Abstract
Background
Both protective and therapeutic functions of insulin-like growth factor-1 (IGF-1) in brain injury have been reported, but its effects on cognitive sequelae after septic encephalopathy (SE) remain unclear.
Materials and methods
This study was divided into three parts, and a septic model was built by cecal ligation and puncture (CLP). First, survival analysis was performed, and IGF-1’s effects on long-term cognition and depressive emotion were assessed. Second, the characteristics of IGF-1 function in cognition were evaluated. Finally, cytochrome C, caspase-9, tumor necrosis factor receptor (TNFR), and caspase-8 levels as well as cell apoptosis in the hippocampus were evaluated.
Results
IGF-1 did not reduce mortality or alleviate depressive symptoms in septic rats, but improved the memory of noxious stimulation and spatial learning and memory. These effects were observed only when IGF-1 was administered within 0–6 hours after CLP. Moreover, cytochrome C and caspase-9 expression levels were increased at 6 hours after CLP in the hippocampus, while TNFR and caspase-8 amounts were not increased until 12 hours after CLP. Cell apoptosis increased at 12 hours after CLP, but was inhibited by IGF-1.
Conclusion
Cognitive impairment in rats recovering from SE is associated with cell apoptosis in the hippocampus. Supplementation of IGF-1 reduces cell apoptosis by preventing the over-expression of cytochrome C and TNFR, and results in improved cognitive function. However, improvement only occurs when IGF-1 is administered at the early stage (within 6 hours) of sepsis. As cytochrome C activation occurs earlier than that of TNFR in this study, cytochrome C may be the main factor inducing apoptosis in early SE.
Introduction
Sepsis is a systemic inflammation caused by severe infection.Citation1–Citation3 Although effective treatments, including administration of antibiotics, antithrombin, and β-2 agonists,Citation4–Citation7 fluid resuscitation and assessment of hemodynamics,Citation8,Citation9 and prophylaxis of stress ulcer and transfusion of red blood cells,Citation10,Citation11 have been adopted to reduce mortality in sepsis, many survivors still suffer from septic encephalopathy (SE), a neurological complication of sepsis characterized by short- and long-term symptoms.Citation12–Citation14 Long-term cognitive impairment and psychiatric disorders, such as memory deficits, learning disabilities, and depressive-like symptoms (not anxiety-like symptoms), can seriously lower the patient’s quality of daily life.Citation12,Citation15,Citation16 Thus, the neurological sequelae caused by sepsis remain important medical problems, and new therapies targeted on them are urgently needed.
Studies indicate that two pathways, initiated by tumor necrosis factor receptor (TNFR) and cytochrome C, respectively, participate in the activation of cell apoptosis, including in hippocampal CA1 neurons.Citation17,Citation18 Thus, we hypothesized that the above neurological sequelae may be related to cell apoptosis, with TNFR and cytochrome C constituting the key factors promoting cell apoptosis in SE. IGF-1, mainly produced by the liver, has important functions on body inflammation and immunity and brain cell differentiation, proliferation, and maturation.Citation19–Citation23 Previous findings indicate that insulin-like growth factor-1 (IGF-1) may be an important anti-apoptosis factor.Citation24 Administration of IGF-1 is associated with reduced oligoden-drocyte apoptosis caused by a variety of insults.Citation25–Citation27 Guan et al found that intraventricular injection of IGF-1 reduces neuronal apoptosis in the hippocampus and has a potential therapeutic impact on hypoxic-ischemic brain injury.Citation28 However, tissue IGF-1 amounts are mainly regulated by serum growth hormone (GH) levels and are decreased during inflammation, especially in sepsis, due to GH secretion and resistance.Citation29,Citation30 Upregulation of proinflammatory cytokines attenuates IGF-1 bioactivity by upregulating insulin-like growth factor binding proteins (IGFBPs), mainly IGFBP-1. Moreover, inflammation of the central nervous system (CNS) suppresses microglia-derived neuronal IGF-1 production.Citation31 All these factors jointly attenuate the anti-apoptosis effect of IGF-1 and make brain cells more sensitive to apoptotic stimuli.Citation32,Citation33
As IGF-1 shows anti-apoptotic benefits and its concentration and bioactivity are decreased in sepsis, it may constitute a potential factor for SE treatment. In this study, the effects of IGF-1 on cognitive deficits and cell apoptosis in the hippocampus of septic rats were clarified. Then, we investigated whether the anti-apoptotic effect of IGF-1 is associated with cytochrome C and TNFR regulation.
Materials and methods
Animals and animal care
All studies were performed in accordance with National Institutes of Health guidelines and approved by the Animal Ethical Committee of Air Force Medical University. The male Sprague Dawley rats (n=435) ranging from 220 to 250 g were obtained from the laboratory animal center of Air Force Medical University. Rats were housed under a 12-hour light–dark cycle with free access to food and water.
Cecal ligation and puncture (CLP) surgery
Rats were deeply anesthetized with ketamine (90 mg/kg body weight) and xylazine (10 mg/kg body weight), and sepsis was induced by CLP as described previously.Citation34 Briefly, lower quadrants of the abdomen were shaved and disinfected with alcohol. A longitudinal midline incision was then made, and the cecum was exteriorized from the abdominal cavity. Cecal contents were pushed to the distal side. Then, ligation was performed between the ileocecal valve and the distal cecum by using a 3-0 nylon filament. As the severity of sepsis is controlled by ligation at different sites between the ileocecal valve and the distal cecum, moderate sepsis was adopted in this study by ligation at the middle of the cecum followed by puncture from side to side at the middle of the ligated cecum. Small droplets of feces were extruded from both holes, and the cecum was then returned to the abdominal cavity. The peritoneum and the skin were closed by simple interrupted sutures. In the sham-operation group, a similar procedure was performed but without ligation and puncture of the cecum. All rats were returned to their cages and housed under a 12-hour light–dark cycle with free access to food and water.
