Abstract
Objective
To evaluate the effect of aripiprazole once-monthly 400 mg (AOM 400; Abilify Maintena®) on personal and social functioning in patients with schizophrenia in both the acute treatment and maintenance therapy settings.
Methods
Post hoc analyses were conducted on data from Study 291 (NCT01663532), a 12-week, randomized, double-blind, placebo-controlled trial conducted in patients who were experiencing an acute psychotic episode, and Study 248 (NCT00731549), a 52-week open-label extension of two randomized, controlled trials of AOM 400 as maintenance therapy. Assessment of functioning was made using the Personal and Social Performance (PSP) scale. In Study 291, results were stratified by age (≤35 years or >35 years).
Results
In Study 291, 340 patients were included in the analysis (n=168 randomized to AOM 400 [n=49 aged ≤35 years, n=119 aged >35 years]; n=172 randomized to placebo [n=54 aged ≤35 years, n=118 aged >35 years]). In Study 248, 1,081 patients entered the open-label maintenance phase and 858 completed the study. In Study 291, AOM 400, compared with placebo, resulted in a significant increase (improvement) in PSP scores based on LSM (SE) changes from baseline to Week 12 in patients aged ≤35 years (20.6 [1.9] for AOM 400 vs 9.5 [2.4] for placebo; P=0.001) and a numerically (but not significantly) larger increase in PSP scores in patients aged >35 years (16.1 [1.7] for AOM 400 vs 12.5 [1.9] for placebo; P=0.093). Improvements in both age groups met criteria for a minimally important clinical difference (7–10 points). In Study 248, AOM 400 resulted in either numerical improvements (increases) from baseline in PSP total score or maintenance of stable baseline values throughout the study.
Conclusion
AOM 400 was effective in improving personal and social functioning during acute treatment and maintaining function during long-term treatment.
Introduction
Schizophrenia is a chronic disorder associated with significant disability and high personal and social costs.Citation1,Citation2 Deficits in psychosocial functioning constitute a core feature of schizophrenia. Therefore, psychosocial functioning is considered an integral outcome parameter for evaluating the success of both acute and long-term treatment of schizophrenia.Citation3 Prevention of illness exacerbation/relapse plays a crucial role in maintaining patient function, and international treatment guidelines have recommended the use of continuous antipsychotic treatment to prevent relapses.Citation4–Citation6 Nonadherence with antipsychotic treatments is commonCitation7,Citation8 and significantly increases the risk of both relapse and hospitalization.Citation9–Citation11 Additionally, nonadherence has been associated with poorer functional outcomes.Citation12
Long-acting injectable (LAI) antipsychotics may be useful in facilitating relapse prevention by allowing monitoring of adherence and removing from the patient the burden of daily dosing with oral formulations. A systematic meta-analysis of results from placebo-controlled trials of antipsychotic medications found that depot formulations were significantly more effective than oral formulations in reducing rates of relapse.Citation13 While traditional typical and atypical antipsychotics that act as full antagonists at D2 receptors are effective at treating positive symptoms, they are ineffective or may worsen negative symptoms, including social withdrawal, blunted affect, and cognitive function, which are thought to contribute to impairment of psychosocial function.Citation14–Citation16
Aripiprazole once-monthly 400 mg (AOM 400; Abilify Maintena®, Otsuka Pharmaceutical Co., Ltd, Tokyo, Japan), an extended-release injectable solution of aripiprazole, is the first dopamine D2 receptor partial agonist available as a LAI formulation for the treatment of schizophrenia.Citation17–Citation19 AOM 400 has shown efficacy in treating both positive and negative symptoms, as well as improvement in measures of psychosocial function and quality of life.Citation20,Citation21
In two randomized, controlled trials of AOM 400 as maintenance treatment in stable patients with schizophrenia (Study 246 [NCT00705783]; Study 247 [NCT00706654]), AOM 400 resulted in delayed time to, and reduced the rate of, impending relapse versus placebo or a subtherapeutic dose of AOM, respectively.Citation18,Citation22 In both studies, AOM 400 resulted in improvement and maintenance of personal and social functioning, as measured by the Personal and Social Performance (PSP) scale.Citation20 A long-term (52-week) open-label extension of these two studies demonstrated that AOM 400 was safe and effective as maintenance treatment for up to 52 weeks in patients with schizophrenia.Citation23 In a randomized, placebo-controlled study in the acute setting, AOM improved symptoms and functioning versus placebo.Citation24
Here, we report post hoc analyses of the effect of AOM 400 on personal and social functioning from the aforementioned study in the acute setting (Study 291 [NCT01663532]) and the open-label maintenance study in patients with symptom stability (Study 248 [NCT00731549]). Based on the natural history of schizophrenia, patients will likely find themselves in both of these scenarios; hence, the two studies described here address dual priorities for preserving functional status in these patients: managing acute exacerbations rapidly to minimize erosion of functional status; and maintaining symptomatic stability in the long term to prevent future exacerbations that will further erode function.
