Abstract
Signs and symptoms of depression can be linked to one or more monoaminergic systems, specifically the norepinephrine (NE), the dopamine (DA), and the serotonin (5-HT) systems. In particular, the modulation of energy, vigilance, and arousal can be directly linked to the NE system. There is, however, a great deal of overlap in the modulation of the symptoms of depression between these monoaminergic systems. There are considerable reciprocal interactions between the NE, DA, and the 5-HT systems. When using a selective serotonin reuptake inhibitor (SSRI), for example, 5-HT transmission is enhanced, but at the same time there is a dampening of the activity of NE and DA neurons through inhibitory 5-HT2A and 5-HT2C receptors, respectively. This could explain the residual symptoms of fatigue, lack of energy, and anhedonia, often seen after patients present an overall positive response to a SSRI. Using a dual 5-HT and NE reuptake inhibitor (SNRI), such as milnacipran, would result in an additional increase in NE activity. Futhermore, inhibiting NE reuptake increases DA availability in the frontal cortex since DA is mainly cleared by the NE transporters in several brain regions. A risk inherent in increased NE activity is that of provoking anxiety. This is avoided however by the attenuation of the phasic reactivity of the firing of NE neurons through prolonged administration of SSRI and SNRI.
Introduction
Noradrenergic pathways in the brain originate mainly in the locus coeruleus and project to the cortex, limbic regions, and hindbrain. They are involved in the regulation of energy levels, vigilance, reactivity, and executive function. Noradrenergic stimulation dampens noise in both excitatory and inhibitory circuits thus enhancing signal to noise in the target areas.Citation1
The functions of norepinephrine (NE) and dopamine (DA) are closely linked. The DA system is extremely complex due to the multitude of DA receptor subtypes, their locations, and functions. The nigrostriatal DA pathway, which projects from the substantia nigra to the basal ganglia, is part of the extrapyramidal system and plays a key role in regulating movement. DA deficiency can thus result in Parkinsonism with tremor, rigidity, and akinesia/bradykinesia. The mesolimbic DA pathway originates from dopaminergic cell bodies in the ventral tegmental area of the brainstem and projects to limbic regions of the brain. These pathways play an important role in emotional behavior, such as pleasurable sensations, the euphoria experienced with drugs of abuse, and the delusions and hallucinations of psychosis. Mesocortical DA projections mediate cognitive functions, such as verbal fluency, focus, serial learning, executive functioning, focusing and sustaining attention, prioritizing behavior, and modulating social behavior.Citation1
The effects of NE, serotonin (5-HT), and DA overlap in the brain and all three transmitters are implicated in the symptoms of depression. The three neurotransmitters are involved in mood, emotion, cognition, and chronic pain. Symptoms associated with vigilance, arousal, interest, and energy are most closely associated with NE neurotransmission while impulsivity is associated with 5-HT neurotransmission, and symptoms associated with drive are related to DA neurotransmission. Thus, depressive symptoms may result from dysfunction of any or all of the monoamine neurotransmitter systems.Citation1
Residual symptoms
Remission, usually defined as a score of 7 or less on the Hamilton depression rating scale (HAMD) or 10 or less on the Montgomery–Asberg depression rating scale (MADRS), does not necessarily mean a total absence of all symptoms. Residual symptoms are present in 30% to 50% of patients in remission. In addition to the reduced quality of life this causes, relapse rates are 3 to 6 times higher in patients with residual symptoms.Citation2,Citation3
The nature of the residual symptoms can vary depending on the nature of the initial depression and antidepressant usedCitation4 but in general, fatigue (both mental and physical), concentration difficulties, decreased interest or pleasure, cognitive impairment, and anxiety are among the most common residual symptoms.Citation3,Citation5 For example, in a study of 215 patients with major depressive disorder who received fluoxetine for 8 weeks, 108 were found to be in remission (HAMD ≤7).Citation6 Of these, however, only 18% had no threshold or subthreshold symptoms of major depression, while 26% had one residual symptom, and over half (57%) still had 2 or more symptoms. The question can be asked as to whether these remaining symptoms are true residual symptoms or iatrogenic symptoms caused by the antidepressants used to treat the disorder.Citation2,Citation3 In order to answer this question it is necessary to examine the interaction between 5-HT and NE and DA neurotransmission.