Experimental groups and drug treatment
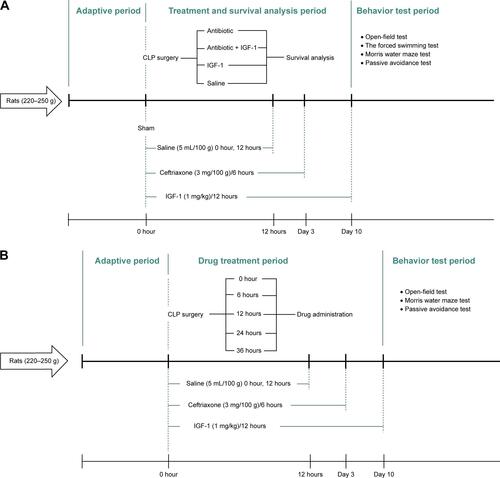
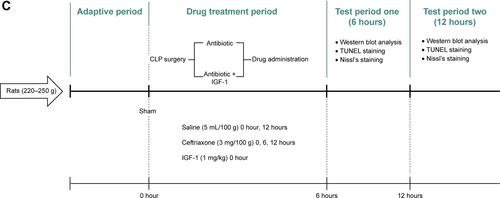
The study was divided into three parts and all rats were subcutaneously injected with saline (37°C, 5 mL/100 g) immediately and 12 hours after surgery. The first part, designed to evaluate the effect of IGF-1 on cognition of septic rats, was divided into five groups, that is sham-operative (n=15), antibiotic (n=30), antibiotic + IGF-1 (n=30), IGF-1 (n=30), and saline (n=30) groups. Rats in the antibiotic and antibiotic + IGF-1 groups were subcutaneously injected with ceftriaxone (Hoffman-La Roche Ltd., Basel, Switzerland) (3 mg/100 g) every 6 hours for 3 days. Rats in the antibiotic + IGF-1 and IGF-1 groups were subcutaneously injected with IGF-1 (ProsPec, Rehovot, Israel) (1 mg/kg) immediately and every 12 hours after surgery for 10 days (). The second part, also divided into five groups, was designed to study the feature of IGF-1’s effects on cognition of septic rats. Antibiotics were given to all rats, but IGF-1 was given at 0 hours (n=30), 6 hours (n=30), 12 hours (n=30), 24 hours (n=30), or 36 hours (n=30) after surgery in each group, respectively, and then supplied every 12 hours for 10 days (). The third part, designed to study the mechanisms of IGF-1’s function on cognition of septic rats, consisted of sham-operative (n=30), antibiotic (n=60), and antibiotic + IGF-1 (n=60) groups. Each group was divided into two subgroups, one for Western blot at 6 hours (n=10 in the sham-operation group; n=20 in both antibiotic and antibiotic + IGF-1 groups) and 12 hours (n=10 in the sham-operation group; n=20 in both antibiotic and antibiotic + IGF-1 groups) after surgery, and another for TUNEL and Nissl’s staining (n=10 in the sham-operation group; n=20 in both antibiotic and antibiotic + IGF-1 groups) 12 hours after surgery. Antibiotics were given immediately and then at 6 hours and 12 hours after surgery, respectively. IGF-1 (1 mg/kg) was given immediately after surgery in the sham-operated and antibiotic + IGF-1 groups (). The dosage selection of IGF-1 was based on previous studies in mice and rats.Citation35,Citation36
Behavioral trial
Behavioral tests were performed 10 days after surgery at 8:00–11:30 am with the instruments provided by the Department of Neurosurgery (Tangdu Hospital, Army Force Medical University, Xi’an, Shannxi, China). Tests were conducted and results were recorded by two colleagues who were blinded to the research.
Open-field tests
The open field was 40×60 cm and surrounded by 50 cm high walls made of black plywood. The ground floor was divided into nine equally spaced squares by black lines. After rats were fully accustomed to the experimental environment, they were placed in the middle of the floor and were allowed to move freely for 5 minutes (training session). Then all rats were put back into cages and at the same time on the second day, they received a similar training in another open field (testing session). The total number of crossings (horizontal activity) and rearings (standing upright), and the total time spent in the center and corner of the open field were recorded. At the end of each test, any olfactory cues on the surface of the arena were carefully eliminated.
The forced swimming test
A cylindrical tank made of transparent glass was used in this test. Rats cannot touch the bottom of the tank or escape when the tank was full of water. At the beginning of tests, water (23°C) was filled into the tank to a depth of 50 cm, and rats were placed in the water to swim for 15 minutes. Then rats were put back into cages and at the same time on the second day, they received a similar test for 5 minutes. In the second test, the periods of immobility, swimming, and struggling time were recorded and depressive-like behavior was evaluated by the immobility time. At the end of each test, water was replaced in order not to affect the behavior of the subsequent animals.
Morris water maze tests
A water maze pool full of opaque water and an escape platform (submerged 2.5 cm, not visible) was employed to evaluate the spatial learning and memory ability of rats. The escape platform was placed in one quadrant of the pool. Rats were randomly released on one side of the pool and 1 minute was given to find the platform. Once finding the platform, they were allowed to stay there for 30 seconds. All rats were trained four times per day for 4 consecutive days, and the time to reach the platform (latency) was recorded. The next day of the last training, rats started to swim from the same place without the platform. The number of “times” rats arrived at the quadrant within 1 minute was recorded.
Passive avoidance test
The apparatus consists of a light and a dark compartment (30×30×30 cm3) with a circular door on the wall between them. The floor of the dark compartment was electrifiable. Rats were fully accustomed to the experimental environment and then placed in the light room. When the rats stepped into the dark room (four paws), a 0.8 mA, 4.0-second scrambled foot shock was applied for 15 seconds and then rats were taken back to cages (training session). At the same time on the second day, rats received the same training (testing session), and the time they spent in the training and testing sessions to enter the dark room was recorded (latency). When rats did not enter the dark room for over 120 seconds, a time of 120 seconds was recorded.
