Abstract
Background and purpose
The study evaluated olfactory performance and pleasantness rating of odors in patients with first episode psychosis (FEP) and chronic schizophrenia (SCH) with regard to the severity of psychopathological symptoms and plasma β-endorphin concentration.
Patients and methods
Twenty patients with FEP, 27 with SCH and 29 healthy individuals, were recruited to the research . The University of Pennsylvania Smell Identification Test (UPSIT), subjective odor hedonic judgment and plasma levels of β-endorphin (BE) assay were performed in all participants.
Results
Individuals with SCH revealed higher BE concentration than other study groups (P=0.000). All patients identified pleasant odors poorer than controls, however, SCH made more identification errors (P=0.000) than those with FEP. Moreover, participants with FEP rated pleasant odors as more pleasant than individuals with chronic schizophrenia and healthy controls (P=0.009). Nevertheless, higher β-endorphin level was related with lower scores in pleasant odor identification (Rs=−0.452; P=0.046) and more severe psychotic symptoms in FEP sample. Chronic schizophrenia patients did not demonstrate any relationship between symptom severity, odor identification performance and β-endorphin concentration. No relationship was found between BE concentration and hedonic judgment of the presented odors among all study groups. Chronically ill subjects identified odors significantly more poorly than those with first episode psychosis. Deficits in identifying pleasant odors might not be the only potential risk factor for undergoing chronic, recurrent schizophrenia. All patients subjectively overrated pleasant odors. Those with SCH and more severe negative symptoms made significantly more identification errors.
Conclusion
The endogenous morphine system deregulation is observed in first episode psychosis as well as in chronic schizophrenia. In first episode schizophrenia higher beta-endorphin concentration is related to pleasant odor identification deficit.
Introduction
Disturbances in olfactory performance, mainly in odor identification, is well known in both chronic schizophrenia (SCH) and first episode psychosis (FEP). It appears to be unrelated to antipsychotic medication, although smoking status might have a marginal impact.Citation1 This phenomenon has been reported independently alike in subjects undergoing neuroleptic treatment and among neuroleptic-naive patients with FEPCitation2 and those with an at-risk mental state (prodromal phase).Citation3,Citation4 Notably, these deficits have been found to be associated with negative symptoms.Citation5 However, the impact of hedonic valence on olfactory performance in schizophrenia still remains unclear. The results of published studies are inconclusive, revealing overrating of odor pleasantness,Citation6 but also lower pleasantnessCitation7 by patients with schizophrenia.
The role of the endogenous opioid system in the etiology and pathogenesis of schizophrenia has been extensively investigated in the past, but remains controversial. The presence of a possible excess of opioid in schizophrenia was indicated in most studies.Citation8 In the brain, endogenous opiate immunoreactivity has been observed in various structures including the hippocampus, the olfactory bulb, the band of Broca, and the basal ganglia and cerebellum.Citation9 Endogenous morphine was mainly deposited in astrocytes and GABAergic cells. Opioid modulation of dopaminergic (DA) functions has already been shown in anatomical and biochemical studies, indicating the opioid receptor interactions have an effect on DA nerve terminals, cell bodies, and afferent nerve endings, culminating in DA activities. Opioid agonists alter dopaminergic transmission including dopamine release, reuptake, and metabolism in the striatum and substantia nigra. It might indicate that a disturbance in opiate–dopamine interaction could play a role in the pathogenesis of schizophrenia.
