Abstract
Objective
One year observation and evaluation of the VNS (vagus nerve stimulation) efficacy and safety for patients with treatment resistant depression in Polish conditions.
Methods
An open label, uncontrolled and one center retrospective study of VNS therapy was implemented with stable pharmacotherapy in 6 patients with treatment resistant depression (TRD). For the first 3 months, only VNS parameters were altered but the pharmacological treatment was unchanged and in the following 9 months, medication and VNS dosing parameters were altered according to the clinical state of the patients.
Results
The baseline 24-item Hamilton Depression Rating Scale (HAMD-24) score averaged 24. Both response (>50% reduction in baseline scores) and remission rates after 3 months of treatment were only 40%. After 1 year of VNS therapy, the response rates increased to 86%. Most frequent side-effects were voice alteration (86% at 3 months of stimulation) and headaches (40%).
Conclusion
VNS treatment was safe and effective in TRD patients and its efficacy increased with time. Efficacy ratings are similar to the previously reported studies using a congenial protocol.
Introduction
Depression is a common illness worldwide, with more than 300 million people affected of all ages.Citation1 Severe intensity of depression may lead, in many cases, to life threatening suicidal behavior. In Poland, according to EZOP 2015 year epidemiological study,Citation2 depression was the third most common mental disorder in terms of the prevalence and occurred in women (4.0%) twice as often than in males (1.9%). In an American study from 2012, mood and other behavioral health disorders were the most common diagnoses for Medicaid-covered and uninsured hospital stays in the United States – respectively 6.1% and 5.2% of stays.Citation3
The group of antidepressant drugs with different mechanisms of action are widely used in treatment for major depressive disorder. According to 2018 Cipriani meta-analysisCitation4 of 522 placebo-controlled and head-to-head trials of 21 antidepressants used for the acute treatment of adults comprising 116 477 participants, all drugs were significantly more effective then placebo.
However, together with psychotherapy and/or electroconvulsive therapy (ECT), these medications are not effective in all patients suffering from the depression.
In Sequenced Treatment Alternatives to Relieve Depression – STAR*D, the largest prospective clinical trial of treatment of major depressive disorder, funded by National Institutes of Health and completed in 2006, at the level 4, where only highly TRD patients were gathered after three failures, 33% of patients did not respond to the treatment despite adequate treatment.Citation5 These results were coherent with previously presented data by Thase, where approximately 30% of patients with treatment-resistant depression (TRD) did not respond to any treatment.Citation6
TRD is defined by lack of response or failure to fully respond or achieve remission after at least two proven antidepressant trials with adequate dosing and duration.Citation8–Citation10
In order to improve the response to antidepressant treatment, add-on augmentation has been developed, which is the combination of first-line antidepressive pharmacotherapy with a second treatment approach, either pharmacological, or neurostimulation techniques.Citation11
Atypical antipsychotics and thyroid augmentation have been proven to have better antidepressive effect combined with first-line antidepressants in TRD.Citation12
Neurostimulation options in TRD include electroconvulsive therapy (ECT), magnetic seizure therapy (MST), transcranial direct current atimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS), deep brain stimulation (DBS), cranial electrotherapy stimulation (CES), and vagus nerve stimulation (VNS). The most common therapy for TRD has been ECT, however its final clinical outcome is diminished through high relapse rates of up to 50%.Citation13 rTMS response rates are low in patients resistant to ECT.Citation14 DBS is a neurosurgical invasive option for TRD treatment that requires brain surgery, with limited clinical data available.Citation15,Citation16
Early clinical observations of mood improvement in first epilepsy treated patients with VNS, drew attention to its antidepressant action potential. Finally, after controlled studies in Europe and Canada in 2001–2005, the FDA approved VNS treatment in chronic depression and in TRD patients.Citation17–Citation20 Patients should have used 4 or more medications before VNS treatment and should be older than or at least 18 years old. Over 100 000 patients/year (psychiatric and neurological indications) have been treated in the whole world. Overall the number needed to treat (NNT) for VNS ranges from 4 to 10, which is clinically significant, considering the treatment resistant depression population.
