Abstract
Purpose
Among other non-motor symptoms, theory of mind (ToM), the ability to recognize, understand and infer others’ mental states, beliefs, intents and wishes, has been shown to deteriorate during the course of Parkinson’s disease (PD). It has been speculated that ToM impairments could be related to cognitive deficits in PD. However, the current state of literature suggests that there is heterogeneity regarding the involvement of cognitive functioning in the relationship of PD and ToM. The study aimed to measure affective ToM abilities and cognitive performance in a sample of PD patients, to explore the link between affective ToM abilities and cognitive status, and to examine the impact of PD on affective ToM through the mediator effect of cognitive performance.
Patients and methods
Sixty-five patients diagnosed with idiopathic PD and 51 healthy controls matched for age, gender and educational level completed a visual affective ToM task (Reading the Mind in the Eyes – RMET), cognitive performance was evaluated with Montreal Cognitive Assessment, and psychiatric symptoms were measured with BPRS-E (Brief Psychiatric Rating Scale).
Results
Affective ToM abilities were preserved in early PD patients, declining as the disease progressed. Deficits in cognitive functioning predicted deficiencies in affective ToM. Although attention (AT), executive functions (EF) and visuospatial abilities (VSA) together mediated the relationship between PD and affective ToM, only VSA impairment had a specific negative impact on affective ToM. Moreover, 41% of the total effect of attention and executive functions on affective ToM was mediated by visuospatial skills.
Conclusion
Cognitive performance may have an impact on the relationship between PD and affective ToM through the involvement of VSA. The influence of AT and EF in this relationship appears to be also exerted by PD patients’ VSA.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder clinically diagnosed due to the presence of several typical motor symptoms, such as resting tremor, bradykinesia and rigidity. These symptoms appear as a result of the degeneration of dopaminergic neurons in the substansia nigra.Citation1 However, numerous other non-motor symptoms are described in PD patients, including depression, anxiety, sleep disturbances, and cognitive decline.Citation2–Citation4 Furthermore, the ability of producing spontaneous emotional expressions,Citation5 as well as recognizing facial emotions has been shown to deteriorate throughout PD progression.Citation6,Citation7 Deficits in identifying emotions from prosody also appear to be present in PD.Citation8,Citation9
Theory of mind (ToM) refers to the ability to deduce others’ mental states, their beliefs, intentions or desires, in order to explain and foresee their behavior.Citation10 As such, two sub-components of ToM have been studied and defined: affective ToM (the ability to ascertain the emotional states of another person: knowledge about emotions) and cognitive ToM (the ability to understand other people’s beliefs, desires and intentions: knowledge about beliefs, intentions and desires).Citation11 Since ToM may be regarded as an essential precondition for an effective human interaction,Citation12 deficits in this area are extremely important, as they can lead to social seclusion, thus impacting negatively on patients’ quality of life.Citation13
ToM is considered to be a complex neuropsychological ability, mediated by an elaborate neuroanatomical network, which includes the medial prefrontal cortex, the temporal lobe (superior temporal sulcus region and the temporal pole), the temporoparietal junction, and the amygdala.Citation10,Citation12 ToM abilities have been investigated in various neurodegenerative diseases, several studies showing impaired ToM performance in PD. Certain authors reveal deficits only regarding the cognitive aspect of ToM,Citation14 while others also suggest supplementary reduced performance in the affective component of ToM.Citation11,Citation15 Studies investigating ToM performance in PD during the early stages have also yielded contradictory results.Citation15 Some authors report preserved affective and cognitive ToM skills in the early stages of PD, deficiencies occurring only in the more advanced stages and concerning the cognitive aspects of ToM.Citation14 At the same time, others argue that affective ToM abilities,Citation16 as well as ToM performance at large (both affective and cognitive)Citation17 might be impaired in early PD patients. These mixed results might be consequences of the specific measurements that were used, and of the differences in the severity of PD. A study using a computerized Yoni task to assess cognitive and affective dimensions of ToM in patients with mild cognitive impairment (MCI), PD and a healthy control (HC) group showed a reduced ToM performance in patients with PD (MCI<PD<HC).Citation18 Studies of affective ToM utilizing the Faux-Pas Test have, in general, offered consistent results, PD patients showing impaired cognitive ToM abilities, but preserved performance regarding the affective component of the Faux-Pas Test. Similar to controls, PD patients could detect inappropriate remarks in the presented stories (the affective component of the task), only having difficulties when trying to infer the reason as to why the character from the story made the inappropriate remark.Citation14,Citation17,Citation19 Certain studies using the Reading the Mind in the Eyes test (RMET) have proposed that affective ToM performance of medicated PD patients might be preserved both in the earlyCitation17,Citation20,Citation21 and in the more advanced stages of the disease.Citation14,Citation22 Moreover, a sample of non-medicated PD patients (de novo) appeared to have a similar performance in the RMET to the medicated PD, and to the control group.Citation17 By contrast, other two studies using the RMET, have reported impaired affective ToM abilities in PD patients when compared to HCs.Citation11,Citation16 However, in both studies, the control group was younger than the PD group, a fact that must be taken into consideration, as performance in RMET has been shown to decrease with age.Citation23