Odors may alter emotions, mood, and behavior, and trigger strong sensations of pleasure or displeasure.Citation10 Olfaction is an important neurobehavioral probe of hedonic capacity. The endogenous opioid system is involved in regulation of the reward system, including the reward value of a food through orosensory stimuli such as taste and smell.Citation11 All types of opioid receptors are localized in the olfactory system in the brain, especially in the olfactory bulb.Citation12 It was observed that remifentanyl (opioid u-receptor agonist) increased the subjective olfactory threshold among healthy individuals; nevertheless, no influence was observed referring to odor identification and discrimination.Citation13 Exploring the biological process of hedonic experience might play a role in understanding schizophrenia pathophysiology and may be helpful in early diagnosis and treatment of mental illness.Citation14 Anhedonia is a component related to negative symptom complex.Citation15 In the rodent brain, specific hedonic “hotspots” have been described, ie, areas where direct stimulation with opioid agonists can cause or amplify “liking” reactions.Citation16 These hotspots have been identified in the nucleus accumbens and ventral pallidum, and in the parabrachial nucleus of the brainstem. Stimulation with opioids in these regions can amplify sensory pleasure by multiplication of the normal number of “liking” reactions to sucrose taste. In the human brain, the mid-anterior orbitofrontal cortex is a crucial structure in the translation of subcortically-driven “liking” reactions into our conscious feelings of pleasure.Citation17
In our previous studyCitation18 we found a significantly higher plasma β-endorphin (BE) concentration in patients with predominant negative symptoms of schizophrenia. It is known that negative symptoms are also related to poorer olfactory performance in the patient population. Therefore, we hypothesized that increased BE levels might be related to olfactory disturbance in those with chronic illness and in FEP. We hypothesize that BE levels may influence the specific perception of odor pleasantness, and be at least indirectly associated with the psychopathological manifestation of psychosis.
Materials and methods
Twenty-seven patients with SCH and 20 individuals with first episode schizophrenia (FEP) diagnosed according to the ICD-10 criteria were recruited to the study, along with 29 healthy controls. Patient samples were recruited from the outpatient unit of Affective and Psychotic Disorders Department of the Medical University of Łódź. They were all in stable mental state (without psychosis exacerbation or relapse). The staff and students of Medical University of Łódź were included in the control group. The patients, psychopathological symptom severity was assessed by a senior psychiatrist during clinical psychiatric examination and also using the Positive and Negative Syndrome Scale (PANSS). The patients with FEP were younger than controls and the subjects with chronic schizophrenia (P=0.000). The study groups did not differ with regard to sex composition (chiCitation2=0.2; P=0.906). The SCH patients demonstrated significantly greater severity of negative symptoms than the FEP group (P=0.029), with the duration of the illness ranging from 5–18 years (SD=±3.2). No significant differences in PANSS and other PANSS subscales were observed (). All patients were treated with first generation or second generation antipsychotic in therapeutic doses (amisulpride, aripiprazole, clozapine, haloperidol, perazine, quetiapine, olanzapine, risperidone).
Table 1 Sample characteristics for schizophrenia with first episode psychosis (FEP), chronic schizophrenia (SCH) and healthy comparison (C) groups
The exclusion criteria for all groups were as follows: a history of drug abuse, a history of psychiatric disorders (other than schizophrenia for the patient sample), neurological disorders, head trauma, loss of consciousness, deviation of the nasal septum, acute upper respiratory infection, or chronic rhinosinusitis. The individuals included to the study had no somatic illnesses. They all underwent olfactory identification tests, and peripheral blood BE measurement. All participants gave their written informed consent prior to their inclusion in the study. The study was approved by the Ethics Committee of the Medical University of Łódź (Nr RNN/92/12/KE).
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013.
Biochemical assay
The BE assay was performed using a DRG Diagnostics β-Endorphin Kit (RIA 3023). The procedure is described in detail by Urban-Kowalczyk et al.Citation19
Olfactory identification and hedonic assessment
The University of Pennsylvania Smell Identification Test (UPSIT) is a commonly used, standardized tool including of a four-choice test with 40 items. The odors were rated birhinally. To perform the test participants scratched each microcapsuled odor patch with a pencil, sniffed it, and then indicated the name of the odor from among four responses. The odors were divided into pleasant, unpleasant, or neutral. UPSIT normative data was used according to the value categorization described by Doty et al.Citation20 The UPSIT items were rated on a Likert scale ranging from 1 to 9, with 5 designated as the neutral point. In total, 16 items (UPSIT P) were categorized as pleasant (bubble gum, cherry, banana, fruit punch, licorice, cinnamon, strawberry, chocolate, root beer, pineapple, lime, orange, wintergreen, watermelon, grape, melon), 15 items (UPSIT N) were categorized as neutral (menthol, mint, clove, coconut, cheddar cheese, cedar, ginger bread, lilac, peach, dill pickle, grass, pine, soap, rose, peanut), and 9 items (UPSIT U) were categorized as unpleasant (pizza, motor oil, leather, onion, gasoline, turpentine, paint thinner, smoke, natural gas). All subjects rated each UPSIT odor subjectively as pleasant (+1 point), neutral (0 points), or unpleasant (−1 point).