Taking into consideration other neurostimulation methods, VNS has better scientific evidence for efficacy compared to MST, tDCS, and CES and for maintenance in long term treatment it is better even than the gold standard – maintenance ECT, and also less invasive.Citation11
After the surgical implantation of the VNS device, it is telemetrically activated by a wand connected to a computer. All the adjustments of stimulus intensity parameters are non-invasive and are executed with the external telemetric wand. VNS settings are programmed to supply intermittent stimulation with a current of 0.25–3.0mA, a frequency of 20–50 Hz, a pulse width of 130–500ms, and a duty cycle.Citation21
In general, the exact mechanism of action of VNS is still unknown. There is a hypothesis of the desynchronization of neuronal activity by the modulation of neurotransmitter release, hippocampal plasticity and anti-inflammation action.Citation22 The long term VNS modulates the neurotransmission of norepinephrine, serotonin, and gamma-aminobutyric acid (GABA).Citation24 Locus coeruleus (LC) activated by VNS is the main source of norepinephrine.Citation25 LC lesions stop both the antiepileptic and antidepressant action of VNS.Citation23
The excitatory projections from LC to the Dorsal Raphe Nucleus (DRN), which is the major source of serotonin in the brain,Citation26 have been shown to activate the excitatory α1- adrenoreceptors on the cell bodies of the serotonergic neurons, thus increasing the activity of these neurons.Citation27 VNS also induces c-fos activity, a nuclear protein expressed under high neuronal activity.Citation29,Citation30 Furmaga compared activity changes in the animal brain after VNS stimulation and that caused by sertraline and desimipramineCitation31 by double labelling of ΔFosB and serotonin, and he found that sertraline had similar effect to VNS in DRN. The effects of VNS were more widespread than those of antidepressants.
In the hippocampal plasticity hypothesis of depression, chronic stress leads to atrophy and synaptic changes in limbic brain. VNS increases the expression of neurotrophic and growth factors – brain derived neurotrophic factor (BDNF) and basic fibroblast growth factor (bFGF),Citation32 which promote the neuroplasticity, ie, the formation and differentiation of neurons, decreased in mood disorders. Another way antidepressants could increase BDNF efficacy is the activation by phosphorylation of its receptor: tropomyosin receptor kinase B (TrkB). Furmaga demonstrated that both long term and short term VNS in rats activated the TrkB receptors.Citation31 Clinical studies have proven that therapeutic effects appear after several months of treatment and the plasticity hypothesis explains its mechanism. It requires weeks for newborn cells to become mature neurons with the functional network of synapses to rebuild the neuronal paths in frontal and limbic structure circuits.Citation33
Method
The VNS treatment was evaluated in an open, unblinded, not sham controlled, one center trial conducted in Department of Psychiatry and Psychotherapy at Katowice Silesian Medical University. Every patient, before the device implantation, underwent baseline assessments. All the patients had their stimulation initiated on the second day after the implantation of device - their set of parameters is shown in . First changes of stimulation parameters were done if needed at the 5th week after the start of stimulation. During the first 3 months after the stimulation onset, no pharmacological treatment changes were made, only the stimulation parameters of VNS were set according to comfort and tolerability of patients every 2 weeks. The pharmacological profile of all patients before the implantation of the VNS device is shown in . At the follow-up period 3 months after VNS implantation, pharmacological medication changes together with stimulation parameter adjustments were performed at one month period visits.
Table 1 For All Patients: Pulse Width 500 Mikroseconds, Stimulation Time 30 Seconds, Interval Between Stimulations 5 mins
Table 2 The Pharmacological Profile Of All Patients Before The Implantation Of The VNS Device
Patients
Patients with TRD signed informed consent to undergo implantation of the VNS device at neurosurgery unit after qualification exam at psychiatry ward. The current major depressive episode had lasted more than 2 years and/or the patient had at least four major depressive episodes in his life. Age of the patients was between 63 and 56, 3 males and 2 women. All patients had experienced at least 2 unsuccessful adequate medication trials for the current major depressive episode with antidepressant drugs, evaluated by Antidepressant Resistance Rating score of ≥3 (30) and one patient had electroconvulsive therapy (ECT) for the current major depressive episode.
None of the patients had history of suicide, atypical or psychotic depression, schizophrenia, schizo-affective disorder or delusional disorder, bipolar disorder or a secondary diagnosis of delirium, dementia, and other cognitive disorders as the health problems that might interfere with surgical and anesthetic procedures.
Stimulation was initiated the day following operation with the parameters presented in . The output current parameters were adjusted according to individual maximal tolerability at each study visit in order to get maximum efficacy of depressive symptoms' treatment but only frequency and intensity were changed. There were no changes of pulse width on stimulation timings throughout the whole observation period of 12 months. Maximum value for frequency was 30Hz in 3 individuals and for intensity was 1 mA in 2 patients.