Interaction between 5-HT and NE neurotransmission
5-HT projections from the raphe nucleus to the locus coeruleus impose a tonic inhibitory tone on the firing of NE neurons. When this inhibition is removed, NE firing and release are increased. This has been shown in animal studies where brain 5-HT levels were reduced by administration of the 5-HT synthesis inhibitor, p-chlorophenylalanineCitation7 or by lesion of the raphe nucleus using the specific 5-HT neuron toxin, 5,7-dihydroxytryptamine (5,7-DHT).Citation8 In both cases removal of the serotonergic influence on the locus coeruleus led to a significant increase in firing of the NE neurons.
If the serotonergic inhibition of NE neurotransmission is increased, as during treatment with a selective serotonin reuptake inhibitor (SSRI), NE firing and release are decreased. Sustained administration of the SSRI, citalopram, for 21 days, (20 mg/kg/d sc using osmotic minipumps) produced a progressive decrease in spontaneous firing activity of NE neurons in the locus coeruleus.Citation9 Similarly, subacute and long-term treatment with the SSRI, escitalopram, decreased NE neuronal firing. Blockade of this effect by selective antagonists showed that the effect was mediated by the 5-HT2A receptor.Citation7
The effects of chronic administration of citalopram are also seen on the levels of extracellular NE in the brain. Brain microdialysis was used to determine the extracellular NE in conscious rats. In rats treated daily for 14 days with citalopram (10 mg/kg/day sc), NE levels in the dialysate showed a significant decrease in the basolateral nucleus of the amygdala.Citation10
Interaction between 5-HT and DA neurotransmission
In addition to its tonic inhibition of NE transmission, 5-HT also inhibits the firing of DA neurons. Selective lesion of 5-HT neurons by 5,7-DHT enhanced the firing activity of DA neurons in the ventral tegmental area by 36%, indicating the presence, under normal conditions, of a tonic inhibitory effect of 5-HT on these DA neurons.Citation11 Sustained administration of escitalopram robustly decreased the firing rate of DA neurons. This inhibition was reversed by a selective 5-HT2C receptor antagonist.Citation7
Thus 5-HT exerts a tonic inhibition on NE neurons through 5-HT2A receptors and a tonic inhibition of DA neurons through 5-HT2C receptors.
Residual or iatrogenic symptoms?
SSRI treatment results in a sustained increase of 5-HT activity and thus an increased inhibitory tone which leads to a decrease of both NE and DA neurotransmission. These two neurotransmitters are closely implicated in the regulation of the many frequent residual symptoms, namely, fatigue, decreased concentration, decreased interest or pleasure, cognitive impairment, and anxiety. Thus it is possible that these “residual” symptoms could be, at least in part, the result of SSRI treatment and thus “iatrogenic”.
The effects of milnacipran
The serotonin norepinephrine reuptake inhibitor (SNRI), milnacipran, is unique in as much as it blocks the reuptake of both 5-HT and NE with similar affinity without any effects on postsynaptic receptors.Citation12,Citation13 As with SSRIs, milnacipran inhibits the firing of NE neurons in the locus coeruleus.Citation14 Unlike SSRIs, however, milnacipran also blocks the reuptake of NE. Microdialysis studies have shown that, unlike SSRIs, which after chronic administration increase 5-HT levelsCitation7,Citation9,Citation10 but decrease NE levels, both acute and subacute administration of milnacipran increase extracellular levels of both 5-HT and NE.Citation15,Citation16
Although it has no affinity with the DA transporter (and does not block the reuptake of DA),Citation12,Citation13 milnacipran increases DA levels, as well as NE and 5-HT levels, in the frontal cortex.Citation16,Citation17 Although the exact nature of this effect has not yet been determined a probable mechanism can be proposed. The NE transporter has similar affinities for NE and DA.Citation18 Since DA is relatively abundant compared with DA transporters in the prefrontal cortexCitation19 it can be taken up non-selectively by NE transporters in this regionCitation20 and co-released with NE.Citation21 The selective NE uptake inhibitors, reboxetine and atomoxetine, which have no affinity with the DA transporter, have been shown to increase extracellular DA levels as well as NE levels in the prefrontal cortex, but not in the nucleus accumbens.Citation22,Citation23 The inhibition of the NE transporter by milnacipran therefore results in an increase in the extracellular levels of both NE and DA.