Western blot analysis
Rats were killed by decapitation at 6 and 12 hours after CLP and hippocampus was immediately isolated on ice and stored at −80°C for further analysis. Samples were homogenized in lysis buffer containing phenylmethanesulfonyl fluoride for 30 minutes and then centrifuged (12,000 rpm) for 20 minutes. Protein quantitation was performed by using bicinchoninic acid protein assay kit (Beyotime, Shanghai, China). The samples were separated using 12% SDS-PAGE and then electrophoresis was adopted to transfer the samples to nitrocellulose membrane. Then, the membranes were blocked using 5% skim milk for 1 hour, and incubated overnight at 4°C with the appropriate primary rabbit anti-rat cytochrome C antibody (1:200; Protein Tech, Chicago, IL, USA), anti-rat caspase-9 antibody (1:300; Protein Tech), anti-rat TNFR1 antibody (1:300; Protein Tech), and anti-rat caspase-8 antibody (1:200; Protein Tech). After washing with Tris-buffered saline with Tween-20, membranes were incubated with horseradish peroxidase-conjugated appropriate secondary antibodies (1:2,000; Jackson, West Grove, PA, USA) for 1 hour. The membranes were washed and the blots were visualized using enhanced chemiluminescence reagents (EMD Millipore, Billerica, MA, USA) and Kodak (Fuji, Japan) radiography film. Bands were digitally scanned and analyzed by using ImageJ software, and the intensity signal of each band in each group was then recorded for statistical comparisons.
TUNEL staining
Rats were killed 12 hours after CLP. Brains were isolated and fixed in formalin for 48 hours. Tissues were embedded in paraffin and sliced into 5 µm sections. After deparaffinization and dexylenation, tissues were stained with fluorescein-dUTP for apoptotic cell nuclei according to the manufacturer’s instructions of In Situ Cell Death Detection Kit (Hoffman-La Roche Ltd). The nuclei were stained with DAPI (1:200; Thermo Fisher Scientific, Waltham, MA, USA) for 10 minutes. The ratio of apoptotic cells in five microscopic fields in the hippocampus region was evaluated manually by two colleagues blinded to the study by using fluorescent microscope (Olympus Corporation, Tokyo, Japan).
Nissl’s staining
To further exhibit the morphology of neuron and quantify it, Nissl’s staining was adopted. Neurons without apoptotic structural changes were counted, and criteria included the presence of a round, open, pale nucleus (not condensed and darkly stained) and granular Nissl staining of the cytoplasm. Early apoptosis of neuron was defined by nascent chromatin condensation into mini-masses, and end-stage apoptosis of neuron was characterized by the presence of at least two discrete, large round nuclear masses. According to these criteria, astrocytes, oligodendrocytes, and microglia were excluded from the counts. One percent toluidine blue was preheated to 50°C and then the samples for TUNEL staining were hydrated in toluidine blue for 20 minutes in a 56°C thermotank. Then they were washed in water and differentiated in 70% ethanol for 1 minute and 95% ethanol for 30 seconds. Then they were dehydrated in gradient ethanol and cleaned with xylene. Five microscopic fields in hippocampus were then selected under optical microscope (Olympus) to take count of the ratio of apoptotic neurons manually by two colleagues blinded to the study.
Statistical analysis
Statistical analysis was performed with SPSS Statistics (SPSS 20; SPSS, Inc, Chicago, IL, USA) and all values are presented as mean ± SD. The survival rate was calculated by the Kaplan–Meier method and compared by log-rank test. The comparisons among multiple groups were performed by one-way classification of ANOVA followed by Bonferroni’s test. The comparisons between two groups were performed by Student’s t-test. Nonparametric statistics were treated by Kruskal–Walls H-test followed by Nemenyi test. Differences were considered statistically different when P-values <0.05.
Ethics approval and informed consent
All studies were performed in accordance with National Institutes of Health guidelines and approved by the Animal Ethical Committee of Air Force Medical University.
Results
After 6 hours of surgery, all the animals administered CLP exhibited the symptoms of sepsis and/or SE, including malaise, chills, piloerection, reduced gross motor activity, tachypnea, and diarrhea. Meanwhile, rats in the sham-operated group showed no signs of sepsis or SE.
Animal survival
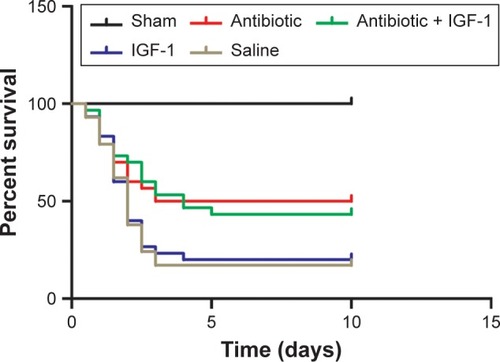
Lethality began as early as 12 hours after CLP. No animal died in the sham-operation group (n=15). In the antibiotic group (n=30), 15 animals died in the first 3 days, indicating a mortality rate of 50%. In the antibiotic + IGF-1 group (n=30), 17 animals died in the first 5 days, indicating a mortality rate of 53.7%. In the saline group (n=30), 25 animals died in the first 3 days, indicating a mortality rate of 83.3%. In the IGF-1 group (n=30), 24 rats died within 4 days, ie, a mortality rate of 80% ().
Figure 1 IGF-1 cannot improve the survival rate of septic rats.
Abbreviation: IGF-1, insulin-like growth factor-1.
Behavioral properties in the first part
Behavior trails were used to evaluate the effect of IGF-1 on cognitive impairment and emotional change during sepsis. Animals that died during the 10 days were excluded.
Open-field test
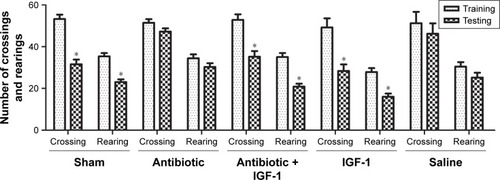
General behavior and habitual memory were tested in the open field. No differences were found in the number of crossings or rearings among groups in the training session, indicating that the ability of exploration and motion was similar among groups. When compared with their own data in training session, the number of crossings and rearings was reduced in sham-operation, antibiotic + IGF-1, and IGF-1 groups in the testing session, while no differences were found in antibiotic and saline groups. These findings indicated an impairment of memory in new environment ().