VNS: Implantation
The implantable system made by Cyberonics Inc., Houston, USA, consisted of the generator VNS DemipulseTM Model 103 and two helicoidal electrodes with lead and connector pin to insert into the generator. The manufacturer also provided the external programing wand for telemetric adjustment of stimulation parameters. The operations were performed with the patients under general anesthesia, placed in supine position with the head slightly extended. Patients received prophylactic intravenous antibiotics preoperatively and for 6 days postoperatively. A transverse incision measuring 30–40 mm was made in left posterior triangle of the neck at the cricothyroid interval level. The lateral margin of sternocleidomastoid (SCM) muscle was retracted medially to visualize cervical neurovascular bundle. The left vagus nerve was exposed by blunt dissection, mobilized over a length of 30–40 mm and retracted superiorly with 2 latex loops. Second transverse incision, long enough for generator placement, was made in the left chest wall, a few centimeters below the clavicle. Between both incisions the subcutaneous tunnel was created using a tunnelling device and the lead was drawn from the neck incision to the chest incision. In the next step the electrodes were wrapped around the vagus nerve. To prevent unwanted traction or dislocation during patients’ neck movements, the lead was looped and placed in a latex collar, which in turn was attached to the internal surface of the SCM by a nonresorbable stitch. The connector pin of lead was connected to the generator and electrodiagnostic testing was performed to confirm a proper function of the system. Finally, the generator was placed in a pocket bluntly created by a surgeon between the pectoralis major and minor muscles and attached to the pectoralis fascia by a nonresorbable stitch. The wounds were closed in layers.
In all cases surgical procedures were noncomplicated with minimal blood loss. The healing was uneventful; all patients were pleased with cosmetic effect and tolerated their subcutaneously placed device well.
Concomitant Therapy
4 weeks prior to the planned VNS implantation and 12 weeks after, antidepressant medications were stable. At the follow-up period 3 months after VNS implantation, all necessary psychotropic treatment changes were performed, similar to the other non-psychiatric drugs (eg, antibiotics).
Outcome Measures
Baseline HAMD-24 score was compared to ratings after 3 months of VNS and after additional 3, 6, and 9 months to assess the depression severity. Response was defined as a ≥50% reduction in HAMD-24 score from the baseline and remission was determined as a HAMD-24 score ≤10.
Results
Recruitment
A total of 6 patients were observed in this study. Their epidemiologic and affective disorder features are summarized in . The unsuccessful current major depressive disorder number of treatments averaged 4,2 ±2,8. One patient had received electroconvulsive therapy (ECT) during the current major depressive episode. The baseline scores of depression scales – HAMD-24, MADRS certified a severe level of depression in all the observed patients.
Table 3 Demographic And Clinical Characteristics Of Patients
Stimulation Parameters
Most of the patients (60%) received stimulation with 30Hz frequency. The others' stimulation was reduced to 20Hz due to adverse events – especially voice alteration, in order to improve its tolerability. The output currents ranged from 0.25 to 1 mA - mean 0,5±0.25 mA during the first 3 months after VNS initiation and mean 0.65±0.35 mA during the follow up period. The pulse width of 500μs with stimulation on for 30 s and off for 5 mins remained unchanged throughout the whole time of observation.
Efficacy
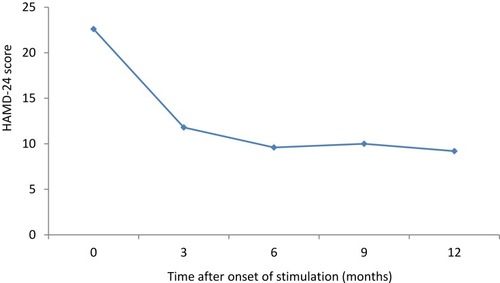
Severity of depression assessed by the HAMD-24 mean scores decreased under VNS as shown in . There is in the HAMD-24 course of scores through 12 months period of every rated patient.
Figure 1 Mean scores of the HAMD-24 at study visits. Severity of depression assessed by the HAMD-24 score decreased under vagus nerve stimulation (VNS). The largest diminishment appeared during the first 3 months of treatment and it was maintained throughout the rest of the observation period.