Methods
Patients
Study 291 enrolled patients aged between 18 and 65 years with schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision [DSM-IV-TR]) who were experiencing an acute psychotic episode at screening and baseline, defined by a Positive and Negative Syndrome Scale (PANSS) total score of ≥80 and specific psychotic symptoms on the PANSS as measured by a score of >4 on each of 4 specific items: conceptual disorganization, hallucinatory behavior, suspiciousness/persecution, and unusual thought content.Citation24 Study 248 enrolled de novo patients aged between 18 and 65 years or patients who participated in Studies 246 and 247, with a diagnosis of schizophrenia (DSM-IV-TR) for at least 3 years prior to screening, who required chronic antipsychotic treatment.Citation18,Citation22,Citation23
All patients who took part in the above studies provided written informed consent prior to study participation. In accordance with the International Conference on Harmonisation (ICH) Good Clinical Practice (GCP) Consolidated Guideline and the Declaration of Helsinki, the protocol and amendments of each study were approved by the governing institutional review board (IRB) or independent ethics committee (IEC) for each respective trial site or country. Full inclusion and exclusion criteria for Studies 291, 246, 247, and 248 have been previously reported.Citation18,Citation22–Citation24
Study designs
Study 291, a 12-week, randomized, double-blind, placebo-controlled study, was conducted in three phases: a screening and washout phase (up to 13 days) requiring hospitalization, an acute treatment phase (12 weeks; hospitalization mandatory for the first 2 weeks) in which patients were randomized 1:1 in double-blind fashion to AOM 400 or placebo, and a safety follow-up phase, in which patients were contacted by phone 14 (±2) days following the final study visit.
Study 248, a 52-week open-label extension of two previous randomized, controlled trials, consisted of a screening phase and three treatment phases. The first treatment phase was an oral conversion phase (4–6 weeks), in which patients who were receiving another antipsychotic were cross-titrated to oral aripiprazole (OA) monotherapy. The second treatment phase was an oral stabilization phase (4–16 weeks), in which patients were stabilized on OA 10–30 mg/day according to predefined stability criteria. The final treatment phase was an AOM 400 maintenance phase (52 weeks), in which patients received AOM 400 with concomitant OA 10–20 mg/day for the initial 2 weeks. During maintenance treatment, patients were seen weekly for the first 4 weeks, biweekly for weeks 6–12, and every 4 weeks thereafter.
Patients enrolled in Study 248 met all of the following baseline stability criteria at screening: outpatient status, PANSS total score of ≤80, lack of specific psychotic symptoms as measured by a score of ≤4 on each of the PANSS items (conceptual disorganization, suspiciousness, hallucinatory behavior, unusual thought content), Clinical Global Impression-Severity (CGI-S) score of ≤4 (moderately ill), and Clinical Global Impression-Severity of Suicidality (CGI-SS) score of ≤2 (mildly suicidal) on Part 1 and ≤5 (minimally worsened) on Part 2.