In contrast to the SSRIs, milnacipran increases extra-cellular levels of NE and DA which may compensate for the decreased firing of NE neurons. Clinically this could translate into less reduced residual (iatrogenic?) symptoms compared with treatment with SSRIs. No studies have yet analyzed the comparative frequencies of residual symptoms with milnacipran and SSRIs. However a recent study comparing the SSRI, escitalopram with the SNRI, duloxetine, found that patients on the SNRI had greater improvement in cognitive function in the remission phase, and in the recovery phase.Citation24
The noradrenergic paradox
There is strong evidence of a close relationship between NE stimulation and the induction of anxiety states.Citation25 If milnacipran increases synaptic levels of NE (and DA) why does it not increase anxiety?
The effects of NE neurotransmission on the function of the prefrontal cortex can be described by an inverted U shape ().Citation26 Very low levels of NE (and DA) transmission result in cognitive impairment, inattention, and drowsiness. Optimal levels of NE neurotransmission lead to enhanced cognitive performance and executive functions that guide behavior, thought, and affect (). Very high levels of NE stimulation produce cognitive impairment, anxiety, and even dysphoric psychosis. Thus one might expect chronic NE reuptake inhibition with milnacipran to increase anxiety symptoms. This, however, does not happen.Citation27 Indeed several studies have shown that milnacipran decreases anxiety both in animal models of anxiety,Citation28 in anxio-depressiveCitation29 and anxio-schizophrenic patients.Citation30
Figure 1 Norepinephrine (NE) and dopamine (DA) neurotransmission in the prefrontal cortex and executive function. NE and DA in arrows represent increasing levels of stimulation.
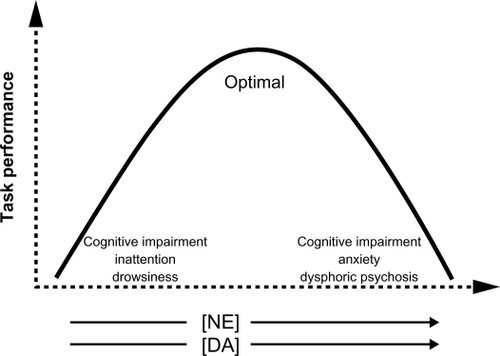
Table 1 Executive function in the prefrontal cortex and its effect on behavior, thought and affect
A possible reason is that there are two types of NE firing pattern in the brain: tonic and phasic.Citation31 Tonic firing is the steady-state, background firing that occurs at rest. It correlates with behavioral arousal at rest (awake, alert). Mechanistically it involves exocytotic release of NE at the nerve terminal. Phasic reactivity, on the other hand, occurs in response to a stimulus. It correlates with threat or stress-induced anxiety-like behavior or, in extreme cases, a panic attack. It can last from seconds to minutes or longer. It increases vigilance (hypervigilance), enhances sensorimotor reflex responses, increases acoustic startle reflex, and reduces immobility and facilitates active escape behaviors (fight or flight response to an aversive stimulus). It is a result of robust increases in NE neuron firing. A certain amount of phasic reactivity is necessary for survival, but too much (as in anxiety disorders and anxious depression and panic disorder) is maladaptive.
In the depressed state, with no threatening stimulus, tonic NE activity (exocytotic release) at rest is low. The low basal firing rate of NE neurons combined with an efficient reuptake may lead to very low extracellular NE levels in the prefrontal cortex resulting in fatigue, somnolence, and cognitive impairment.Citation31–Citation33 This corresponds to the left-hand end of the inverted-U curve ().