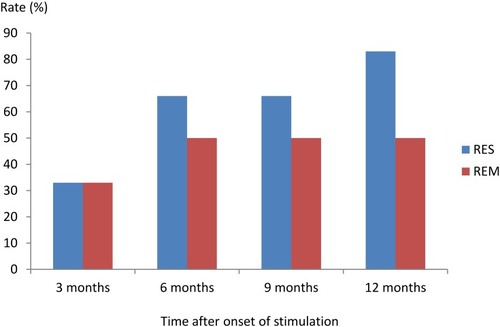
During the first 3 months the number of patients who responded to the treatment equaled those with remission. The rest of the patients needed an extra 3 months to pass the response criteria threshold – as shown in , we could divide patients in 3 groups: 1) no response, 2) fluctuating response and 3) early remission. Only one patient did not achieve remission after 6 months. 2 patients could be assigned to the fluctuating response group and 2 patients to early remission.
Figure 2 Response RES and remission rates REM (%), defined as reduction of >50% in the HAMD-24 score compared to baseline HAMD-24 score; remission was defined as a HAMD-24 score of <10%.
Finally, after 12 months' observation, 83% (5/6) of patients reached criteria of response, but the number of remitted patients remained the same 50% (3/6).
Adverse Events
The adverse events recorded during the 12 months observation period after the VNS stimulation onset are summarized in . The most common adverse event during the first 3 months was voice alterations during 30 second VNS stimulation (80% of patients) and headache and pain complaints (40% of patients). After 12 months, 83% of patients still reported voice alterations only during the time of stimulation but no one suffered pain and headaches. According to the adverse event classification, these voice alterations were definitely related to the VNS and they were moderate, influencing the patients' quality of life as they had some problems in social relations resulting from the voice hoarseness. During 12 months observation period, there were no serious adverse events that might have resulted in patient hospitalization.
Table 4 Adverse Events Recorded During The Observation Period After The VNS Stimulation Onset
Discussion
Carreno and FrazerCitation35 noticed in their review in 2017, that the evidence for efficacy of VNS in TRD is quite substantial, however more research is still needed before efficacy could be established conclusively.
Thus, we compared our results to the EuropeanCitation34 and American multi-center VNS studyCitation17 as both studies had similar protocol in methodology and design. The size of our observed group was very small but it was quite homogenous as there were only patients with unipolar diagnosis by DSM V, and the same as in both studies, unsuccessful adequate medication trials for the current major depressive episode was 4,2 ± 2,8. The remission rate after 3 months was 33%, compared to 17% in the two studies and after 1 year of VNS therapy the response rate increased to 83%, compared to 53%. We observed the increase in remission rates after one year observation, compared to the first 3 months.
In a Sperling open-label case control study with a follow-up period up to 12 months, where the group consisted of 18 patients suffering from TRD, with the same VNS settings, a significant improvement of HAMD to a mean of 10.2 points was observed,Citation36 as in our study – 9.6 points.
In the case control retrospective study published by Mueller,Citation19 a group of 20 patients with TRD was treated with low-strength/high-frequency VNS (≤1,5 mA, 20 Hz) and high-strength/low-frequency (>1,5 mA, 15 Hz) VNS. Significant decrease in the HAMD was observed in patients who were treated with the low-strength/high-frequency stimulation parameters. The scores of the patients treated with high-strength/low-frequency combination did not change. 60% of our patients had low-strength/high-frequency stimulation with frequency 30Hz and 0.65±0.35 mA during the follow up period. The frequency was the first parameter to be increased when the patient demonstrated HAMD deterioration. Only the adverse events, like voice changes, were stopping us from using 30Hz stimulation in some patients.
In another open, uncontrolled European multi-center study with 28 chronic TRD unipolar patients, 35.7% met criteria for response after 12 months' treatment time.Citation37
In the longest – 5 years and the largest (795 patients) naturalistic study of VNS efficacy in TRD by Aaronson, 5 year cumulative response rate was 67.6% with remission rate of 43.3%.Citation38
Conclusion
After 1 year of VNS therapy, the response rates increased to 83%. Most frequent side-effects were voice alteration (83% at 3 months stimulation, decreased to 50% after 6 months) and headaches (33%). Thus, VNS treatment was safe and effective in TRD patients and its efficacy increased with time. Efficacy ratings were similar to the previously reported studies using a congenial protocol.
As Helge Mueller stated in the review of augmentation strategies for TRD,Citation11 DBS or VNS should be strongly recommended as promising adjunctive options to ECT – the gold standard. VNS has strong scientific evidence for efficacy and safety, although first treatment symptoms are 3–12 months delayed and patience is needed.