Psychiatric symptoms are part of the non-motor manifestations of PD.Citation24 It has been reported that a large percentage of PD individuals complained about at least one psychiatric comorbidity such as depression, anxiety, or apathy.Citation25 Even newly diagnosed and untreated PD patients exhibit higher rates of depression-anxiety symptoms and apathy than HCs recruited from the general population.Citation26 As it is well known that ToM performance is deficient in many psychiatric disorders, such as depression, anxiety, schizophrenia and other related psychotic disorders,Citation27–Citation30 the influence of psychiatric symptoms on ToM performance in PD was also tested. While the severity of apathy seems to be related to affective ToM impairments,Citation16 data on the influence of depressive symptoms on ToM in PD are inconsistent, ToM dysfunctions either appearing to be independent,Citation11 or to be accentuated by depressive symptomatology.Citation27
It has been proposed that ToM impairments may be consequences of cognitive deficits,Citation31 which are also found to appear early throughout the course of PD.Citation2 Cognitive domains generally affected in PD consist of executive functions, visuospatial skills, attention, and memory. To date, the relationship between cognitive functions and ToM remains controversial, as certain authors report no significant correlations,Citation11,Citation13,Citation17 whereas others regard affective and cognitive ToM as at least partially dependent on other cognitive functions (particularly executive and visuospatial abilities).Citation14,Citation15,Citation31,Citation32 These differences could be partly explained by the varying degrees of difficulty that each utilized test places upon executive processing. Significant associations were reported between ToM abilities and executive functions,Citation13 and in a sample of advanced PD patients, a significant correlation has been obtained between the Stroop Interference score and the “explanation” sub-score of the Faux-Pas Test.Citation14 By contrast, even if executive functions were significantly affected in their PD patients, some studies revealed no significant correlations between executive dysfunctions and ToM performance (affective and cognitive).Citation11,Citation27 More recently, it has been demonstrated that visuospatial dysfunctions might have a mediating role in the relationship between executive dysfunctions and affective ToM impairments in PD patients, and that deficiencies in visuospatial functioning may have a direct contribution to the affective ToM difficulties observed in patients with PD.Citation32 Nevertheless, the link between cognition and affective ToM in PD needs further exploring.
The main objectives of this research are assessing affective ToM abilities (using a visual ToM task) and cognitive performance in a sample of PD patients (using a HC group for comparison, and also analyzing performances taking into account the stage of PD), exploring the relationship between affective ToM abilities and cognitive status, and examining the role of PD on affective ToM abilities, considering the mediator effect of cognitive performance (global cognitive status and specific cognitive domains).
Materials and methods
Participants and procedure
The study was conducted between April 2017 and April 2019, and included 116 participants, of which 65 were patients diagnosed with idiopathic PD (the PD sample) and 51 were HCs matched for age, gender and educational level (the HC sample). The diagnosis and progression (Hoehn and Yahr [H&Y] staging) of idiopathic PD were established by neurologists. As suggested by the Movement Disorder Society Task Force report on the H&Y staging criteria,Citation33 the severity of PD was defined as follows: mild PD (stages 1, 1.5, and 2), moderate (stages 2.5 and 3), and advanced (stages 4 and 5). All of our PD patients were in mild or moderate stages of PD and were receiving optimal doses of anti-Parkinsonian medication throughout the study.
Exclusion criteria for both samples were: (a) history of a major somatic or psychiatric illness that could negatively affect their cognitive performance; (b) history of severe brain trauma; (c) dementia (defined by a Mini-Mental State Examination [MMSE] score <24, adjusted for age and education level);Citation34 (d) alcohol and/or other substance abuse; (e) uncorrected hearing and visual impairments; (f) chronic treatment with psychotropic substances (other than medication prescribed for PD); (g) illiteracy. Patients with atypical parkinsonism and those with a history of stroke or neurological disorders other than PD were also excluded from the PD sample.