Statistical analysis
The structural characteristics were calculated for the qualitative analysis, and the arithmetic mean (x) and median (Me) were calculated for the quantitative characteristics to describe the studied group. To measure dispersion, the standard deviation (SD) was used. The range of the variables tested, ie, the minimum and maximum values, was also calculated. The asymmetry coefficient was also calculated to better represent the empirical distribution.
The empirical distributions of the tested parameters were analyzed using the Wilk–Shapiro test. Non-parametric tests, such as the Pearson’s chi-squared test, were used for non-measurable characteristics. For non-parametric features, a non-parametric equivalent of the Student’s t-test was used, and the Mann–Whitney U-test was applied for unrelated variables. Levene’s test was used to test the homogeneity of variance for a set of variables.
The non-parametric Kruskal-Wallis test, a multidimensional statistical analysis for comparison between groups, was used, while Dunn’s test was used to compare the groups. The Spearman’s rank correlation coefficient was also used. The threshold of statistical significance was assumed to be P=0.05.
Statistical and graphical work was performed using Statistica 12 software.
Results
β-endorphin concentration
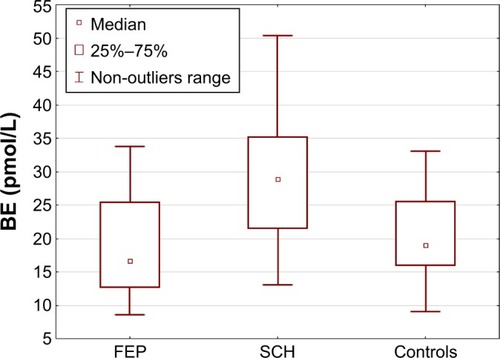
Patients with chronic schizophrenia revealed significantly higher BE concentrations than other study groups (H2,76=17.168; P=0.000). The BE concentrations were similar among patients with FEP and healthy controls (). No correlation was found between duration of illness and BE concentration in the chronic group.
Olfactory performance
FEP and SCH participants identified less number of odors correctly vs controls while FEP patients identified more numbers correctly vs chronically ill patients (P=0.000). In general, both patient samples were not as good at identifying pleasant odors as the controls. However, those with chronic schizophrenia made more identification errors in pleasant odors than FEP (H2,76=33.422; P=0.000) (). The SCH sample also achieved the lowest score for identifying neutral odors (H2,76=16.738; P=0.000). No differences were found between FEP and controls. All study groups identified unpleasant odors with similar accuracy (H2,76=7.914; P=0.055).
Figure 2 Pleasant odor (UPSIT P) identification accuracy in study samples.
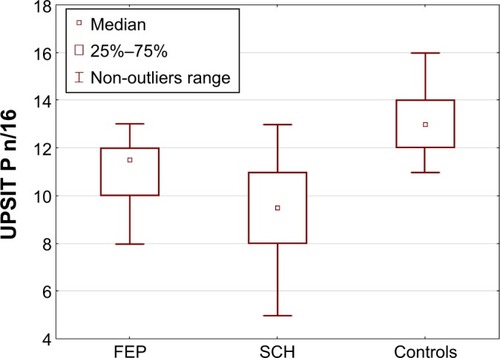
All participants judged the pleasantness of the neutral and unpleasant odors to be at around the same level (H2,76=3.06; P=0.217 vs H2,76=4.47; P=0.107 vs H2,76=1.27; P=0.530). Patients with FEP rated the pleasant odors as more pleasant than those with chronic schizophrenia and healthy controls (H2,76=9.309; P=0.009).