Figure 2 IGF-1 can improve the memory in a new environment of septic rats.
Abbreviation: IGF-1, insulin-like growth factor-1.
Morris water maze results
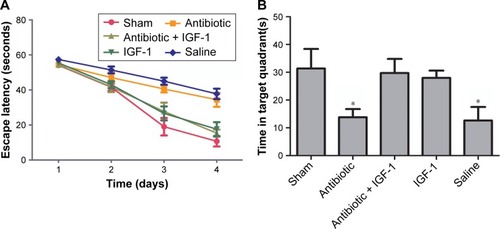
Spatial learning and memory ability were evaluated by the Morris water maze test. On the first 2 days, no differences were found in time spent by the animals to reach the platform among the five groups. However, the antibiotic and saline groups spent more time reaching the platform on the third and fourth days compared with the sham-operation group, which indicated an impairment of learning ability and a protective effect of IGF on learning (). The number of times rats reached the target quadrant within 1 minute was recorded on the fifth day; the results showed that this number was lower in the antibiotic and saline groups compared with the sham-operation group, while no differences were found among sham-operation, antibiotic + IGF-1, and IGF-1 groups, or between the antibiotic and saline groups. These results indicated memory impairment during sepsis, which could be relieved by IGF-1 ().
Figure 3 IGF-1 can improve the spatial learning and memory of septic rats.
Abbreviation: IGF-1, insulin-like growth factor-1.
Passive avoidance results
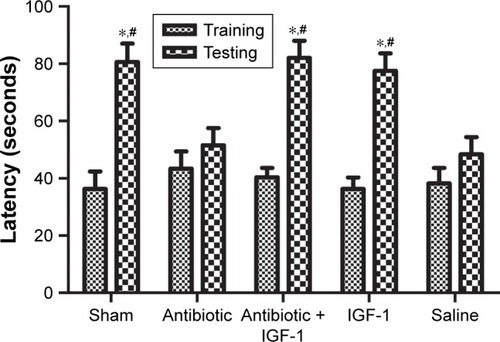
The memory of noxious stimulation was evaluated by the passive avoidance test. In the training session, rats spent similar times stepping into the dark compartment. Compared with their own data in the training session, rats in the sham-operation, antibiotic + IGF-1, and IGF-1 groups took more time to step into the dark compartment in the testing session, while no differences were found in the antibiotic and saline groups, indicating that IGF-1 could relieve the impairment of noxious memory after sepsis ().
Figure 4 IGF-1 can relieve the impairment of noxious memory in septic rats.
Abbreviation: IGF-1, insulin-like growth factor-1.
Emotional changes assessed by the forced swim test
The forced swim test was used to evaluate whether emotional changes occurred in septic rats. Compared with the sham-operative group, immobility time was prolonged in the remaining four groups administered CLP, with no significant differences among them. These findings indicated that septic rats exhibited depressive-like symptoms which might be caused by SE, and IGF-1 could not improve them ().
Figure 5 IGF-1 could not improve the depressive-like symptoms which might be caused by SE.
Abbreviations: CLP, cecal ligation and puncture; IG F-1, insulin-like growth factor-1; SE, septic encephalopathy.
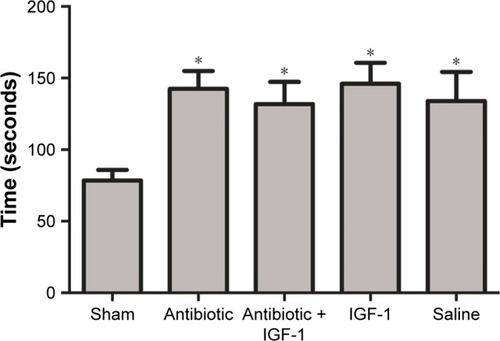
Behavior properties in the second part
Behavior trials in this part were performed to investigate whether the protective effects of IGF-1 were time-dependent. A total of 10 days after CLP, there were 15, 11, 12, 14, and 12 rats left in the 0 hour, 6 hours, 12 hours, 24 hours, and 36 hours groups, respectively. Rats which died during the 10 days were excluded.
Open-field test results
No differences were found among groups in the number of crossings and rearings in the training session, indicating that the ability of exploration and motion was similar among groups. Compared with their own data in the training session, the number of crossings and rearings in the testing session was decreased in the 0-hour and 6-hour IGF-1 groups. However, no statistically significant differences were found in other groups. These findings indicated that administration of IGF-1 at 12, 24, or 36 hours after CLP could not protect memory in a new environment ().
Figure 6 IGF-1 cannot improve the memory of septic rats in a new environment when it is administrated at 12, 24, or 36 hours after CLP.
Abbreviations: CLP, cecal ligation and puncture; IGF-1, insulin-like growth factor-1.
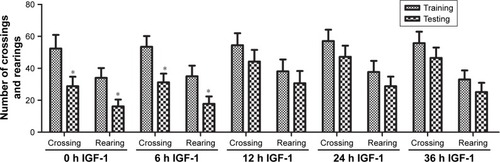
Morris water maze results
On the first 2 days of training, the time required by rats to reach the platform had no significant differences among the five groups. On the third and fourth days, animals in the 0-hour and 6-hour IGF-1 groups spent less time to reach the platform compared with the 12-hour, 24-hour, and 36-hour groups (). The numbers of times rats reached the target quadrant within 1 minute was recorded on the fifth day. The results showed that the number of times rats reached the target quadrant within 1 minute was higher in the 0-hour and 6-hour IGF-1 groups compared with the remaining three groups. No significant differences were observed among the 12-hour, 24-hour, and 36-hour IGF-1 groups or between the 0-hour and 6-hour IGF-1 groups (). These findings indicated that injection of IGF-1 at 12, 24, or 36 hours after CLP could not protect learning ability and spatial memory.