Detailed study designs for Studies 291 and 248 have been previously reported.Citation23,Citation24
Assessment of personal and social functioning
Personal and social functioning were measured using the PSP Scale, a validated clinician-rated scale that measures personal and social functioning in four domains: (1) Socially Useful Activities, including work and study; (2) Personal and Social Relationships; (3) Self-Care; and (4) Disturbing and Aggressive Behaviors.Citation25 Impairment in each domain was rated on a 6-point scale (0= absent, 1= mild, 2= manifest, 3= marked, 4= severe, and 5= very severe); ratings were converted to a total score based on a 100-point scale, with algorithms used to identify the appropriate 10-point interval within the 100-point range, and the total score within the 10-point interval determined by the rater’s judgment. On the PSP 100-point scale, 71–100 indicates mild functional difficulty; 31–70, varying degrees of disability; 1–30, minimal functioning needing intense support and/or supervision. Research suggests that the minimal clinically important difference (MCID) for the PSP is in the range of 7‒10 points.Citation26–Citation29
In Study 291, PSP total score was assessed at endpoint (Week 10) and Week 12 or last visit, with results stratified by age (≤ vs >35 years) to assess the benefits of early treatment in patients with recent-onset schizophrenia. The likely upper age threshold for recent-onset schizophrenia would be in the late twenties. Because the sample of younger patients was smaller and inclusion criteria included a 3-year history of disease, however, the line of demarcation between younger and older patients was set higher, at 35 years of age, to ensure that as many recent-onset patients as possible were included in the appropriate cohort. In Study 248, mean changes from baseline in PSP total score were tabulated by visit for the AOM 400 maintenance phase without respect to age or duration of disease.
Statistical analyses
Efficacy analyses for Study 291 included patients in the efficacy sample defined as the modified intent-to-treat population, which included all randomized patients who received at least one dose of AOM 400 or placebo and had at least one postbaseline efficacy assessment. Analyses were performed in both observed case (OC) and last observation carried forward datasets. Within-group difference in least squared mean (LSM) change from baseline for AOM 400 versus placebo was calculated in the efficacy sample to determine treatment effect using mixed-model repeated measures (MMRM, including treatment group, pooled centers, week, and treatment-by-week interaction as factors, and baseline-by-week interaction as covariate) and analysis of covariance (ANCOVA, including treatment group and pooled centers as factors, and baseline value as covariate) of OC data. The LSM treatment effects for patients ≤35 years and >35 years are reported by treatment week for week 10 and 12 for the PSP scale. Safety for both studies was assessed using descriptive statistics. Efficacy analyses in Study 248 included all patients who received at least one dose of AOM 400 and who had at least one postbaseline efficacy evaluation during the maintenance phase. Efficacy endpoints were summarized for OC using descriptive statistics.
Results
Patient disposition and characteristics
Study 291
A total of 340 patients were included in the present analysis of Study 291 (n=168, AOM 400; n=172, placebo). In the AOM 400 group, 49 patients were aged ≤35 years and 119 patients were aged >35 years; in the placebo group, 54 patients were aged ≤35 years and 118 patients were aged >35 years. Baseline demographics, patient disposition, and baseline disease severity are shown by age group in . Among patients aged ≤35 years in the AOM 400 and placebo groups, 44 (89.8%) and 48 (88.9%), respectively, were male; among patients aged >35 years in the AOM 400 and placebo groups, 86 (72.2%) and 48 (77.1%), respectively, were male. The majority of patients in the study (~66%) were black/African American. A total of 94 (55.9%) patients in the AOM 400 group and 65 (37.7%) patients in the placebo group completed the study. Withdrawal of consent was the most common reason for discontinuation in the AOM 400 group (n=35, 20.8%) and lack of efficacy was the most common reason for discontinuation in the placebo group (n=60, 34.8%).
Table 1 Baseline demographics and patient disposition in subgroups of randomized patients stratified by age from a 12-week randomized, placebo-controlled study of AOM 400 in adults experiencing an acute psychotic episode
Study 248
Baseline demographics and patient disposition for Study 248 have been reported previously.Citation23 Of the 1,178 patients enrolled, 1,081 (91.8%) entered the 52-week maintenance phase (n=464, Study 246; n=474, Study 247; n=143, de novo patients). Of those entering the maintenance phase, 934 (86.4%) received AOM 400 for 6 months or longer, and 858 (79.4%) completed the study. The primary reason for discontinuation was withdrawal of consent (n=89, 8.2%). A total of 31 (2.9%) patients withdrew due to adverse events (AEs). Mean age (SD) at baseline was 41.2 (10.6); 63.1% of patients were white.