β-endorphin concentration vs the severity of psychopathological symptoms and olfactory performance
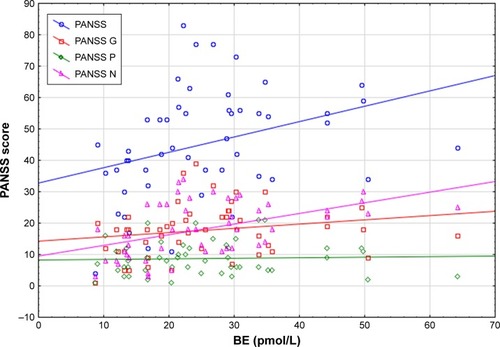
In the FEP sample, a significant positive correlation was observed between BE concentration and PANSS (rs=0.499; P=0.025), PANSS G (rs=0.567; P=0.009), and PANSS N (rs=0.577; P=0.008) (). The BE concentration was not related to PANSS P score (rs=0.264; P=0.261). Patients with FEP and a higher BE level obtained lower scores in pleasant odor identification (rs=−0.452; P=0.046). BE concentration had no impact on the identification accuracy of other UPSIT items. No correlation was found between UPSIT performance and the severity of schizophrenia symptoms in the FEP group.
Figure 3 Correlation between BE concentration and psychopathological symptom severity among patients with FEP.
The BE concentration in patients with chronic schizophrenia did not correlate with the severity of psychopathological symptoms in PANSS, and with odor identification performance. SCH individuals with a higher PANSS score obtained a lower total UPSIT score (rs=−0.488; P=0.006), but also lower scores for identification of neutral (rs=−0.382; P=0.037) and unpleasant odors (rs=−0.436; P=0.016). No correlation was observed between PANSS and identification accuracy of pleasant odors (rs=−0.237; P=0.207). Similarly, a negative correlation was observed between PANSS G score and total UPSIT (rs=−0.541; P=0.002), UPSIT N (rs=−0.437; P=0.016), and UPSIT U (rs=−0.419; P=0.024), but no correlation was found between PANSS G and UPSIT P (rs=−0.243; P=0.195). Patients with more severe negative symptoms obtained a lower score for total UPSIT odor identification (rs=−0.462; P=0.010) and for unpleasant odor identification (rs=−0.415; P=0.023). No relationship was found between PANSS N and UPSIT N or UPSIT P. Furthermore, no correlation was observed between the severity of positive symptoms of schizophrenia (PANSS P) and UPSIT performance.
No relationship was found between BE concentration and hedonic judgment of presented odors in any study group.
Psychopathological symptom severity and hedonic judgment of odors
FEP patients with greater psychopathological symptoms severity (PANSS score) rated general UPSIT odors (rs=0.474; P=0.035) and pleasant odors (rs=0.468; P=0.037) as subjectively more pleasant. PANSS score was not related to hedonic judgment of neutral and unpleasant odors. FEP individuals with higher PANSS P score judged UPSIT total odors (rs=0.517; P=0.019) and pleasant odors (rs=0.446; P=0.049) as more pleasant. There was no effect of positive symptoms severity on the subjective rating of neutral and unpleasant odors. Among this sample, no relationship was found between PANSS G, PANSS N, and the hedonic judgment of the presented odors.
The severity of psychopathological symptoms, measured using PANSS, did not appear to have any influence on the hedonic judgment of odors in patients with chronic schizophrenia. Furthermore, no relationship was observed between duration of illness and olfactory performance.
Smoking status
The study groups were divided into smokers and non-smokers (chi2=1.04; P=0.592). No smoking influence was found on odor identification ability or hedonic judgment among study samples. Additionally, there was no relationship between BE concentration and smoking.
Discussion
The majority of studies indicated that about 80% of schizophrenia patients revealed such significant odor identification impairment causes a disturbance in daily functioning. In contrast, such defects are present in less than 15% of the general population.Citation1 This result was reflected in the present study. Both patient samples made more UPSIT errors than controls; however, the chronically ill subjects identified odors significantly more poorly than the FEP subjects. All individuals with psychosis had the most difficulty in identifying pleasant odors. SCH patients also received a low score in neutral odor identification. All study groups demonstrated similar accuracy when identifying unpleasant odors.