All subjects received information about the purpose of the study and gave a written informed consent prior to study inclusion. This research was conducted in accordance to the guidelines set by the Helsinki Declaration for experiments involving human beings. The study was approved by the Scientific Research Ethics Committee of “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania (Reference number 16/2017).
Measurements
Demographic and clinical data
Demographic characteristics such as age upon study entry, gender, level of education, marital and professional status were collected for each participant. For patients with PD, we also documented disease duration (months) and disease severity according to Hoehn & Yahr (H&Y) staging criteria.
Affective theory of mind (ToM)
Affective ToM abilities were evaluated using the Romanian revised version of the RMET.Citation35 Participants were presented with 36 photographs depicting the eye region of a Caucasian actor/actress. Each photograph was printed with four possible mental state descriptors around it (eg, “suspicious,” “ashamed,” “frightened,” “serious”), and only one of them was the correct portrayal of the mental state of the person in the photograph. Subjects were asked to choose from the four words the one that best describes what the actor illustrated in the photograph might be feeling or thinking. There was no time limit for choosing, participants continuing to the next item when feeling ready. Test performance was calculated by summing the correct responses offered by each participant. The maximum score that could be obtained was 36. RMET assesses not only the ability to process visual information, but also the capacity to hypothesize mental empathic conclusions about other peoples’ feelings/thoughts. Literature data regarding RMET’s internal validity are heterogeneous. Whilst some authors may criticize its validity and internal construct (reporting low alpha coefficients of 0.37,Citation36 or ranging between 0.60 and 0.63Citation37), others describe satisfactory reliability and internal consistency (alpha coefficients of 0.70 or above),Citation38,Citation39 good test–retest reliability (0.74)Citation36 and split-half reliability (0.55),Citation40 concluding that RMET is a suitable instrument for detecting subtle deficiencies in social awareness, both in patients and in normal adults.
Cognitive performance
Cognitive performance was examined with the Romanian version of MoCA (Montreal Cognitive Assessment), a 30-item test, which can assess six different cognitive sub-domains: executive functions, visuospatial abilities, language, attention, memory, and orientation.Citation41 MoCA measures executive functions using an adapted form of the Trail Making B task (1 point), a verbal abstraction task (2 points) and a phonemic fluency test (1 point). Visuospatial abilities are explored using a cube-drawing copy task (1 point) and a clock-drawing test (3 points). Language is tested utilizing a three-animal (lion, rhinoceros and camel) naming task (3 points), and repetition of two complex sentences (2 points). Attention and working memory are evaluated using the following: repeating a list of digits forwards and backward (2 points), a finger tapping test (1 point) and a serial subtraction test (3 points). Short-term memory is assessed with a delayed recall task of five nouns (after two trials of hearing and repeating them). Lastly, time and place orientation are evaluated with 6 points. The maximum total score that could be obtained by each patient was 30. As suggested by previous studies, MCI was considered if MoCA total score was under 26.Citation42 Six MoCA sub-scores (executive functions – EF, visuospatial abilities – VSA, attention – AT, memory – MEM, language – L, and orientation – O) and a MoCA total score were computed for each patient. Literature shows that MoCA is extensively used as a screening test for detecting MCIs in PD patients, having a higher sensitivity than other neurocognitive assessment scales (eg, MMSE – Mini Mental Status ExaminationCitation34), particularly for detecting milder cognitive symptoms.Citation41 Studies examining the validity of MoCA revealed a good internal consistency (a Cronbach alpha on the standardized items of 0.83) and a high test–retest reliability (a correlation coefficient of 0.92).Citation41
Psychiatric symptoms
Both samples were assessed for the presence and severity of psychiatric symptoms using the Romanian version of BPRS-E (Brief Psychiatric Rating Scale, Expanded version 4.0), administered and interpreted by a trained psychiatrist during the clinical interview, following the 24-item BPRS administration manual.Citation43 BPRS-E is a semi-structured interview developed for the evaluation of overall psychopathology, comprised of 24 items that address the following psychiatric symptoms: somatic concerns, anxiety, depression, suicidality, guilt, hostility, elated mood, grandiosity, suspiciousness, hallucinations, unusual thought content, bizarre behavior, self-neglect, disorientation, conceptual disorganization, blunted affect, emotional withdrawal, motor retardation, tension, uncooperativeness, excitement, distractibility, motor hyperactivity, mannerisms and posturing. Each symptom is rated on a 7-point Likert scale from 1 (symptom not present) to 7 (extremely severe symptom). The maximum score that can be