Application of VNS in TRD is recommended in such national guidelines as: CANMAT 2016Citation39 and NICE 2012,Citation7 but long-term naturalistic observational studies are needed for the future evaluation of VNS efficacy and safety in TRD.Citation40
Disclosure
The authors report no conflict of interest in this work.
References
- World Health Organisation. Depression. (2018) Available from: http://www.who.int/news-room/fact-sheets/detail/depression Accessed 1022, 2019.
- Kiejna A, Piotrowski P, Adamowski T, et al. The prevalence of common mental disorders in the population of adult poles by sex and age structure – an EZOP Poland study. Psychiatr Pol. 2015;49(1):15–27. doi:10.12740/PP/3081125844407
- Lopez-Gonzalez L, Pickens GT, Washington R, Weiss AJ. Characteristics of Medicaid and uninsured hospitalisations, 2012 In: HCUP Statistical Brief #183. Rockville, MD: Agency for Healthcare Research and Quality; 2014.
- Cipriani A, Furukawa T, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi:10.1016/S0140-6736(17)32802-729477251
- Gaynes BN, Rush AJ, Trivedi MH, Wisniewski SR, Spencer D, Fava M. The STAR*D study: treating depression in the real world. Cleve Clin J Med. 2008;75(1):57–66. doi:10.3949/ccjm.75.1.5718236731
- Thase ME, Rush JA. Treatment-resistant depression In: Bloom FE, Kupfer DJ, editors. Psychopharmacology. New York, NY: Raven; 2005.
- Cleare A, Pariante CM, Young AH, et al.; Members of the Consensus M. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459–525. doi:10.1177/026988111558109325969470
- Bschor T. Therapy-resistant depression. Expert Rev Neurother. 2010;10:77–86. doi:10.1586/ern.09.13720021322
- Wiles N, Thomas L, Abel A, et al. Clinical effectiveness and cost-effectiveness of cognitive behavioural therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care: the CoBalT randomised controlled trial. Health Technol Assess. 2014;18:1–167. doi:10.3310/hta18310
- Holtzmann J, Richieri R, Saba G, et al. Quelle définition pour la dépression résistante? [How to define treatment-resistant depression?]. La Presse Médicale. 2016;45(3):323–328. doi:10.1016/j.lpm.2016.02.00226970938
- Müller H, Moeller S, Lam A, Lücke C, Braun N, Philipsen A. Vagus Nerve Stimulation (VNS) and other augmentation strategies for Therapy-Resistant Depression (TRD): review of the evidence and clinical advice for use. Front Neurosci. 2018;12(239). doi:10.3389/fnins.2018.00239
- Fornaro M, Stubbs B, De Berardis D, et al. Atypical antipsychotics in the treatment of acute bipolar depression with mixed features: a systematic review and exploratory meta-analysis of placebo-controlled clinical trials. Int J Mol Sci. 2016;17:241. doi:10.3390/ijms1702024126891297
- Charlson F, Siskind D, Doi SA, McCallum E, Broome A, Lie DC. ECT effcacy and treatment course: a systematic review and metaanalysis of twice vs. thrice weekly schedules. J Affect Disord. 2012;138:1–8. doi:10.1016/j.jad.2011.03.03921501875
- Kedzior KK, Schuchinsky M, Gerkensmeier I, Loo C. Challenges in comparing the acute cognitive outcomes of high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) vs. electroconvulsive therapy (ECT) in major depression: a systematic review. J Psychiatr Res. 2017;91:14–17. doi:10.1016/j.jpsychires.2017.03.00228288306
- Schlaepfer TE, Lieb K. Deep brain stimulation for treatment of refractory depression. Lancet. 2005;366:1420–1422. doi:10.1016/S0140-6736(05)67582-416243078
- Buhmann C, Huckhagel T, Engel K, et al. Adverse events in deep brain stimulation: a retrospective longterm analysis of neurological, psychiatric and other occurrences. PLoS One. 2017;12(7). doi:10.1371/journal.pone.0178984
- Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: a multicenter study. Biol Psychiatry. 2000;47:276–286. doi:10.1016/S0006-3223(99)00304-210686262
- Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatmentresistant depression: effcacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25:713–728. doi:10.1016/S0893-133X(01)00271-811682255