Efficacy
Study 291
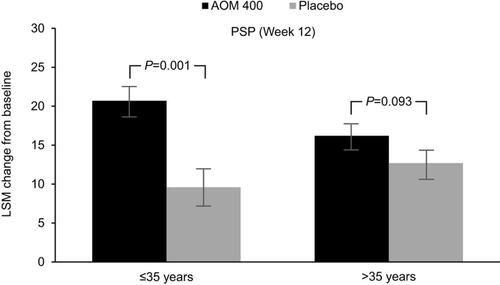
In Study 291, in patients aged ≤35 years, AOM 400 resulted in significantly greater LSM (SD) changes (improvement) in PSP score from baseline to Week 12 versus placebo (20.6 [1.9] for AOM 400 versus 9.5 [2.4] for placebo; P=0.001). In patients aged >35 years, LSM changes in PSP score were numerically largely for AOM 400 compared with placebo (16.1 [1.7] for AOM 400 versus 12.5 [1.9] for placebo; P=0.093) (). Although response was more pronounced in younger patients, improvements in both age groups met MCID criteria. Notably, while younger patients treated with AOM 400 showed greater functional improvement than older ones; older patients treated with placebo showed numerically greater functional improvement than younger ones; thus, both the magnitude of improvement and the disparity between treatment and nontreatment were greater in younger patients. At Week 10, significant improvements with AOM 400 versus placebo were found both in patients aged ≤35 years and those >35 years (P<0.05). Similar results were seen with ANCOVA for the OC dataset. At the last visit, AOM 400 resulted in a mean change from baseline in PSP total score of 15.7 (2.2) versus 4.6 (2.2) for placebo (P<0.0001) among patients aged ≤35 years and 11.8 (1.5) versus 5.9 (1.4) for placebo (P=0.0013) among patients aged >35 years.
Figure 1 Change from baseline in PSP total score in subgroups stratified by age in Study 291.
Abbreviations: ANCOVA, analysis of covariance; AOM 400, aripiprazole once-monthly 400 mg; LSM, least square means; MMRM, mixed model repeated measures; OC, observed cases; PSP, Personal and Social Performance.
Study 248
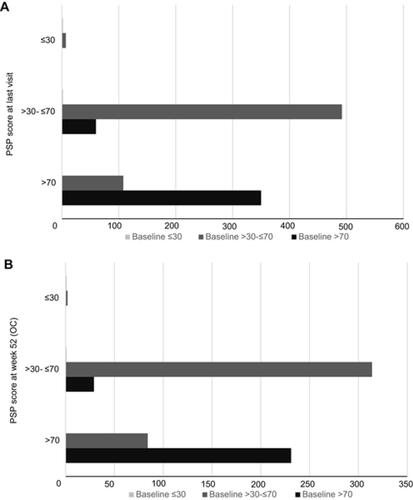
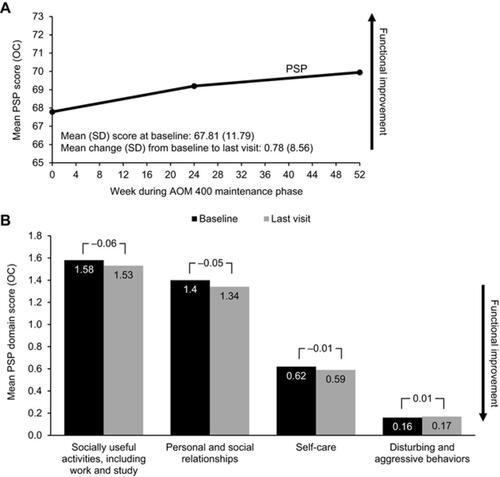
In Study 248, patients on AOM 400 demonstrated long-term maintenance and stability of function, with small consistent numerical improvements in functioning as measured by mean change (increase) in PSP total score from baseline over the course of the study (). The mean (SD) PSP total score increased from 67.81 (11.79) at baseline to 68.62 at last visit – a mean change (SD) from baseline of 0.78 (8.56). Among OCs, mean (SD) PSP total score was 69.23 (11.73) at Week 24 and 69.97 (11.78) at Week 52 – mean changes (SD) from baseline of 1.09 (7.13) and 2.15 (6.93), respectively (). Mean PSP domain scores at baseline were less than 2, indicating no greater than mild impairment. AOM 400 resulted in either small decreases or slight increases in scores for all PSP domains, indicating maintenance of function over the course of the 52-week study (). Mean change (SD) from baseline through the last visit in individual domain scores included Socially Useful Activities, −0.06 (0.72), Personal and Social Relationships, −0.05 (0.69), Self-Care, −0.01 (0.64), and Disturbing and Aggressive Behaviors, 0.01 (0.54) ().