Good et alCitation21 found that patients with FEP who met the remission criteria for the negative symptoms after a 1-year follow-up achieved significantly better olfactory test performance than those who did not. Among patients identifying odors similar to healthy population, 80% achieved remission, in contrast to 56% of patients with olfactory identification deficit. However, UPSIT performance was not related with persistent positive or affective symptoms. Brewer et alCitation2 described a significant deficit in odor identification in patients with FEP compared with healthy controls, and this deficit remained stable in a 6-month follow-up. They also reported more negative symptoms related to more identification errors in UPSIT, but no association between olfactory deficits and duration of untreated psychosis or the prodromal phase period. After a 6-month follow-up, the patients achieved significant improvement in positive symptoms, but not in negative symptoms. Brewer et alCitation3 concluded that ultra-high-risk individuals who developed psychosis made more odor identification errors than non-converters and healthy controls. Moreover, significantly impaired identification of pleasant odors was also observed among at-risk mental state for schizophrenia (ARMS) individuals who subsequently transitioned to psychosis.Citation22 It has been suggested that schizophrenia disturbs normal frontal lobe development and secondarily might impair the development of neuropsychological functions dependent on this brain structure.
Some studies reveal that olfactory deficits in patients with FEP are related to poorer functional outcome.Citation23 Corcoran et alCitation24 found poor odor identification similar to that reported in adult schizophrenics in a population of children and adolescent with psychotic symptoms. However, an inverse association was observed between UPSIT score and severity of negative symptoms, but no correlation with positive symptoms. Based on previous reports and our present findings, we hypothesize that the presence of deficits in pleasant odor identification may not only be a potential indicator of psychosis development, but also a risk factor for developing chronic, recurrent schizophrenia.
Hedonic value and arousal are basic informational input parameters in the motivational system that modulate avoidance/defense or approach behaviors. Potentially unpleasant olfactory input initiates and motivates the patient to engage in possible avoidance behaviors, while pleasant sensations would mainly induce a “non-action” attitude intended to maintain exposure to the stimulus.Citation25 Similar to our results, Doop and ParkCitation6 found that patients with chronic schizophrenia tend to overrate the pleasantness of odorants. More pleasant judgment was positively correlated with some negative symptoms, such as affective flattening. Moreover, correctly identified UPSIT odors were judged as more pleasant, and authors suggested that familiarity is associated with liking. Among healthy individuals, Hawkes et alCitation26 report that, with age, odor hedonic score increased and identification ability decreased. Nevertheless, some studies indicate that unpleasant odors showed age invariance, whereas pleasant ones exhibited age sensitivity. In older subjects, this could be attributed to the loss of olfactory receptor neuron selectivity.Citation27 Our present findings indicate that all patients, even young ones with FEP, overrated pleasant, but not unpleasant odors, which can reflect the early failure in olfactory neurons related to psychosis development. Some research results found a relationship between olfactory identification deficits and persistent negative symptoms.Citation5 However, our findings in the FEP sample fail to indicate any relationship between olfactory performance and negative symptoms. Despite this, the SCH group members with more severe negative symptoms made significantly more identification errors, and this might be characteristic of chronic schizophrenia.
Previous studies display no consensus regarding the subjective hedonic judgment of odors in schizophrenia. Some indicate that patients with schizophrenia do not generally show reduced hedonic experience of olfactory stimuli in the laboratory.Citation6,Citation28 It is, hence, frequently observed that evaluation of the familiarity of a given odor is positively associated with judgment of pleasantness.Citation29 However, self-reporting does not always reflect the real state of the underlying pleasure networks. Schizophrenic patients do not tend to show lower hedonic reactivity to pleasurable stimuli in comparison to healthy ones.Citation30,Citation31 It should be noted that the discovery that here-and-now measures of liking are surprisingly intact in schizophrenic patients is based only on self-reported ratings. In contrast, studies measuring prospective, retrospective, and hypothetical experiences of pleasure described decreased levels of liking in these patients. Our observations suggest that schizophrenia might preclude the individual from feelings of unpleasantness, and this deficit is exacerbated by the severe psychopathological symptoms already present in the first episode of the disease. Meanwhile, in chronic schizophrenia, symptom severity has no impact on the hedonic judgment of odors.