Figure 7 IGF-1 cannot improve the spatial learning and memory of septic rats when it is administrated at 12, 24, or 36 hours after CLP.
Abbreviations: CLP, cecal ligation and puncture; IGF-1, insulin-like growth factor-1.
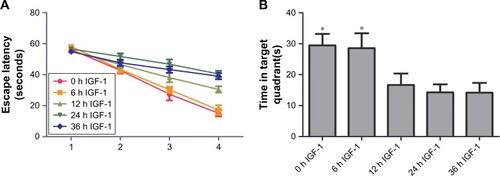
Passive avoidance test
Compared with training session, rats in testing session spent more time stepping into the dark compartment in 0-hour and 6-hour IGF-1 groups, while no differences were observed in 12-hour, 24-hour, and 36-hour IGF-1 groups. Besides, the time rats spent stepping into the dark compartment was similar among all groups in the training session. These results indicated that supplement of IGF-1 at 12, 24, or 36 hours after CLP could not improve memory of noxious stimulation ().
Figure 8 IGF-1 cannot improve the impairment of noxious memory of septic rats when it is administrated at 12, 24, or 36 hours after CLP.
Abbreviations: CLP, cecal ligation and puncture; IGF-1, insulin-like growth factor-1.
Cell apoptosis in the hippocampus
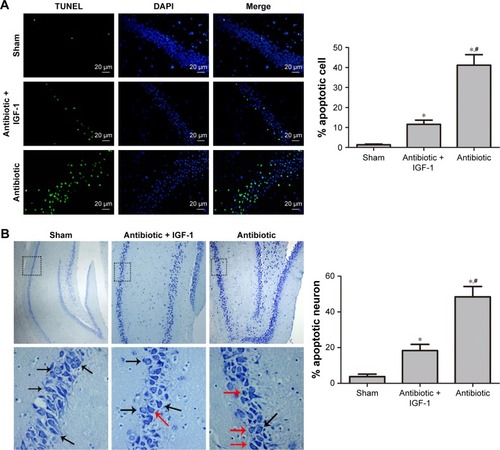
TUNEL staining showed that apoptotic rates in the antibiotic and antibiotic + IGF-1 groups were significantly higher than those of the sham-operation group, while the apoptotic rate in the antibiotic group was significantly higher than that of the antibiotic + IGF-1 group (). Nissl’s staining showed that neurons with normal morphology were predominant in the sham and antibiotic + IGF-1 groups, while neurons with early or end-stage apoptotic morphology were common in the antibiotic group. Moreover, the rates of neuronal apoptosis in the antibiotic and antibiotic + IGF-1 groups were significantly higher than that of the sham-operation group; meanwhile, the rate of neuronal apoptosis in the antibiotic group was significantly higher than that of the antibiotic + IGF-1 group (). These findings revealed the association of cell apoptosis inhibition in the hippocampus with memory and learning improvements during sepsis.
Figure 9 Inhibition of cell apoptosis in the hippocampus is associated with improvement of memory and learning during sepsis.
Abbreviation: IGF-1, insulin-like growth factor-1.
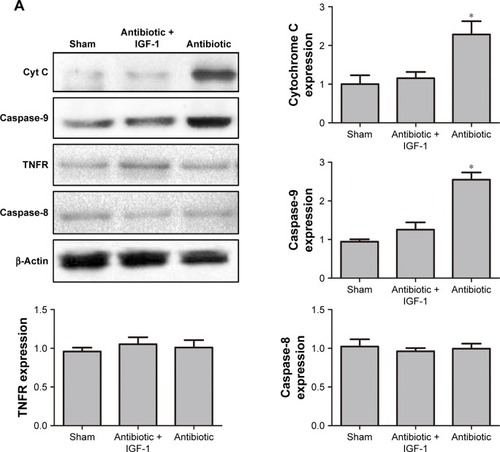
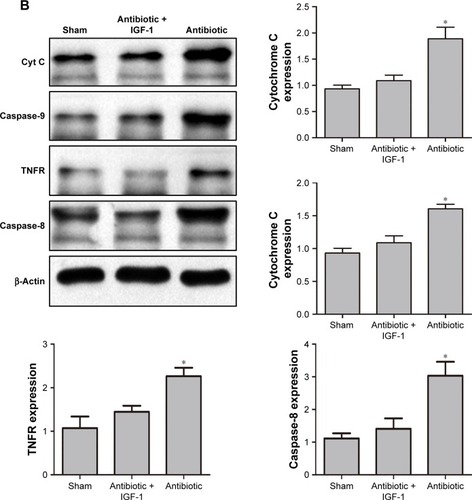
Protein quantification of cytochrome C and TNFR
As cell apoptosis in brain is tightly associated with the apoptotic pathway mediated by cytochrome C and TNFR,Citation17,Citation18 Western blot was performed to detect their expression in hippocampus. Results showed that 6 hours after CLP, the expression of cytochrome C and caspase-9 was higher in antibiotic group than in sham-operative and antibiotic + IGF-1 groups, while the expression of TNFR and caspase-8 was very low and had no differences among the three groups (). Twelve hours after CLP, the expression of TNFR and caspase-8 was increased and their expression was higher in antibiotic group than in sham-operative and antibiotic + IGF-1 groups. Moreover, the expression of cytochrome C and caspase-9 was still higher in antibiotic group (). These results indicated that both the pathways, initiated by cytochrome C and TNFR, participated in cell apoptosis during SE, but the activation of cytochorme C was earlier than TNFR. Moreover, IGF-1 could inhibit their expression.
Figure 10 Cytochorme C and TNFR are activated at different time points and IGF-1 can inhibit their expression.
Abbreviations: Caspase, cysteinyl aspartate specific proteinase; CLP, cecal ligation and puncture; Cyt C, cytochrome C; IGF-1, Insulin-like growth factor-1; TNFR, tumor necrosis factor receptor.