Table 2 Mean change from baseline to last visit in PSP total score and domain scores: 52-week, open-label extension study of AOM 400 as maintenance treatment
Figure 2 Change from baseline in PSP total score (A) and individual domain scores (B) from Study 248.
Abbreviations: AOM 400, aripiprazole once-monthly 400 mg; OC, observed cases; PSP, Personal and Social Performance; SD, standard deviation.
From baseline prior to OA stabilization through 52 weeks of open-label treatment with AOM 400, among OCs 108 patients with PSP total score >30–≤70 at baseline improved to PSP scores >70 (indicating mild to no impairment of function) at last visit, whereas only 60 patients decreased from >70 (baseline visit) to >30–≤70 (). Among OCs at Week 52, 84 patients with PSP total score >30–≤70 at baseline had improved to PSP scores >70, while only 29 patients with PSP score >70 (baseline visit) had decreased to >30–≤70 ().
Safety
Key safety and tolerability findings have been reported previouslyCitation23,Citation24 and are summarized in . In Study 291, the most common (>10%) AEs associated with AOM 400 during acute treatment were weight increase, headache, and akathisia, which occurred in similar proportions of patients in both age-stratified treatment groups.Citation30 Serious treatment-emergent AEs observed in ≥1% of patients included schizophrenia (1.8%, AOM 400; 1.7%, placebo) and psychotic disorder (1.2% in both groups), and all serious treatment-emergent AEs resolved. In Study 248, the most frequent (>5%) AEs during the maintenance phase were headache, nasopharyngitis, anxiety, and insomnia, which were more frequent among de novo patients than those enrolled from the earlier 52-week and 38-week studies.Citation23 Serious AEs were infrequent and generally unrelated to treatment; all resolved with continued treatment or withdrawal of AOM 400. Serious treatment-emergent AEs that occurred in ≥1% of patients included schizophrenia (1.9%) and psychotic disorder (1.4%). In both studies, most AEs associated with AOM 400 were mild or moderate in severity and seldom necessitated discontinuation of AOM 400.
Table 3 Adverse events during acute and maintenance treatment in Studies 291 and 248
Discussion
Social functioning encompasses the capacity of the individual to function in a variety of social roles, including worker, student, spouse, friend, and family member, as well as the capacity to engage in self-care, leisure and recreational activities, and to derive satisfaction from their various social roles.Citation31 Functional deficits are a core feature of schizophrenia, observable in early disease stages, during acute exacerbations, and even among patients in symptomatic remission. As such, appropriate functioning, along with symptomatic remission, is an important goal of treatment. However, while 30–70% of patients with schizophrenia currently achieve symptomatic remission, a lower percentage of patients attain adequate social functioning.Citation32–Citation35 While there is a close relationship between function and psychopathology in the natural history of schizophrenia, the congruence between control of symptoms and status of function is only a partial one.Citation36,Citation37 While remission of symptoms does predict improved function, it is not a guarantee of improvement.Citation38 Conversely, among patients with schizophrenia who experience recovery of function, 5–7% fail to achieve remission of symptoms.Citation39
The importance of functional deficits in schizophrenia, independent of psychopathology, highlights the importance of accurate measurement of social functioning in assessment of the efficacy of drug treatments. Findings from two post hoc analyses reported here suggest that AOM 400 is effective in improving and maintaining social functioning as measured by the PSP scale in both the maintenance and acute treatment settings.
