Abstract
Objective
Spontaneous supratentorial intracerebral hemorrhage (SSICH) is one of the deadliest diseases, and neuroendoscopic surgery (NE) is a minimally invasive and promising treatment that might improve the functional recovery of patients. This study analyzed patient’s experience with this treatment in terms of safety, efficacy, and surgical technique.
Patients and methods
Forty-two patients with SSICHs treated by transcranial neuroendoscopic approach were retrospectively reviewed from June 2016 to July 2018 in our department. Patients were classified into four groups according to the main location of the hematoma on CT scans: Group A (basal ganglia hemorrhage), Group B (subcortical hemorrhage), Group C (thalamic hemorrhage), and Group D (intraventricular hemorrhage [IVH]). The clinical data were collected, and the outcomes were analyzed.
Results
All procedures were successfully completed, and no patient died in the perioperative period. The average hematoma evacuation rate was 90.1%, and the highest hematoma evacuation rate was achieved in Group B which was 92.7%. No severe complications occurred, and the average GCS score improvement was 4.0 at discharge.
Conclusion
These data suggest that evacuation hemorrhage by neuroendoscopy might be an effective and safe approach for SSICH. For better efficiency of this treatment, some details needed to be emphasized, such as setting up a good working channel, using of suction and bipolar forceps accurately.
Introduction
Spontaneous supratentorial intracerebral hemorrhage (SSICH) is the deadliest disease and has high morbidity rate, high mortality rate, high disability rate, and high economic burden.Citation1 The 30-day mortality is approximately 40%,Citation2 and up to 75%Citation3 of the long-term survivors suffer significant disability. Unfortunately, the use of the traditional craniotomy for SSICH remains a matter of controversy and has not been demonstrated to significantly improve outcome compared with medical management.Citation4–Citation6
Recent reports have demonstrated that neuroendoscopic surgery (NE) is a minimally invasive treatment that is an attractive alternative and a promising procedure that may improve the rate of good functional recovery from intracerebral hemorrhage (ICH).Citation7–Citation9 However, according to the American Heart Association/American Stroke Association Guidelines, supporting evidence for this method from controlled trials is still lacking.Citation10 Therefore, we present our series of patients with SSICH who underwent neuroendoscopic hematoma evacuations and discuss the safety, efficacy, and surgical technique of this approach.
Patients and methods
Surgical indications and patient selection
Forty-two patients with SSICH treated by the transcranial neuroendoscopic approach were retrospectively reviewed from June 2016 to July 2018 in our department. The clinical data were collected, and the outcomes (including the average surgery time, hematoma evacuation rate, perioperative mortality, and Glasgow Coma Scale [GCS] improvement) and complications (including rebleeding, pneumonia, and intracranial infection) were analyzed. Patients were classified into four groups according to the main location of the hematoma on CT scans: Group A (basal ganglia hemorrhage), Group B (subcortical hemorrhage), Group C (thalamic hemorrhage), and Group D (intraventricular hemorrhage [IVH]) (). The intracerebral hematoma volumes were analyzed with 3D Slicer software (http://www.slicer.org). All patients underwent a CT scan before the operation, a post-operation CT scan within 24 hours after the surgery, and a follow-up CT or MRI scan 3 days to 1 month after the operation. The hematoma evacuation rate was calculated as follows: ([preoperative hematoma volume - postoperative hematoma volume]/preoperative hematoma volume) ×100% (). After surgery, the patients were managed in the intensive care unit, the blood pressure was controlled, and the consumption of excessive fluid was not allowed.
Figure 1 Calculation and analysis of the hematoma volumes and hematoma evacuation rates in the neuroendoscopic surgery patients with the 3D Slice software.
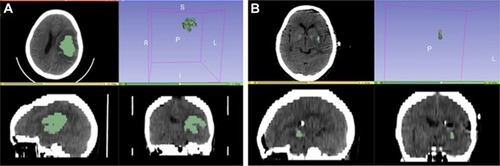
Table 1 Demographic and clinical characteristics of the 42 patients with SSICH
The inclusion criteria for this study were the following: 1) diagnosis of acute SICH, 2) hematoma volume greater than 30 mL for a putaminal ICH, 3) hematoma volume greater than 20 mL for a thalamic ICH, 4) IVH with acute hydrocephalus, 5) subcortical hemorrhage greater than 30 mL with a significant mass effect (midline shift greater than 5 mm), 6) a GCS score ≥5, and 7) stable vital signs. The exclusion criteria were the following: 1) the ICH was caused by secondary factors (eg, arteriovenous malformation, aneurysm, tumor stroke, or head injury); 2) serious visceral disease or clotting disorders (ie, a prothrombin time >12.2 seconds, a partial thromboplastin time >35.5 seconds, or a platelet count <100×103/mL); 3) a GCS of <5; and 4) multiple intracranial hemorrhages.