obtained is 168. The total score of BPRS-E is an indicator of the subject’s overall symptom severity (high scores indicating higher levels of severity). The separate analysis of the 24 items may offer an insight as to the type of psychopathology and symptom representation.Citation44 The BPRS-E has proven to be a sensitive and reliable instrument for gathering information about the possible presence and severity of several psychiatric symptoms.Citation45 To date, there are several BPRS-E factor models available in the literature, the majority finding 4 or 5 factor solutions which refer to positive psychotic symptoms (thought disturbances, suspiciousness-hostility), negative symptoms (apathy, anergia), agitation (animation, activation, disorganization) and affective symptomatology (depression-anxiety, affect, mood disturbances).Citation44,Citation46 Some authors reported a moderate internal consistency for their four-factor BPRS-E scale, with a Cronbach alpha ranging from 0.76 (for the depression factor) to 0.64 (for the mania – agitation factor).Citation47 In another study, the internal consistency (alpha coefficient) of the BPRS-E total score was estimated at 0.75. For the factors underlying its structure, the reported alpha coefficients were 0.70 for “thought disturbances,” 0.79 for “animation,” 0.80 for “mood disturbances” and 0.64 for “apathy.” These results reveal a satisfactory internal consistency for the BPRS-E.Citation48
Statistical analysis
Data analysis was performed using IBM SPSS Statistics (version 20; IBM Corporation, Armonk, NY, USA) and the PROCESS macro for SPSS.Citation49 Because of the non-Gaussian data distribution, as revealed by the Shapiro-Wilk normality test (), differences between groups were assessed using non-parametric tests (the Mann–Whitney U test with the Bonferonni correction, the Kruskal–Wallis test and Dunn-Bonferonni’s post hoc test). Spearman’s correlation coefficient was used to examine associations between scale scores. Differences between categorical data were examined with the χ2 (chi-square) test. The level of significance was considered to be 0.05 and all the results were two-tailed.
Table 1 Shapiro–Wilk test results (testing for normality of distribution)
The relationship between cognitive functions and affective ToM was assessed through a series of ordinary least squares (OLS) regression analyses, for which we reported values for: R2 (coefficient of determination), F (F-test of overall model significance), p (level of significance) and β (unstandardized coefficients). Notwithstanding the non-normality of data distribution, the main assumptions needed for running regression analyses were otherwise satisfied. The regressions were computed taking into account the presence of disease (PD), which was introduced as a covariate (where HC participants were coded as 0 and PD patients were coded as 1), so that its impact on the results might be controlled for.
To explore whether PD influences affective ToM abilities (assessed with a visual task – RMET) through its effect on cognitive performance (assessed using MoCA), non-parametric bootstrapping analyses were performed, following the procedures recommended by Hayes.Citation49 Before computing the mediational analyses, the influence of PD on affective ToM abilities and cognitive performance had to be also tested using a series of logistic regressions, for which we reported β (unstandardized coefficients) and p (level of significance). Next, a simple mediation was conducted using global cognitive status (MoCA total score) as the mediator. The OLS regression analyses showed that three specific cognitive domains (as examined with MoCA) had a significant influence on affective ToM performance: AT, EF and VSA. Therefore, the effect of these cognitive domains on the relationship of PD with affective ToM was also tested using a serial mediation model (as literature data suggested that the three mediators might causally influence each other). In both the simple and the serial mediation models, the presence of disease (PD) constituted the independent variable (where HC participants were coded as 0, and PD patients were coded as 1), while the dependent variable consisted of RMET scores (number of correct answers). Model 4 of the PROCESS function was used for the simple model, while model 6 was applied for the serial mediation model.Citation49 Mediation was considered significant if the 95% bias-corrected CI for the indirect effects based on 10,000 bootstrap samples did not include 0.Citation50,Citation51
Results
Demographic and clinical characteristics are presented separately for each sample in . As shown in , PD and HC were matched for age, gender, educational level and marital status (no significant differences were observed between the two samples regarding the aforementioned data). All PD patients were in mild-moderate stages of PD (stages 1–3, according to the modified classification of H&Y), with a mean duration of the disease of 76.43±38.04 months (range: 12–180 months). Mean values and the results of the Mann–Whitney U test comparing BPRS-E, MoCA total and sub-scores and RMET scores between the two samples are presented in . Of all subjects, 44 presented MCI (defined as a MoCA total score <26Citation41): 43 (66.2%) PD patients and 1 (1.9%) HC participant. The HC participant with MCI was excluded from the analysis.