The present analysis of results from Study 291 (12-week, placebo-controlled study) examined the effect of AOM 400 on social functioning in the efficacy sample of patients experiencing an acute psychotic episode, stratified by age ≤35 years or >35 years. As previously reported, LSM change from baseline in PSP total score for the overall sample was statistically significantly greater for AOM 400 versus placebo at Week 10 (endpoint) and Week 12 (P<0.0001).Citation24 Our subgroup analysis by age (≤35 years or >35 years) showed a pattern of improvement in PSP total score at Week 12 for both age groups, with AOM 400 resulting in statistically significant improvements in PSP total scores versus placebo for patients ≤35 years (P=0.001). Improvements with AOM 400 versus placebo in patients aged >35 years were not statistically significant. Nonetheless, functional improvements in both age groups met criteria for clinically meaningful improvements in functional status (MCID 7–10 points).Citation26–Citation29
The three-fold larger treatment effects on the PSP scale for patients aged ≤35 years versus those >35 years suggest that perhaps patients earlier in the course of their illness may be more sensitive to improvements in psychosocial functioning and highlight the need for treatment in younger patients. The magnitude of functional improvement was largest in patients aged ≤35 years on AOM 400 and negligible in patients aged ≤35 years on placebo. The greater disparity between active treatment and placebo in patients aged ≤35 versus >35 years further suggests a greater sensitivity to improvements in social functioning in younger patients treated with AOM 400. These differences between age groups agreed with our finding of numerically larger treatment effects for patients ≤35 years on the PANSS negative subscale,Citation30 which is often associated with impaired functioning.Citation40 To confirm these findings, further study is required using sample sizes that are adequately powered to detect age-related differences in symptoms and functioning. However, these preliminary results do suggest the importance of protecting patients from deterioration via rapid control of acute symptoms and effective maintenance treatment to prevent relapse. The results of our analysis of Study 291, consistent with overall results for the study, indicate clinically important improvements in functioning across age groups and support the utility of AOM 400 in the acute treatment setting across age groups.
Analysis of results from Study 248, a 52-week extension of two randomized controlled trials of AOM 400 as maintenance treatment (Studies 246 and 247), extends the results of those previous studies with additional long-term data. A separate analysis of Studies 246 and 247 demonstrated that AOM 400 improved PSP total scores during OA and AOM 400 stabilization phases in both studies and maintained those scores in stabilized patients throughout the course of each study.Citation20 In the present analysis of Study 248, mean change (SD) in PSP total score from baseline to the last visit during the open-label treatment phase was 0.78 (8.56) indicating long-term stability and improvement in functioning. This mean change in PSP total score was comparable to mean changes from baseline in PSP total score seen during the double-blind treatment phases of Studies 246 and 247.Citation20
Safety and tolerability results from post hoc analyses reported here were consistent with the established AOM 400 safety/tolerability profile. Safety results from Study 291 suggest that AOM 400 has a similar tolerability profile in patients ≤35 years versus those >35 years. In agreement with overall safety results from the 52-week open-label extension of the two pivotal maintenance trials, there were no new safety signals that arose in analysis of long-term results from this study.Citation23
Our analysis of Study 291 is limited by its post hoc nature, including the lack of sufficient power in terms of sample size to detect age-related differences in functioning. Our analysis of Study 248 was limited by the design of the study, including the lack of a control group, which restricted attribution of efficacy outcomes to AOM 400. Additionally, the study was conducted among patients who were stabilized on OA before the AOM 400 open-label maintenance phase, which may have limited the ability of the study detect large improvements in PSP scores.
Conclusion
Results from two post hoc analyses of data from studies in both the acute treatment and maintenance settings suggest that AOM 400 improves and maintains personal and social functioning in patients with schizophrenia. In the acute setting, improvements in functioning were consistent across age groups (≤35 years vs >35 years), with numerically larger improvements in PSP scores seen in the younger group of patients, suggesting a greater sensitivity to improvements in social functioning in younger patients. Safety/tolerability data were consistent with previous reports.