Neuroendoscopic surgical management
This study was approved by the ethics committee of Renmin Hospital of Wuhan University. All patients or their family provided written informed consent, and this procedure was conducted in accordance with the Declaration of Helsinki.
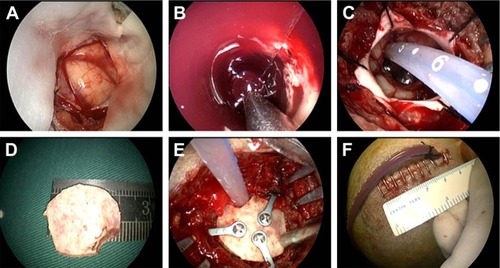
All surgeries were performed under general anesthesia with the patients in the supine position. The surgical approaches were chosen according to the locations of the hematomas. Typically, a linear scalp incision (4–5 cm) was made, and then, a small bone flap (~2.5 cm) was created. After tenting the dura, it was opened in a cruciate fashion. A 1.5 cm cortical incision was made, and a transparent plastic sheath was inserted into the hematoma cavity. A 0° rigid endoscopy (Karl Storz, Germany) was introduced into the space that was created by the hematoma, and suction was used to keep surgical field clear. Usually, most of the clot and blood gushed out due to high pressure and were removed under direct vision by endoscopy. During the operation, obvious bleeding was stopped using a bipolar coagulator with a low output power.
After evacuation of the hematoma, a soft catheter was laid inside the hematoma cavity to drain any residual liquid hematoma, the bone flap was recovered, and the skin incision was closed ().
Figure 2 Surgical approach for a basal ganglia hemorrhage.
Results
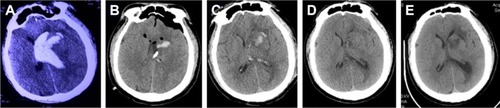
The 42 patients in this study included 28 men and 14 women with a median age of 59.7 years (range 41–79 years). Among these patients, there were 22 cases in Group A (52.3%), 10 cases in Group B (23.8%), six cases in Group C (14.3%), and four cases in Group D (9.5%) (). In Group A, we usually selected the “temporal” approach, and the post-operation CT scans indicated that the hematomas were nearly completely evacuated by the neuroendoscopy. The follow-up CT scans revealed that these patients recovered well based on the images (). In Group B, the corridor shortest distance to the hematoma was used and also the hematoma was evacuated satisfactorily (). In Group C, a midline approach in which the ipsilateral Kocher point served as the entry point and the follow-up CT scans indicated that no further damage to the thalamus was caused by neuroendoscopy (). In Group D, also the ipsilateral Kocher point was selected and the post-operation CT scans showed well (). The preoperative hematoma volume was 49.5 mL (24.3–122.5 mL), and the average hematoma evacuation rate was 90.1%. The highest hematoma evacuation rate was achieved in Group B (92.7%), the second highest in Group A (90.7%), the third highest in Group D (87.9%), and the lowest in Group C (84.6%).
Figure 3 Pre- and post-operation CT scans of a basal ganglia hemorrhage that was evacuated by neuroendoscopy.
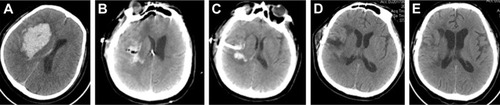
Figure 4 Pre- and post-operation CT scans of a subcortical hemorrhage that was evacuated by neuroendoscopy.
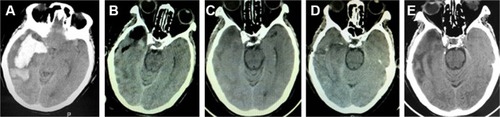
Figure 5 Pre- and post-operation CT scans of a thalamic hemorrhage breaking into the ventricles that was evacuated by neuroendoscopy.