Some studies suggest the involvement of endogenous opioids in reward-related aspects of ingestion and olfaction. The subjective hedonic experience of reward has been shown to correlate with activity in the insular and rostral anterior cingulate cortex.Citation32 Moreover, this activity in the rostral anterior cingulate cortex is in part suppressed after naloxone administration. Yeomans and WrightCitation33 found that hedonic judgment of the smell and the taste of foods was significantly lower in nalmefene-treated healthy individuals compared to those who received placebo. We therefore hypothesize that endogenous opioid systems are reciprocally dysregulated in schizophrenia and are linked to the olfactory process. Significantly increased concentrations of BE were found in those with chronic schizophrenia, which was unrelated to the duration of illness, severity of psychopathological symptoms and olfactory performance. Although BE concentration was similar between the members of the FEP sample and healthy controls, those with higher BE concentrations had more severe negative symptoms and demonstrated poorer identification of pleasant odors. It has even been suggested that long-term neuroleptic use stimulate the synthesis and release of pituitary BE and prolactin through DA blockade.Citation34 Research exploring the involvement of opioids on psychotic symptoms should attempt to determine the importance of opioid modulation of DA activity in these patients.
An important limitation of the study is that it does not compare the findings, particularly endorphin level, with those of patients in prodromal phase. However, it does include patients with chronic schizophrenia, albeit those without persistent negative symptoms or deficit syndrome; it is possible that this subpopulation develops a distinct pattern of olfactory performance and BE status. Our results should, hence, be interpreted cautiously. In addition, the study groups are relatively small, and it would be advisable to confirm the results on larger samples. Previous studies have assessed BE levels in various biological materials, ie, plasma,Citation35 cerebrospinal fluid,Citation36 and peripheral blood mononuclear cells,Citation37 as plasma BE level does not directly reflect central endogenous opioid status. Additionally, gastrointestinal tissues contain small amounts of BE which could be released into circulation. In addition, gynecological studies have reported unstable BE levels in women during the menstrual cycle,Citation38 and it is thought that gonadal steroids and luteinizing hormone might influence BE concentration. However, all female participants in our study were in the premenopausal period, but no data was collected regarding their gynecological status, and their hormonal profile was not evaluated as part of the study. It should be noted that many aspects of BE involvement in schizophrenia remain unclear or unknown, such as the impact of long-term neuroleptic treatment on BE concentration. Further investigation in this area might reveal interesting findings.
Acknowledgments
This work was supported by the Medical University of Łódź (502-03/1-155-02/502-14-269).
Disclosure
The authors report no conflicts of interest in this work.
References
- MobergPJAgrinRGurREGurRCTuretskyBIDotyRLOlfactory dysfunction in schizophrenia: a qualitative and quantitative reviewNeuropsychopharmacology199921332534010457530
- BrewerWJPantelisCAndersonVStability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosisAm J Psychiatry2001158110711511136641
- BrewerWJWoodSJMcgorryPDImpairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophreniaAm J Psychiatry2003160101790179414514492
- KamathVMobergPJCalkinsMEAn odor-specific threshold deficit implicates abnormal cAMP signaling in youths at clinical risk for psychosisSchizophr Res20121382–328028422537567
- IshizukaKTajindaKColantuoniCNegative symptoms of schizophrenia correlate with impairment on the University of Pennsylvania smell identification testNeurosci Res201066110611019819272
- DoopMLParkSOn knowing and judging smells: identification and hedonic judgment of odors in schizophreniaSchizophr Res2006812–331731916181773
- StraussGPAllenDNRossSADukeLASchwartzJOlfactory hedonic judgment in patients with deficit syndrome schizophrenia. Olfactory hedonic judgment in patients with deficit syndrome schizophreniaSchizophr Bull201036486086819223658
- VolavkaJDavisLGEhrlichYHEndorphins, dopamine, and schizophreniaSchizophr Bull19795222723937595