In the face of acute stress or a threatening stimulus, NE neurons (with low tonic, baseline levels of NE exerting only a minimal inhibition, via the somatodendritic α2-receptors, on the firing of neurons in the locus coeruleus) would respond with very high phasic reactivity (a sharp increase in neuronal firing). This important increase in firing would greatly increase extracellular NE to levels corresponding to the far right-hand end of the inverted-U curve (). Clinically this would produce cognitive impairment, anxiety/panic, and dysphoric psychosis.Citation14,Citation31–Citation33
Although it increases NE (and DA) neurotransmission in the prefrontal cortex, milnacipran does not cause anxiety. This NE paradox is explained by the different adaptation to chronic drug treatment of the two types of firing.Citation31
The effect of acute and long-term milnacipran treatment on 5-HT and NE neurotransmission
Acute treatment with milnacipran () results in increased synaptic concentrations of 5-HT but decreased firing of the 5-HT neurons in the raphe nucleus possibly as a result of increased stimulation of the inhibitory somatodendritic autoreceptors. Long-term treatment results in a further increase in synaptic concentrations of 5-HT due to desensitization of terminal autoreceptors controlling the release of 5-HT. Neuronal firing has been shown to recover to normal levels in the presence of SSRI or milnacipran.Citation14 The ensuing enhanced 5-HT neurotransmission may thus contribute to its antidepressant effect. The increased 5-HT transmission by SSRI would also act to inhibit NE neurotransmission. Decreased NE phasic reactivity (NE firing) would lead to an anxiolytic effect, but the decreased tonic NE activity (NE release) could potentially lead to side effects (or residual symptoms) such as, fatigue, apathy, low interest, indifference, and cognitive dulling.
Figure 2 Scheme of the activity of 5-HT (A) and norepinephrine (NE) (B) neurotransmission during treatment with milnacipran. A) 5-HT system. Acute treatment with milnacipran results in a decreased firing of 5-HT neurons from the raphe nucleus possibly due in part to increased stimulation of somatodendritic autoreceptors. Long-term treatment with milnacipran results in a full recovery in the firing rate of 5-HT neurons in the presence of milnacipran thereby leading to a net increase in 5-HT neurotransmission. Contributing to this enhancement are: 1) the normalized firing rate of 5-HT neurons in the presence of milnacipran 2) the desensitization of the terminal 5-HT1B autoreceptor, and 3) the desensitization of the α2-adrenergic heteroreceptors on 5-HT terminals. B) NE system. Acute treatment with milnacipran results in increased synaptic concentrations of NE but decreased firing of the NE neurons of the locus coeruleus due to increased stimulation of the somatodendritic α2-adrenergic autoreceptors. Long-term treatment with milnacipran results in a further increase in synaptic concentrations of NE due to desensitization of presynaptic autoreceptors. The somatodendritic α2-adrenergic autoreceptors do not desensitize.
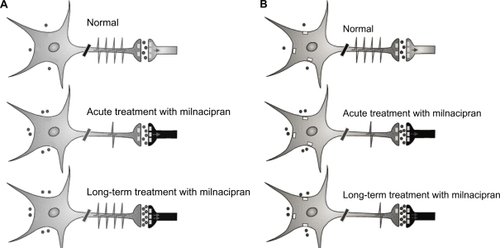
Treatment with milnacipran, however, will also act upon NE neurotransmission. Acute treatment will () result in increased synaptic concentrations of NE but decreased firing of the NE neurons in the locus coeruleus through stimulation of inhibitory somatodendritic autoreceptors. Long-term treatment results in a further increase in synaptic concentrations of NE due to desensitization of terminal autoreceptors (controlling the release of NE). The enhanced NE neurotransmission may thus contribute to the antidepressant effect. In contrast to terminal α2-adrenergic autoreceptors controlling NE release, long-term blockade of the NE transporter does not desensitize somatodendritic α2-adrenergic autoreceptors which control NE firingCitation34,Citation35 so that the firing of the NE neurons remains suppressed. This phenomenon has been recently reviewed.Citation36 This decreased phasic NE reactivity may contribute to the anxiolytic effect. The enhanced tonic NE activity however, should potentially reduce the risk of the long-term side effects (residual symptoms?) seen with SSRIs. In addition the low phasic NE reactivity may protect against the acute stress-induced anxiety or panic attacks following long-term NE reuptake inhibition in part also due to the desensitization of excitatory β-adrenergic receptors on postsynaptic neurons.