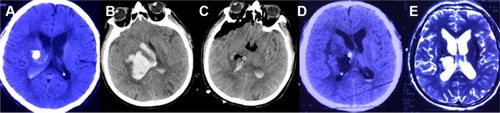
Figure 6 Pre- and post-operation CT scans of an intraventricular hemorrhage that was evacuated by neuroendoscopy.
All procedures were successfully completed, and the average surgery time ranged from 47.5 to 85.6 minutes with a mean length of 63.1 minutes. The median GCS scores were 7.3 before surgery and 11.3 at discharge; thus, the average GCS score improvement was 4.0.
No patients died within 3 days of surgery, and the surgery-related mortality was 0%. However, three patients were discharged at GCS 3 and died in their home for no improvement after surgery and their family decided to give up further treatment. Thus, the total mortality rate was 7.1%. One patient experienced rebleeding due to malignant hypertension 4 days after the surgery, and the rebleeding rate was 2.4%. The hematoma of this rebleeding patient was 25 mL, and conservative treatment was administered by fibrinolysis agent (containing 20,000 U–40,000 U urokinase/2–3 mL saline solution) for 3 days. The patient’s GCS score improved gradually after draining and reached 14 at discharge. Pneumonia happened in three cases, and no patient died from this complication. There were no cases of intracranial infection.
Discussion
Although spontaneous ICHs account for only 15% of all strokes, this condition is the most dangerous and disabling form of stroke.Citation11 The high mortality rate of SSICH patients has been found to be significantly associated with the hematoma volume, treatment method, and location of the hematoma. Rost et alCitation12 concluded that ICH volumes are frequently used in clinical decision-making and categorized the volumes as <30 mL, 30–60 mL, and >60 mL. In Bhaskar’s study, the mortality rate at 3 months was found to be directly proportional to hematoma volume (P=0.039); the mortality rates reported in this study were 56.4% among patients with hematoma volumes in the range of 31–60 mL, 81% for volumes in the range of 61–90 mL, and 100% for volumes >90 mL.Citation13
The choice of treatment for SSICH is difficult, but medical treatment is usually recommended for patients with small cerebral hemorrhages. However, the best therapeutic option for those with medium-to-large-sized SSICHs remains controversial.Citation14 Juvela et alCitation15 found that there were no significant differences in mortality or morbidity rates between patients who were treated surgically and those who were treated conservatively; however, the mortality rate was significantly lower in the surgical group among patients with GCS scores between 7 and 10.
Moreover, the Surgical Trial in Intracerebral Hemorrhage (STICH) studied 1,033 patients with SSICH and reported that there was no overall benefit of surgical intervention compared with nonsurgical management.Citation4 However, in Mukesh’s study,Citation13 the mortality rate at 3 months was significantly lower in the surgical group (61.8%) than in the conservative group (85.2%; P=0.043), and the mortality rate was significantly higher (80.5%) among patients with GCS scores of 4–8 compared with those with GCS scores of 9–14 (55%) among both the surgical and conservative treatment groups (P=0.037). Furthermore, among patients with GCS scores of 4–8, the mortality rate of the surgical group (65.2%) was found to be significantly lower than that of the conservative therapy group (100%; P=0.005). Despite the findings of multiple prior surgical trials, the current conservative treatment is insufficient, especially for patients with hemorrhages occupying large spaces and secondary deterioration.Citation16 Thus, Gregson and Mendelow concluded that there is no convincing evidence of the benefit of any medical treatment, and the role of surgery remains controversial.Citation17 Furthermore, the efficacy of any medical or surgical treatment has yet to be proven in a large randomized trial.
The main drawback of traditional surgery is the invasive procedure, which causes too much damage and fails to protect the still-functional brain tissue. An analysis of more than 45,000 patients revealed that the in-hospital mortality rate for this method is 27.2%, and the complication rate (eg, pulmonary, renal, and thromboembolic complications) is 41.2%.Citation18 Compared with conventional craniotomy, endoscopic surgery offers a minimally invasive treatment option with a more favorable safety profile than traditional approaches that do not compromise efficacy. Accumulating evidence indicates that the endoscopic approach might provide minimal invasiveness, maximum efficiency in clot removal, and maximum patient safety.Citation19,Citation20 Prasad et alCitation21 reported that endoscopic evacuation decreased the risk of death and dependence in patients with hematoma volumes greater than 50 mL. Auer et alCitation22 reported lower mortality (3%) and lower morbidity (3%) in an endoscopic group, and Cho et alCitation23 reported that the mortality rate was decreased to 0% via the use of the endoscopic approach. In terms of complications, the study from Nagasaka indicated that the endoscopic approach was associated with a minimal rebleeding rate (0%–3.3%) compared with the traditional craniotomy approach (5%–10%).Citation24 In the treatment of basal ganglia hemorrhages, Zhang et alCitation8 reported a lower rebleeding rate in an endoscopic group than in a control group (4.76% versus 10%), but this result was not statistically significant (P=0.50).
