depression-PTSD comorbidity: implications for clinical practice guidelines and primary care-based interventionsJ Gen Intern Med200722671171817503104
- DunnerDLAaronsonSTSackeimHAA multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up periodJ Clin Psychiatry201475121394140125271871
- SteenkampMMLitzBTHogeCWMarmarCRPsychotherapy for military-related PTSD: a review of randomized clinical trialsJAMA2015314548950026241600
- LeungAFallahAShuklaSrTMS in alleviating mild TBI related headaches–a case seriesPain Physician2016192E347E35426815263
- LeungAShuklaSFallahARepetitive transcranial magnetic stimulation in managing mild traumatic brain injury-related headachesNeuromodulation: Technology at the Neural Interface2016192133141
- LeungAMetzger-SmithVHeYLeft dorsolateral prefrontal cortex rTMS in alleviating MTBI related headaches and depressive symptomsNeuromodulation201821439040128557049
- KoskiLKolivakisTYuCChenJKDelaneySPtitoANoninvasive brain stimulation for persistent postconcussion symptoms in mild traumatic brain injuryJ Neurotrauma2015321384424955920
- ChoiGSKwakSGLeeHDChangMCEffect of high-frequency repetitive transcranial magnetic stimulation on chronic central pain after mild traumatic brain injury: a pilot studyJ Rehabil Med201850324625229392332
- ManganottiPFormaggioEStortiSFEffect of high-frequency repetitive transcranial magnetic stimulation on brain excitability in severely brain-injured patients in minimally conscious or vegetative stateBrain Stimul20136691392123928101
- NevilleISHayashiCYEl HajjSARepetitive transcranial magnetic stimulation (rTMS) for the cognitive rehabilitation of traumatic brain injury (TBI) victims: study protocol for a randomized controlled trialTrials201516144026438108
- LevkovitzYRabanyLHarelEVZangenADeep transcranial magnetic stimulation add-on for treatment of negative symptoms and cognitive deficits of schizophrenia: a feasibility studyInt J Neuropsychopharmacol201114799199621524336
- Drumond MarraHLMyczkowskiMLMaia MemóriaCTranscranial magnetic stimulation to address mild cognitive impairment in the elderly: a randomized controlled studyBehavioural Neurology201520151113
- BrunoniARChaimaniAMoffaAHRepetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysisJAMA Psychiatry201774214315228030740
- PhilipNSBarredoJAikenECarpenterLLNeuroimaging mechanisms of therapeutic transcranial magnetic stimulation for major depressive disorderBiol Psychiatry Cogn Neurosci Neuroimaging20183321122229486862
- BaekenCDe RaedtRvan HoveCClerinxPDe MeyJBossuytAHF-rTMS treatment in medication-resistant melancholic depression: results from 18FDG-PET brain imagingCNS Spectr200914843944819890238
- ListonCChenACZebleyBDDefault mode network mechanisms of transcranial magnetic stimulation in depressionBiol Psychiatry201476751752624629537
- KangJILeeHJhungKFrontostriatal connectivity changes in major depressive disorder after repetitive transcranial magnetic stimulation: a randomized sham-controlled studyJ Clin Psychiatry2016779e1137e114327379563