Discussion
Although hippocampus is considered to be the center of learning and memory,Citation37,Citation38 the exact mechanisms of learning and memory remain unclear. Thus, treatment of learning and memory impairment remains a medical challenge. As a factor regulated by GH, previous reports have assessed the effect of IGF-1 on learning and memory impairment.Citation39 Aging is considered to be the commonest cause of cognitive deficits.Citation40,Citation41 However, Erraji-Benchekroun et al found that it is not only aging but also age-related transcriptional changes of a variety of genes, including IGF-1, that are tightly related to cognitive deficits.Citation42 A study by Bellar et al showed that intracerebroventricular injection of IGF-1 could alleviate cognitive deficits in elderly rats.Citation43 These findings are supported by other studies indicating that adults with childhood-onset GH deficiency also exhibit memory deficits.Citation44,Citation45 Brain injury is another important cause of cognitive deficits, and IGF-1 has been reported to improve long-term cognition by reducing hypoxia-ischemia, oxidative-stress, and neuron apoptosis.Citation46,Citation47 Moreover, studies revealed the protective effect of IGF-1 on the brain undergoing ischemic injury in the context of age-related cerebrovascular diseases.Citation48–Citation50 Because SE is a common disease in the intensive care unit which frequently leads to learning and memory deficits, it is essential to develop new medicines or therapies to alleviate such deficits. The significance of the current study is that we were the first to investigate the effect of IGF-1 on the brain during sepsis, especially on cognitive and emotional changes.
As no previous study has assessed the role of IGF-1 in SE, we performed this research using a “whole to part” procedure, and divided it into several steps. First, the effects of IGF-1 on survival, and cognitive and emotional changes of septic rats were observed. As shown earlier, IGF-1 could improve learning and memory. Second, we evaluated whether the protective role of IGF-1 was time-dependent and preliminarily investigated the potential mechanism. The “whole to part” experimental design ensured a comprehensive, objective, credible, and accurate study.
We demonstrated for the first time that IGF-1 could protect spatial learning and memory, noxious memory, and memory in a new environment, which were impaired by sepsis, by inhibiting cell apoptosis in the hippocampus. Although Nissl’s staining indicated that neuron apoptosis is probably associated with memory and learning impairment, such conclusion cannot be definitely drawn without immunofluorescent staining using the neuron-specific nuclear protein. Moreover, we found that the anti-apoptotic role of IGF-1 might be associated with cytochrome C and TNFR downregulation. Interestingly, we also found that the protective effects of IGF-1 on spatial learning and memory, noxious memory, and memory in a new environment occurred only when the molecule was administered at the early stage of sepsis (less than 6 hours). These findings corroborated those reported by Barichello et al,Citation51 who observed that oxidative stress in CNS is restricted to the earlier stage of sepsis, mainly before 6 hours. As oxidative stress is associated with mitochondrial permeability transition pore, the main regulator of cytochrome C release from the mitochondria to the cytoplasm,Citation52 we believe that cognitive impairment is closely related to oxidative stress in CNS. In another study supporting our findings, Messaris et al demonstrated that mitochondrial-mediated apoptosis and cytochrome C release in CNS begin at the early stage (6–12 hours) of sepsis.Citation17 As demonstrated earlier, it was the expression levels of cytochrome C and caspase-9 that were increased at 6 hours after surgery, but not of TNFR and caspase-8, and early injury of the hippocampus may be primarily caused by cytochrome C. Moreover, although IGF-1 was supplied during the whole study period, only the 0-hour and 6-hour IGF-1 groups showed improvement in learning and memory, indicating that cytochrome C expression at the early stage of sepsis plays a key role in hippocampal injury, and results in memory and learning impairment. Besides, mortality during sepsis was decreased by antibiotic treatment, and not IGF-1, as described previously.
Since IGF-1 is beneficial to cognitive function, selecting an appropriate time for drug administration seems important. In a study by Deijen et al, individuals with childhood-onset GH deficiency had improvement in memory deficit after GH administration for 6 consecutive months,Citation53 indicating that although memory deficit development is a chronic process, it can be improved by GH when given for a certain period of time. Zhong et al found that administration of IGF-1 at 24 and 48 hours after hypoxia-ischemia could significantly reduce brain injury and improve long-term memory and cognition, but such effects were not observed with IGF-1 when administered after the first 2 days.Citation46 In the current study, IGF-1 showed a protective effect on cognitive function when administered during the initial 6 hours after CLP, and no effect was observed in groups treated after 12 hours. In acute diseases such as SE and hypoxia-ischemia, cell apoptosis might be the main reason for learning and memory impairment; therefore, IGF-1 was provided early to relieve the impairment. In chronic diseases such as childhood-onset GH deficiency and aging-related cognitive deficits, learning and memory impairment is caused by IGF-1 and GH deficiencies, and these deficits can be reversed whenever IGF-1 is supplied.
This study had several limitations. First, as the study duration was shorter than that of other reports,Citation12,Citation54–Citation56 the present findings only indicate that IGF-1 may protect from learning and memory impairment caused by sepsis. Further studies of longer duration (1 month or even longer) should be performed to evaluate the effect of IGF-1 on long-term cognition, as long-term cognitive impairment is common in patients recovering from sepsis. Second, as tolerance to sepsis and cecal contents are different among animals, it is difficult to achieve a uniform disease severity in models of sepsis. Third, since high mortality is associated with sepsis, the present sample size was not enough, especially in the saline group, which finally had only five survivors. Fourth, we only assessed cytochrome C and TNFR changes during sepsis, and whether other factors participated in hippocampal cell apoptosis deserves further attention. Fifth, as the forced swim test is mainly used in depressive disorders and associated treatments, the positive results in this study may only indicate that septic rats exhibit depressive-like symptoms which might be caused by SE; further studies should reveal the relationship between psychiatric disorders and SE. Finally, as DAPI is a fluorescent label for the nucleus of all kinds of cells, we could not distinguish apoptotic neurons from other apoptotic cells such as astrocytes and microglia; further studies are required to assess neuronal apoptosis by using the neuron-specific nuclear protein.