In recent decades, some studies have demonstrated high evacuation rates following the treatment of SSICHs via the endoscopic approach. Zhang et alCitation8 reported that the hematoma evacuation rate in an endoscopic group was higher than that in a traditional craniotomy group (P<0.05). Cho et alCitation23 successfully evacuated 92% of a 120 mL hematoma using endoscopic surgery,Citation23 and Nishihara et alCitation25 achieved almost complete evacuation (86%–100%) of a hematoma greater than 40 mL without complications.
Additionally, several series have reported lower rates of rebleeding, morbidity, and mortality, following endoscopic surgery compared with traditional craniotomy.Citation23,Citation26 The main reasons for these findings are reduced adjacent tissue injury, less blood loss, and reduced operation times. Truly minimally invasive surgery involves not only a minimal wound size but also minimal brain tissue trauma during surgery.Citation27 In our study, we achieved a similar result, and the average hematoma evacuate rate was 90.1%, which confirms the effectiveness of the neuroendoscopic technique. In conclusion, the application of the neuroendoscopic approach might be an effective and safe approach for SSICH.
To achieve these advantages, some of the surgical techniques of the endoscopic approach require an emphasis on the protection of the surrounding brain regions to achieve better prognoses. Setting up a good working channel is the first and important step in NE, and many working channels, such as transparent sheaths or other handmade sheaths, have been developed and reported in the literature.Citation27 These techniques allow for the dynamic, multiangled evacuation of the hematoma, clot cavity control, and hemostasis without harming the brain parenchyma.
Conclusion
The key advantage of the minimally invasive approach is the drastically reduced manipulation of viable brain tissue; thus, the use of suction and a bipolar coagulator are also important in the neuroendoscopic approach for the treatment of SSICH. Similar to the report of Wang et al,Citation27 in our practice, we used suction only to evacuate the hematoma, and a thin hematoma over the surrounding brain tissue could be left intact. The power applied with the bipolar forceps was the minimum possible that still achieved the coagulation of the bleeding vessel, and hemostatic agents were used instead of coagulation if there was merely minor oozing.
Abbreviations
| GCS | = | Glasgow Coma Scale |
| ICH | = | intracerebral hemorrhage |
| NE | = | neuroendoscopic surgery |
| SSICH | = | Spontaneous Supratentorial Intracerebral Hemorrhage |
Acknowledgments
This work was supported by National Natural Science Foundation of China (81671306) and the Foundation of China Scholarship Council.
Disclosure
The authors report no conflicts of interest in this work.
References
- AkhigbeTZolnourianARole of surgery in the management of patients with supratentorial spontaneous intracerebral hematoma: critical appraisal of evidenceJ Clin Neurosci201739353810.1016/j.jocn.2017.02.02228258905
- van AschCJLuitseMJRinkelGJvan der TweelIAlgraAKlijnCJIncidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysisLancet Neurol20109216717610.1016/S1474-4422(09)70340-020056489
- AndersonCSHuangYWangJGINTERACT InvestigatorsIntensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trialLancet Neurol20087539139910.1016/S1474-4422(08)70069-318396107
- MendelowADGregsonBAFernandesHMSTICH investigatorsEarly surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trialLancet2005365945738739710.1016/S0140-6736(05)17826-X15680453
- MendelowADGregsonBARowanENMurrayGDGholkarAMitchellPMEarly surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trialLancet2013382989039740810.1016/S0140-6736(13)60986-123726393
- PrzybylowskiCJDingDStarkeRMWebsterCRLiuKCEndoport-assisted surgery for the management of spontaneous intracerebral hemorrhageJ Clin Neurosci201522111727173210.1016/j.jocn.2015.05.01526238692
- LongattiPBasaldellaLEndoscopic management of intracerebral hemorrhageWorld Neurosurg2013792SupplS17e11S17e1710.1016/j.wneu.2012.02.025