Author contributions
TP-S was project lead and medical monitor for Studies 246, 247, 248, and 291; RAB was responsible for the clinical management of studies 246, 247, 248, and 291. All authors—TP-S, RAB, PS, PZ, and JJM—contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
Editorial support for this manuscript was provided by BioScience Communications (New York City, NY, USA), with funding from Otsuka Pharmaceutical Development & Commercialization, Inc.
Disclosure
TP-S, RAB, PZ, and JJM are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. PS is an employee of H. Lundbeck A/S. The authors report no other conflicts of interest in this work.
References
- Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29(2):63–76. doi:10.1097/YIC.0b013e32836508e6
- Millier A, Schmidt U, Angermeyer MC, et al. Humanistic burden in schizophrenia: a literature review. J Psychiatr Res. 2014;54:85–93. doi:10.1016/j.jpsychires.2014.03.021
- Juckel G, Schaub D, Fuchs N, et al. Validation of the Personal and Social Performance (PSP) scale in a German sample of acutely ill patients with schizophrenia. Schizophr Res. 2008;104(1–3):287–293. doi:10.1016/j.schres.2008.05.016
- Buchanan RW, Kreyenbuhl J, Kelly DL, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71–93. doi:10.1093/schbul/sbp116
- Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2–44. doi:10.3109/15622975.2012.739708
- Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB; Schizophrenia Patient Outcomes Research Team. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94–103. doi:10.1093/schbul/sbp130
- Offord S, Lin J, Mirski D, Wong B. Impact of early nonadherence to oral antipsychotics on clinical and economic outcomes among patients with schizophrenia. Adv Ther. 2013;30(3):286–297. doi:10.1007/s12325-013-0016-5
- Remington G, Teo C, Mann S, Hahn M, Foussias G, Agid O. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261–263. doi:10.1097/JCP.0b013e31828568bc
- Ayuso-Gutierrez JL, del Rio Vega JM. Factors influencing relapse in the long-term course of schizophrenia. Schizophr Res. 1997;28(2–3):199–206. doi:10.1016/S0920-9964(97)00131-X
- Olfson M, Mechanic D, Hansell S, Boyer CA, Walkup J, Weiden PJ. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216–222. doi:10.1176/appi.ps.51.2.216
- Valenstein M, Ganoczy D, McCarthy JF, Myra Kim H, Lee TA, Blow FC. Antipsychotic adherence over time among patients receiving treatment for schizophrenia: a retrospective review. J Clin Psychiatry. 2006;67(10):1542–1550. doi:10.4088/JCP.v67n1008
- Ascher-Svanum H, Faries DE, Zhu B, Ernst FR, Swartz MS, Swanson JW. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006;67(3):453–460. doi:10.4088/JCP.v67n0317
- Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. doi:10.1016/S0140-6736(12)60239-6
- Casey AB, Canal CE. Classics in chemical neuroscience: aripiprazole. ACS Chem Neurosci. 2017;8(6):1135–1146. doi:10.1021/acschemneuro.7b000
- Li P, Snyder GL, Vanover KE. Dopamine targeting drugs for the treatment of schizophrenia: past, present and future. Curr Top Med Chem. 2016;16(29):3385–3403. doi:10.2174/1568026616666160608084834
- Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251–267. doi:10.2165/00023210-200418040-00005
- Burris KD, Molski TF, Xu C, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302(1):381–389. doi:10.1124/jpet.102.034280
- Kane JM, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(5):617–624. doi:10.4088/JCP.11m07530
- Shapiro DA, Renock S, Arrington E, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28(8):1400–1411. doi:10.1038/sj.npp.1300203
- Fleischhacker WW, Baker RA, Eramo A, et al. Effects of aripiprazole once-monthly on domains of personal and social performance: results from 2 multicenter, randomized, double-blind studies. Schizophr Res. 2014;159(2–3):415–420. doi:10.1016/j.schres.2014.09.019
- Naber D, Hansen K, Forray C, et al. Qualify: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res. 2015;168(1–2):498–504. doi:10.1016/j.schres.2015.07.007
- Fleischhacker WW, Sanchez R, Perry PP, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry. 2014;205(2):135–144. doi:10.1192/bjp.bp.113.134213
- Peters-Strickland T, Baker RA, McQuade RD, et al. Aripiprazole once-monthly 400 mg for long-term maintenance treatment of schizophrenia: a 52-week open-label study. NPJ Schizophr. 2015;1:15039. doi:10.1038/npjschz.2015.39
- Kane JM, Peters-Strickland T, Baker RA, et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(11):1254–1260. doi:10.4088/JCP.14m09168
- Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323–329.