Conclusion
The signs and symptoms of depression are modulated by changes in NE, 5-HT, and DA neurotransmission. Reduced NE activity may contribute to symptoms such as fatigue, apathy, low interest, indifference, and decreased cognitive performance.
SSRIs may contribute to residual symptoms by dampening NE and DA neuronal activity. Milnacipran, which increases NE (and DA) as well as 5-HT neurotransmission, is expected to improve depressive symptoms without causing iatrogenic residual symptoms. Milnacipran increases tonic NE activity, while attenuating phasic NE reactivity. Its action on the three monoamine neurotransmitters contributes to its antidepressant action while its attenuation of phasic NE reactivity may decrease the risk of anxiety.
Disclosures
Dr Pierre Blier has received research support from, spoken for, or sits on advisory boards for the following companies: AstraZeneca, Biovail, Bristol-Myers Squibb, Eli Lilly, Cyberonics, Janssen-Ortho, Jazz Pharmaceuticals, Pierre Fabre Medicaments, Labopharm, Lundbeck/Takeda, Merck, Schering-Plough, Sanofi-Synthélabo, Servier, Shire and Wyeth. Dr Mike Briley is a consultant for Pierre Fabre Médicament, Asahi Kasei Pharma, Germania Pharmaceutica, Janssen Pharmaceutica, and Cypress BioScience.
References
- StahlSMStahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications3rd edCambridge, UKCambridge University Press2008
- NuttDDemyttenaereKJankaZThe other face of depression, reduced positive affect: the role of catecholamines in causation and cureJ Psychopharmacol20072146147117050654
- TrivediMHHollanderENuttDBlierPClinical evidence and potential neurobiological underpinnings of unresolved symptoms of depressionJ Clin Psychiatry20086924625818363453
- KurianBTGreerTLTrivediMHStrategies to enhance the therapeutic efficacy of antidepressants: targeting residual symptomsExpert Rev Neurother2009997598419589048
- ConradiHJOrmelJde JongePPresence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective studyPsychol Med2011In press.
- NierenbergAAKeefeBRLeslieVCResidual symptoms in depressed patients who respond acutely to fluoxetineJ Clin Psychiatry19996022122510221281
- DremencovEEl MansariMBlierPNoradrenergic augmentation of escitalopram response by risperidone: electrophysiologic studies in the rat brainBiol Psychiatry20076167167816934772
- HaddjeriNde MontignyCBlierPModulation of the firing activity of noradrenergic neurones in the rat locus coeruleus by the 5-hydroxtryptamine systemBr J Pharmacol19971208658759138693
- SzaboSTde MontignyCBlierPProgressive attenuation of the firing activity of locus coeruleus noradrenergic neurons by sustained administration of selective serotonin reuptake inhibitorsInt J Neuropsychopharmacol2000311111343573
- KawaharaYKawaharaHKanekoFTanakaMLong-term administration of citalopram reduces basal and stress-induced extra-cellular noradrenaline levels in rat brainPsychopharmacology (Berl)2007194738117534604
- GuiardBPEl MansariMMeraliZBlierPFunctional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesionsInt J Neuropsychopharmacol20081162563918205979
- MoretCCharveronMFinbergJPCouzinierJPBrileyMBiochemical profile of midalcipran (F 2207), 1-phenyl-1-diethyl-aminocarbonyl-2-aminomethyl-cyclopropane (Z) hydrochloride, a potential fourth generation antidepressant drugNeuropharmacology198524121112193005901
- KochSHemrick-LueckeSKThompsonLKComparison of effects of dual transporter inhibitors on monoamine transporters and extracellular levels in ratsNeuropharmacology20034593594414573386
- MongeauRWeissMde MontignyCBlierPEffect of acute, short- and long-term milnacipran administration on rat locus coeruleus noradrenergic and dorsal raphe serotonergic neuronsNeuropharmacology1998379059189776386
- MoretCBrileyMEffects of milnacipran and pindolol on extracellular noradrenaline and serotonin levels in guinea pig hypothalamusJ Neurochem1997698158229231743