- ZhangHZLiYPYanZCWangXDSheLDongLEndoscopic evacuation of basal ganglia hemorrhage via keyhole approach using an adjustable cannula in comparison with craniotomyBiomed Res Int2014201489876224949476
- AngileriFFEspositoFPriolaSMFully endoscopic freehand evacuation of spontaneous supratentorial intraparenchymal hemorrhageWorld Neurosurg20169426827210.1016/j.wneu.2016.07.01527423197
- HemphillJC3rdGreenbergSMAndersonCSAmerican Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical CardiologyGuidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke AssociationStroke20154672032206010.1161/STR.000000000000006926022637
- QureshiAIMohammadYMYahiaAMA prospective multicenter study to evaluate the feasibility and safety of aggressive antihypertensive treatment in patients with acute intracerebral hemorrhageJ Intensive Care Med2005201344210.1177/088506660427161915665258
- RostNSSmithEEChangYPrediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC scoreStroke20083982304230910.1161/STROKEAHA.107.51220218556582
- BhaskarMKKumarROjhaBA randomized controlled study of operative versus nonoperative treatment for large spontaneous supra-tentorial intracerebral hemorrhageNeurol India201765475275810.4103/neuroindia.NI_151_1628681745
- MorgensternLBFrankowskiRFSheddenPPasteurWGrottaJCSurgical treatment for intracerebral hemorrhage (STICH): a single-center, randomized clinical trialNeurology1998515135913639818860
- JuvelaSHeiskanenOPoranenAThe treatment of spontaneous intracerebral hemorrhage. A prospective randomized trial of surgical and conservative treatmentJ Neurosurg198970575575810.3171/jns.1989.70.5.07552651586
- MishraPNaithaniRDolaiTIntracranial haemorrhage in patients with congenital haemostatic defectsHaemophilia200814595295510.1111/j.1365-2516.2008.01814.x18637845
- GregsonBAMendelowADSTICH InvestigatorsInternational variations in surgical practice for spontaneous intracerebral hemorrhageStroke200334112593259710.1161/01.STR.0000097491.82104.F314563963
- PatilCGAlexanderALHayden GephartMGLadSPArrigoRTBoakyeMA population-based study of inpatient outcomes after operative management of nontraumatic intracerebral hemorrhage in the United StatesWorld Neurosurg201278664064510.1016/j.wneu.2011.10.04222120557
- PerneczkyAFriesGEndoscope-assisted brain surgery: part 1– evolution, basic concept, and current techniqueNeurosurgery1998422219224 discussion 224–2159482171
- FriesGPerneczkyAEndoscope-assisted brain surgery: part 2–analysis of 380 proceduresNeurosurgery1998422226231 discussion231–2229482172
- PrasadKBrowmanGSrivastavaAMenonGSurgery in primary supratentorial intracerebral hematoma: a meta-analysis of randomized trialsActa Neurol Scand19979521031109059730
- AuerLMDeinsbergerWNiederkornKEndoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized studyJ Neurosurg198970453053510.3171/jns.1989.70.4.05302926492
- ChoDYChenCCChangCSLeeWYTsoMEndoscopic surgery for spontaneous basal ganglia hemorrhage: comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patientsSurg Neurol2006656547555 discussion 555–54610.1016/j.surneu.2005.09.03216720167
- GaabMRIntracerebral hemorrhage (ICH) and intraventricular hemorrhage (IVH): improvement of bad prognosis by minimally invasive neurosurgeryWorld Neurosurg201175220620810.1016/j.wneu.2010.10.00321492714
- NishiharaTTeraokaAMoritaAUekiKTakaiKKirinoTA transparent sheath for endoscopic surgery and its application in surgical evacuation of spontaneous intracerebral hematomas. Technical noteJ Neurosurg20009261053105510.3171/jns.2000.92.6.105310839271
- NagasakaTTsugenoMIkedaHOkamotoTInaoSWakabayashiTEarly recovery and better evacuation rate in neuroendoscopic surgery for spontaneous intracerebral hemorrhage using a multifunctional cannula: preliminary study in comparison with craniotomyJ Stroke Cerebrovascular Dis201120320821310.1016/j.jstrokecerebrovasdis.2009.11.021
- WangWHHungYCHsuSPEndoscopic hematoma evacuation in patients with spontaneous supratentorial intracerebral hemorrhageJ Chin Med Assoc201578210110710.1016/j.jcma.2014.08.01325467795