Conclusion
In conclusion, we demonstrate that IGF-1 can reduce cell apoptosis by preventing the over-expression of cytochrome C and TNFR, and lead to the improvement of cognitive function. Interestingly, the improvement only occurs with IGF-1 administered at the early stage of sepsis. As cytochrome C activation occurs earlier than that of TNFR, it may be the cause of apoptosis in early SE. As early IGF-1 administration relieves memory and learning impairment in SE, it may constitute a potential drug to improve cognitive function in septic patients.
Acknowledgments
The work was supported by the Department of Neurosurgery, Tangdu Hospital, Xi’an, China, and they provided all the experimental equipment for behavior tests. The work was also supported by the Department of Neurosurgery, Xijing Hospital, Xi’an, China, and they provided the experimental equipment for Western blot analysis and TUNEL staining. The authors thank Yuefan Yang, PhD, Department of Neurosurgery, Xijing Hospital, for his assistance in performing the Western blot analysis. The authors also thank Xiaoyan Chen, technician, Department of Neurosurgery, Xijing Hospital, for her technical guidance in performing TUNEL staining. We also thank Xingchen Zhao, PhD, Department of Genetics, Air Force Medical University, Xi’an, China, for his assistance in all the statistical work.
Supplementary material
Figure S1 Experimental procedures.
Notes: (A) Experimental procedure for the first part. IGF-1, ceftriaxone, and saline were given and different duration and behavior tests were conducted 10 days after CLP. (B) Experimental procedure for the second part. IGF-1 was given at 0, 6, 12, 24, or 36 hours after surgery in each group, respectively, and then supplied every 12 hours for 10 days. Ceftriaxone and saline were given the same way as that in the first part and then behavior tests were conducted 10 days after CLP. (C) Experimental procedure for the third part. IGF-1, ceftriaxone, and saline were given at different time points. Western blot, TUNEL staining, and Nissl’s staining were conducted at 6 and 12 hours after surgery.
Abbreviations: CLP, cecal ligation and puncture; IGF-1, insulin-like growth factor-1.
Disclosure
The authors report no conflicts of interest in this work.
References
- SingerMDeutschmanCSSeymourCWThe Third International Consensus definitions for sepsis and septic shock (Sepsis-3)JAMA2016315880181026903338
- FleischmannCScheragAAdhikariNKAssessment of global incidence and mortality of Hospital-treated sepsis. Current estimates and limitationsAm J Respir Crit Care Med2016193325927226414292
- HotchkissRSKarlIEThe pathophysiology and treatment of sepsisN Engl J Med2003348213815012519925
- ZhangDMicekSTKollefMHTime to appropriate antibiotic therapy is an independent determinant of postinfection ICU and hospital lengths of stay in patients with sepsisCrit Care Med201543102133214026121071
- KlompasMCalandraTSingerMAntibiotics for Sepsis-Finding the equilibriumJAMA201832014143330242350
- AllingstrupMWetterslevJRavnFBMøllerAAfshariAAnti-thrombin III for critically ill patientsCochrane Database Syst Rev201682CD005370
- SinghBTiwariAKSinghKβ2 agonist for the treatment of acute lung injury: a systematic review and meta-analysisRespir Care201459228829623777655
- ARISE Investigators, ANZICS Clinical Trials GroupPeakeSLGoal-directed resuscitation for patients with early septic shockN Engl J Med2014371161496150625272316
- RhodesAEvansLEAlhazzaniWSurviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016Intensive Care Med201743330437728101605
- KragMPernerAWetterslevJPrevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patientsIntensive Care Med201541583384525860444
- HolstLBHaaseNWetterslevJLower versus higher hemoglobin threshold for transfusion in septic shockN Engl J Med2014371151381139125270275
- IwashynaTJElyEWSmithDMLangaKMLong-term cognitive impairment and functional disability among survivors of severe sepsisJAMA2010304161787179420978258
- WidmannCNHenekaMTLong-term cerebral consequences of sepsisLancet Neurol201413663063624849863
- SalluhJIWangHSchneiderEBOutcome of delirium in critically ill patients: systematic review and meta-analysisBMJ2015350may19 3h253826041151
- IwashynaTJCookeCRWunschHKahnJMPopulation burden of long-term survivorship after severe sepsis in older AmericansJ Am Geriatr Soc20126061070107722642542
- GoftonTEYoungGBSepsis-associated encephalopathyNat Rev Neurol201281055756622986430
- MessarisEMemosNChatzigianniETime-dependent mitochondrial-mediated programmed neuronal cell death prolongs survival in sepsisCrit Care Med20043281764177015286556
- ZhanRZWuCFujiharaHBoth caspase-dependent and caspase-independent pathways may be involved in hippocampal CA1 neuronal death because of loss of cytochrome c from mitochondria in a rat forebrain ischemia modelJ Cereb Blood Flow Metab200121552954011333363
- AuernhammerCJStrasburgerCJEffects of growth hormone and insulin-like growth factor I on the immune systemEur J Endocrinol199513366356458548046
- BeckKDPowell-BraxtonLWidmerHRValverdeJHeftiFIGF1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neuronsNeuron19951447177307718235
- Vicario-AbejónCYusta-BoyoMJFernández-MorenoCde PabloFLocally born olfactory bulb stem cells proliferate in response to insulin-related factors and require endogenous insulin-like growth factor-I for differentiation into neurons and gliaJ Neurosci200323389590612574418
- CaoPMaximovASüdhofTCActivity-dependent IGF-1 exocytosis is controlled by the Ca(2+)-sensor synaptotagmin-10Cell2011145230031121496647
- FeldmanELSullivanKAKimBRussellJWInsulin-like growth factors regulate neuronal differentiation and survivalNeurobiol Dis199743–42012149361296