- Laux-BiehlmannAMouheicheJVérièpeJGoumonYEndogenous morphine and its metabolites in mammals: history, synthesis, localization and perspectivesNeuroscience20132332339511723266549
- RoyetJPHudryJZaldDHFunctional neuroanatomy of different olfactory judgmentsNeuroimage200113350651911170816
- TahaSANorstedELeeLSEndogenous opioids encode relative taste preferenceEur J Neurosci20062441220122616925586
- MansourAKhachaturianHLewisMEAkilHWatsonSJAnatomy of CNS opioid receptorsTrends Neurosci19881173083142465635
- LötschJDarimontJSkarkeCZimmermannMHummelTGeisslingerGEffects of the opioid remifentanil on olfactory function in healthy volunteersLife Sci200169192279228511669470
- ZouLQvan HarteveltTJKringelbachMLCheungEFChanRCThe neural mechanism of hedonic processing and judgment of pleasant odors: an activation likelihood estimation meta-analysisNeuropsychology201630897097927195988
- BlanchardJJCohenASThe structure of negative symptoms within schizophrenia: implications for assessmentSchizophr Bull200632223824516254064
- SmithKSBerridgeKCOpioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidumJ Neurosci20072771594160517301168
- KringelbachMLBerridgeKCTowards a functional neuroanatomy of Pleasure and happinessTrends Cogn Sci2009131147948719782634
- Urban-KowalczykMStrzeleckiDŚmigilelskiJComparison of beta-endorphin and CGRP levels before and after treatment for severe schizophreniaNeuropsychiatr Dis Treat20161512863868
- Urban-KowalczykMPigońskaJŚmigielskiJPain perception in schizophrenia: influence of neuropeptides, cognitive disorders, and negative symptomsNeuropsychiatr Dis Treat2015112023203126273205
- DotyRLShamanPDannMDevelopment of the University of Pennsylvania smell identification test: a standardized microencapsulated test of olfactory functionPhysiol Behav19843234895026463130
- GoodKPWhitehornDRuiQMillikenHKopalaLCOlfactory identification deficits in first-episode psychosis may predict patients at risk for persistent negative and disorganized or cognitive symptomsAm J Psychiatry2006163593293316648339
- Kotlicka-AntczakMPawełczykAKarbownikMSDeficits in the identification of pleasant odors predict the transition of an at-risk mental state to psychosisSchizophr Res2017181495427765522
- GoodKPTibboPMillikenHAn investigation of a possible relationship between olfactory identification deficits at first episode and four-year outcomes in patients with psychosisSchizophr Res20101241–3606520692126
- CorcoranCHWhitakerAColemanEOlfactory deficits, cognition and negative symptoms in early onset psychosisSchizophr Res20051580
- SorokowskaANegoiasSHärtwigSDifferences in the central-nervous processing of olfactory stimuli according to their hedonic and arousal characteristicsNeuroscience2016324324626826968764
- HawkesCHFogoAShahMSmell identification declines from age 36 years and mainly affects pleasant odoursMov Disorders200520Supplement 10160
- KonstantinidisIHummelTLarssonMIdentification of unpleasant odors is independent of ageArch Clin Neuropsychol200621761562116920329
- AusterTLCohenASCallawayDABrownLAObjective and subjective olfaction across the schizophrenia spectrumPsychiatr Interpers Biol Process20147715766
- DelplanqueSGrandjeanDChreaCEmotional processing of odors: evidence for a nonlinear relation between pleasantness and familiarity evaluationsChem Senses200833546947918403383
- HeereyEAGoldJMPatients with schizophrenia demonstrate dissociation between affective experience and motivated behaviorJ Abnorm Psychol2007116226827817516760
- StraussGPGoldJMA new perspective on anhedonia in schizophreniaAm J Psychiatry2012169436437322407079
- PetrovicPPlegerBSeymourBBlocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and lossesJ Neurosci20082842105091051618923027
- YeomansMRWrightPLower pleasantness of palatable foods in nalmefene-treated human volunteersAppetite19911632492591883251
- KreamRMStefanoGBPtáčekRPsychiatric implications of endogenous morphine: up-to-date reviewFolia Biol2010566231241
- BrambillaFGenazzaniARFacchinettiFBeta-endorphin and beta-lipotropin plasma levels in chronic schizophrenia, primary affective disorders and secondary affective disordersPsychoneuroendocrinology1981643213306275438
- DomschkeWDickschasAMitzneggPC.S.F. beta-endorphin in schizophreniaLancet1979121024
- MauriMCRudelliRVanniSCholecystokinin, beta-endorphin and vasoactive intestinal peptide in peripheral blood mononuclear cells of drug-naive schizophrenic patients treated with haloperidol compared to healthy controlsPsychiatry Res1998201–24550
- FerrerJMtnez-GuisasolaJDíazFAlonsoFGuerreroMMarínBPlasma levels of beta-endorphin during the menstrual cycleGynecol Endocrinol199711275829174847