- Lee SC, Tang SF, Lu WS, et al. Minimal detectable change of the personal and social performance scale in individuals with schizophrenia. Psychiatry Res. 2016;246:725–729. doi:10.1016/j.psychres.2016.10.058
- Nasrallah H, Morosini P, Gagnon DD. Reliability, validity and ability to detect change of the personal and social performance scale in patients with stable schizophrenia. Psychiatry Res. 2008;161:213–224. doi:10.1016/j.psychres.2007.11.012
- Nicholl D, Nasrallah H, Nuamah I, Akhras K, Gagnon DD, Gopal S. Personal and social functioning in schizophrenia: defining a clinically meaningful measure of maintenance in relapse prevention. Curr Med Res Opin. 2010;26:1471–1484. doi:10.1185/03007991003798927
- Patrick DL, Burns T, Morosini P, et al. Reliability, validity and ability to detect change of the clinician-rated personal and social performance scale in patients with acute symptoms of schizophrenia. Curr Med Res Opin. 2009;25:325–338. doi:10.1185/03007990802611919
- Fleischhacker WW, Baker RA, Eramo A, et al. Effects of aripiprazole once-monthly on symptoms and functioning of patients with an acute episode of schizophrenia stratified by age. Presented at: The 63rd annual American College of Neuropsychopharmacology congress; December 11–14; 2014; Phoenix, AZ. (Poster T125).
- Priebe S. Social outcomes in schizophrenia. Br J Psychiatry Suppl. 2007;50:s15–20. doi:10.1192/bjp.191.50.s15
- Bobes J, Ciudad A, Alvarez E, San L, Polavieja P, Gilaberte I. Recovery from schizophrenia: results from a 1-year follow-up observational study of patients in symptomatic remission. Schizophr Res. 2009;115(1):58–66. doi:10.1016/j.schres.2009.07.003
- Boden R, Sundstrom J, Lindstrom E, Lindstrom L. Association between symptomatic remission and functional outcome in first-episode schizophrenia. Schizophr Res. 2009;107(2–3):232–237. doi:10.1016/j.schres.2008.10.004
- Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71(6):716–728. doi:10.4088/JCP.08m04846yel
- Novick D, Haro JM, Suarez D, Vieta E, Naber D. Recovery in the outpatient setting: 36-month results from the Schizophrenia Outpatients Health Outcomes (SOHO) study. Schizophr Res. 2009;108(1–3):223–230. doi:10.1016/j.schres.2008.11.007
- Karow A, Moritz S, Lambert M, Schottle D, Naber D, Initiative E. Remitted but still impaired? Symptomatic versus functional remission in patients with schizophrenia. Eur Psychiatry. 2012;27(6):401–405. doi:10.1016/j.eurpsy.2011.01.012
- Wunderink L, Nieboer RM, Wiersma D, Sytema S, Nienhuis FJ. Recovery in remitted first-episode psychosis at 7 years of follow-up of an early dose reduction/discontinuation or maintenance treatment strategy: long-term follow-up of a 2-year randomized clinical trial. JAMA Psychiatry. 2013;70(9):913–920. doi:10.1001/jamapsychiatry.2013.19
- Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441–449. doi:10.1176/appi.ajp.162.3.441
- Shrivastava A, Johnston M, Shah N, Bureau Y. Redefining outcome measures in schizophrenia: integrating social and clinical parameters. Curr Opin Psychiatry. 2010;23(2):120–126. doi:10.1097/YCO.0b013e328336662e
- Rabinowitz J, Levine SZ, Garibaldi G, Bugarski-Kirola D, Berardo CG, Kapur S. Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophr Res. 2012;137(1–3):147–150. doi:10.1016/j.schres.2012.01.015