- SingletonJRDixitVMFeldmanELType I insulin-like growth factor receptor activation regulates apoptotic proteinsJ Biol Chem19962715031791317948943217
- CaoYGunnAJBennetLInsulin-like growth factor (IGF)-1 suppresses oligodendrocyte caspase-3 activation and increases glial proliferation after ischemia in near-term fetal sheepJ Cereb Blood Flow Metab200323673974712796722
- LinSFanLWPangYRhodesPGMitchellHJCaiZIGF-1 protects oligodendrocyte progenitor cells and improves neurological functions following cerebral hypoxia-ischemia in the neonatal ratBrain Res200510631152616259966
- MasonJLJonesJJTaniikeMMorellPSuzukiKMatsushimaGKMature oligodendrocyte apoptosis precedes IGF-1 production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelinationJ Neurosci Res200061325126210900072
- GuanJBeilharzEJSkinnerSJWilliamsCEGluckmanPDIntra-cerebral transportation and cellular localisation of insulin-like growth factor-1 following central administration to rats with hypoxic-ischemic brain injuryBrain Res2000853216317310640614
- SonntagWERamseyMCarterCSGrowth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive agingAgeing Res Rev20054219521216024298
- LangCHFanJCooneyRVaryTCIL-1 receptor antagonist attenuates sepsis-induced alterations in the IGF system and protein synthesisAm J Physiol19962703E430E4378638689
- SuhHSZhaoMLDericoLChoiNLeeSCInsulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediatorsJ Neuroinflammation20131013723497056
- RossRMiellJFreemanECritically ill patients have high basal growth hormone levels with attenuated oscillatory activity associated with low levels of insulin–like growth factor-IClin Endocrinol19913514754
- BuckbinderLTalbottRVelasco-MiguelSInduction of the growth inhibitor IGF-binding protein 3 by p53Nature199537765506466497566179
- BarichelloTMartinsMRReinkeACognitive impairment in sepsis survivors from cecal ligation and perforationCrit Care Med200533122122315644673
- SaatmanKEContrerasPCSmithDHInsulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injuryExp Neurol199714724184279344566
- ContrerasPCStefflerCYuECallisonKStongDVaughtJLSystemic administration of rhIGF-1 enhanced regeneration after sciatic nerve crush in miceJ Pharmacol Exp199527414431449
- ZeidmanPMaguireEAAnterior hippocampus: the anatomy of perception, imagination and episodic memoryNat Rev Neurosci201617317318226865022
- García-GarcíaRCruz-GómezÁJUriosALearning and memory impairments in patients with minimal hepatic encephalopathy are associated with structural and functional connectivity alterations in hippocampusSci Rep201881966429941971
- YanHMitschelenMTothPEndothelin-1-induced focal cerebral ischemia in the growth Hormone/IGF-1 deficient Lewis dwarf ratJ Gerontol A Biol Sci Med Sci201469111353136225098324
- SonntagWEDeakFAshpoleNInsulin-like growth factor-1 in CNS and cerebrovascular agingFront Aging Neurosci201352723847531
- HeddenTGabrieliJDInsights into the ageing mind: a view from cognitive neuroscienceNat Rev Neurosci200452879614735112
- Erraji-BenchekrounLUnderwoodMDArangoVMolecular aging in human prefrontal cortex is selective and continuous throughout adult lifeBiol Psychiatry200557554955815737671
- BellarDGlickmanELJuvancic-HeltzelJGunstadJSerum insulin like growth factor-1 is associated with working memory, executive function and selective attention in a sample of healthy, fit older adultsNeuroscience201117813313721256932
- ClaytonPGleesonHMonsonJPopovicVShaletSMChristiansenJSGrowth hormone replacement throughout life: insights into age-related responses to treatmentGrowth Horm IGF Res200717536938217560153
- ArwertLIVeltmanDJDeijenJBvan DamPSDrentMLEffects of growth hormone substitution therapy on cognitive functioning in growth hormone deficient patients: a functional MRI studyNeuroendocrinology2006831121916707911
- ZhongJZhaoLDuYWeiGYaoWGLeeWHDelayed IGF-1 treatment reduced long-term hypoxia-ischemia-induced brain damage and improved behavior recovery of immature ratsNeurol Res200931548348919500451
- WangJTangYZhangWInsulin-like growth factor-1 secreted by brain microvascular endothelial cells attenuates neuron injury upon ischemiaFEBS J2013280153658366823721666
- LeinningerGMFeldmanELInsulin-like growth factors in the treatment of neurological diseaseEndocr Dev2005913515915879695
- MackayKBLoddickSANaeveGSVanaAMVergeGMFosterACNeuroprotective effects of insulin-like growth factor-binding protein ligand inhibitors in vitro and in vivoJ Cereb Blood Flow Metab200323101160116714526226
- SchäbitzWRHoffmannTTHeilandSDelayed neuroprotective effect of insulin-like growth factor-I after experimental transient focal cerebral ischemia monitored with MRIStroke20013251226123311340238
- BarichelloTFortunatoJJVitaliAMOxidative variables in the rat brain after sepsis induced by cecal ligation and perforationCrit Care Med200634388688916505668
- GhafourifarPSchenkUKleinSDRichterCMitochondrial nitric-oxide synthase stimulation causes cytochrome c release from isolated mitochondria. Evidence for intramitochondrial peroxynitrite formationJ Biol Chem199927444311853118810531311
- DeijenJBde BoerHvan der VeenEACognitive changes during growth hormone replacement in adult menPsychoneuroendocrinology199823145559618751
- SemmlerAFrischCDebeirTLong-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent modelExp Neurol2007204273374017306796
- YuXJiaLYinKLvJYuWDuHSrc is implicated in hepatic ischemia reperfusion-induced hippocampus injury and long-term cognitive impairment in young mice via NMDA receptor subunit 2A activationNeuroscience201839111230213765
- FreemanBDMartinsYCAkide-NdungeOBEndothelin-1 mediates brain microvascular dysfunction leading to long-term cognitive impairment in a model of experimental cerebral malariaPLoS Pathog2016123e100547727031954