- KitaichiYInoueTIzumiTNakagawaSKatoAKoyamaTSubchronic milnacipran treatment increases basal extracellular noradrenaline concentrations in the medial prefrontal cortex of ratsEur J Pharmacol2005520374216153636
- KitaichiYInoueTNakagawaSIzumiTKoyamaTEffect of milnacipran on extracellular monoamine concentrations in the medial prefrontal cortex of rats pre-treated with lithiumEur J Pharmacol200551621922615963494
- RaiteriMDel CarmineRBertolliniALeviGEffect of sympathomimetic amines on the synaptosomal transport of noradrenaline, dopamine and 5-hydroxytryptamineEur J Pharmacol197741133143832672
- SesackSRHawrylakVAMatusCGuidoMALeveyAIDopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporterJ Neurosci199818269727089502827
- YamamotoBKNovotneySRegulation of extracellular dopamine by the norepinephrine transporterJ Neurochem1998712742809648875
- DevotoPFloreGPaniLGessaGLEvidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortexMol Psychiatry2001665766411673793
- LinnérLEnderszHOhmanDBengtssonFSchallingMSvenssonTHReboxetine modulates the firing pattern of dopamine cells in the ventral tegmental area and selectively increases dopamine availability in the prefrontal cortexJ Pharmacol Exp Ther200129754054611303041
- BymasterFPKatnerJSNelsonDLAtomoxetine increases extra-cellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorderNeuropsychopharmacology20022769971112431845
- Herrera-GuzmánIGudayol-FerréEHerrera-AbarcaJEMajor Depressive Disorder in recovery and neuropsychological functioning: effects of selective serotonin reuptake inhibitor and dual inhibitor depression treatments on residual cognitive deficits in patients with Major Depressive Disorder in recoveryJ Affect Disord201012334135019896719
- ItoiKSugimotoNThe brainstem noradrenergic systems in stress, anxiety and depressionJ Neuroendocrinol20102235536120210846
- HainsABArnstenAFMolecular mechanisms of stress-induced prefrontal cortical impairment: implications for mental illnessLearn Mem20081555156418685145
- StahlSMGradyMMMoretCBrileyMSNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressantsCNS Spectr20051073274716142213
- MoojenVKMartinsMRReinkeAEffects of milnacipran in animal models of anxiety and memoryNeurochem Res20063157157716758367
- SelitskiĭGVKaprinADIzvozchikovSBBolotovAVTroshinaAVMilnacipran (ixel) in the treatment of anxiodepressive and sexual disorders in patients with noninflammatory syndrome of chronic pelvic painTer Arkh2007798184
- GamaCSZanattoVCPiconFLobatoMIBelmonte-de-AbreuPSEfficacy of milnacipran in treating anxiety symptoms in schizophrenic patients receiving clozapine: a case series studyRev Bras Psiquiatr20062833934017242816
- MorilakDAFrazerAAntidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disordersInternational Journal of Neuropsychopharmacology2004719321815003145
- SzaboSTBlierPFunctional and pharmacological characterization of the modulatory role of serotonin on the firing activity of locus coeruleus norepinephrine neuronsBrain Res200192292011730697
- PariniSRenoldiGBattagliaAInvernizziRWChronic reboxetine desensitizes terminal but not somatodendritic alpha2-adrenoceptors controlling noradrenaline release in the rat dorsal hippocampusNeuropsychopharmacology2005301048105515668723
- CrewsFTSmithCBPresynaptic alpha-receptor subsensitivity after long-term antidepressant treatmentScience1978202322324211589
- McMillenBAWarnackWGermanDCShorePAEffects of chronic desipramine treatment on rat brain noradrenergic responses to alpha-adrenergic drugsEur J Pharmacol1980612392466102522
- El MansariMGuiardBPChernolozOGhanbariRKatzNBlierPRelevance of norepinephrine-dopamine interactions in the treatment of major depressive disorderCNS Neurosci Ther201016e1e1720406250
- ArnstenAFLiBMNeurobiology of executive functions: catecholamine influences on prefrontal cortical functionsBiol Psychiatry2005571377138415950011