- Kosel M, Schlaepfer TE. Beyond the treatment of epilepsy: new applications of vagus nerve stimulation in psychiatry. CNS Spectr. 2003;8:515–521. doi:10.1017/S109285290001898812894032
- Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry. 2002;51:280–287. doi:10.1016/S0006-3223(01)01343-911958778
- Cusin C, Dougherty DD. Somatic therapies for treatmentresistant depression: ECT, TMS, VNS, DBS. Biol Mood Anxiety Disord. 2012;2:14. doi:10.1186/2045-5380-2-1422901565
- Aalbers M, Vles J, Klinkenberg S, Hoogland G, Majoie M, Rijkers K. Animal models for vagus nerve stimulation in epilepsy. Exp Neurol. 2011;230:167–175. doi:10.1016/j.expneurol.2011.04.01421565191
- Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol. 2017;289:21–30. doi:10.1016/j.expneurol.2016.12.00527988257
- Ruffoli R, Giorgi FS, Pizzanelli C, Murri L, Paparelli A, Fornai F. The chemical neuroanatomy of vagus nerve stimulation. J Chem Neuroanat. 2011;42:288–296. doi:10.1016/j.jchemneu.2010.12.00221167932
- Takigawa M, Mogenson GJ. A study of inputs to antidromically identified neurons of the locus coeruleus. Brain Res. 1977;135:217–230. doi:10.1016/0006-8993(77)91027-7922473
- Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59(6 Suppl. 4):S3–S14. doi:10.1212/WNL.59.6_suppl_4.S3
- Grimonpreza A, Raedta R, Baekenb C, Boona P, Voncka K. The antidepressant mechanism of action of vagus nerve stimulation: evidence from preclinical studies. Neurosci Biobehav Rev. 2015;56:26–34. doi:10.1016/j.neubiorev.2015.06.01926116875
- Prudic J, Sackeim HA, Devanand DP. Medication resistance and clinical response to electroconvulsive therapy. Psychiatry Res. 1990;31:287–296. doi:10.1016/0165-1781(90)90098-P1970656
- Naritoku DK, Terry WJ, Helfert RH. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res. 1995;22:53–62. doi:10.1016/0920-1211(95)00035-98565967
- Cunningham JT, Mifflin SW, Gould GG, Frazer A. Induction of c-Fos and DeltaFosB immunoreactivity in rat brain by vagal nerve stimulation. Neuropsychopharmacology.;. 2008;33:1884–1895. doi:10.1038/sj.npp.130157017957222
- Furmaga H, Carreno FR, Frazer A. Vagal nerve stimulation rapidlyactivates brain-derived neurotrophic factor receptor TrkB in rat brain. PLOSONE. 2012;7(5):e34844. doi:10.1371/journal.pone.0034844
- Follesa P. Vagus nerve stimulation increases norepinephrineconcentration and the gene expression of BDNF and bFGF in the rat brain. BrainRes. 2007;1179:28–34.
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi:10.1038/npp.2009.10419693001
- Schlaepfer T, Frick C, Zobel A, et al. Vagus nerve stimulation for depression: efficacy and safety in a European study. Psychol Med. 2008;38:61–661. doi:10.1017/S0033291707001924
- Carreno F, Frazer A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics. 2017;14:716–727. doi:10.1007/s13311-017-0537-828585221
- Sperling W, Reulbach U, Kornhuber J. Clinical benefits and cost effectiveness of vagus nerve stimulation in a long-term treatment of patients with major depression. Pharmacopsychiatry. 2009;42(3):85–88. doi:10.1055/s-0028-110329419452375
- Christmas D, Steele JD, Tolomeo S, Eljamel MS, Matthews K. Vagus nerve stimulation for chronic major depressive disorder: 12-month outcomes in highly treatment-refractory patients. J Affect Disord. 2013;150(3):1221–1225. doi:10.1016/j.jad.2013.05.08023816447
- Aaronson S, Sears P, Ruvuna F, et al. A 5-year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am J Psychiatry. 2017;174:7. doi:10.1176/appi.ajp.2017.16010034
- Milev RV, Giacobbe P, Kennedy SH; Group CDW. et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 4. neurostimulation treatments. Can J Psychiatry. 2016;61(9):561–575. doi:10.1177/070674371666003327486154
- Cimpianu CL, Strube W, Falkai P, Palm U, Hasan A. Vagus nerve stimulation in psychiatry: a systematic review of the available evidence. J Neural Transm. 2017;124:145–158. doi:10.1007/s00702-016-1642-227848034
- Keller MB. Issues in treatment-resistant depression. J Clin Psychiatry. 2005;66(Suppl 8):5–12.