Table 2 Sample characteristics
Table 3 Results of the neuropsychiatric assessment (comparison between patients with Parkinson’s disease and healthy controls)
As revealed by , compared to the HC group, PD patients presented significantly lower RMET scores, MoCA total scores, MoCA sub-scores for EF, VSA, AT and MEM, and significantly higher BPRS-E total scores. Further analysis regarding the BPRS-E scores revealed that significant differences were present between PD and HC subjects regarding the following BPRS-E specific items: somatic concerns, anxiety, depression, guilt, suicidality, self-neglect, motor retardation, and tension. These symptoms are usually found in depression-anxiety syndromes (depression, guilt, suicidality as symptoms of depression, and somatic concerns, anxiety and tension as symptoms of anxiety). Although self-neglect and motor retardation can be classified as negative symptoms, they can also be categorized as part of the depressive syndrome. Therefore, considering the structure of BPRS-E, a sub-score for depression-anxiety symptoms (affective symptoms) was calculated for both samples by summing the scores for somatic concerns, anxiety, depression, guilt, suicidality, self-neglect, motor retardation, and tension (). Symptoms of depression-anxiety were present in 58 (89.2%) of PD patients and in 12 (23.5%) of HC subjects. BPRS-E sub-scores for depression-anxiety symptoms correlated significantly with MoCA total scores (Spearman’s rho [r]=−0.29, P=0.001), but not with RMET scores (P=0.57).
Based on the severity (stage), PD patients were divided into two categories: mild PD patients (H&Y stages 1 and 2) and moderate PD patients (H&Y stages 2.5 and 3). The Kruskal–Wallis test showed significant differences between HC participants, mild PD patients and moderate PD patients regarding the following scale scores: BPRS-E total scores (H=57.0, P<0.0001), BPRS-E sub-scores for depression-anxiety symptoms (H=45.29, P<0.0001), RMET scores (H=36.57, P<0.0001), MoCA total scores (H=64.71, P<0.0001), MoCA sub-scores for EF (H=15.59, P<0.0001), VSA (H=52.66, P<0.0001), AT (H=15.05, P=0.001), and MEM (H=32.35, P<0.0001). Therefore, multiple post hoc comparisons were used to further assess these differences. The results are presented in .
Table 4 Results of Dunn’s post hoc test for multiple comparisons of scale scores (HC – mild PD – moderate PD)
The influence of demographic and clinical characteristics on all the main assessment scales (BPRS-E, MoCA, RMET) is shown in .
Table 5 Influence of demographic and clinical characteristics on participants’ neuropsychiatric assessment
Because of the significant differences between PD and HC regarding BPRS-E sub-scores for depression-anxiety (), the significant interaction between MoCA total scores and BPRS-E sub-scores for depression-anxiety, the significant differences between males and females regarding RMET scores (females > males, ), and also the significant association between age and RMET/MoCA scores (), BPRS-E sub-scores for depression-anxiety, age and gender were introduced as covariates for all main statistical procedures.
Relationship between cognitive functions and affective theory of mind
To assess the relationship between cognitive functions and affective ToM, a series of OLS regressions was performed. To see if global cognitive status may be predictive of affective ToM, independent of PD presence, MoCA total scores were regressed on RMET scores, independent of PD presence, and also controlling for depression-anxiety symptomatology (BPRS-E scores for depression-anxiety), age, and gender. The results showed that MoCA total scores were significant predictors of affective ToM (R2=0.50, F=28.07, β=1.004, P<0.0001), deficits in cognitive functioning predicting deficiencies in affective ToM. Next, multiple OLS regressions were conducted to investigate whether the six cognitive domains of MoCA (EF, VSA, AT, MEM, L, and O) could significantly predict affective ToM, independent of PD presence, and controlling for depression-anxiety symptoms, age and gender as well. While EF (R2=0.37, F=16.08, β=3.18, P<0.0001), VSA (R2=0.63, F=47.31, β=2.59, P<0.0001), and AT (R2=0.35, F=15.14, β=1.58, P=0.001) appeared to be significant predictors of affective ToM abilities, MEM, L and O did not (P>0.05). A multiple regression model containing all of three cognitive domains (AT, EF and VSA) explained 64% of the variance and was a significant predictor of RMET scores (F=32.004, P<0.0001). However, only VSA scores remained significant predictors of affective ToM when the other two variables were controlled for (β=2.48, P<0.0001 for VSA; β=0.19, P=0.83 for EF; β=0.59, P=0.22 for AT). Moreover, when MoCA total scores and VSA abilities were regressed together on RMET scores, global cognitive performance also lost its predictive value (R2=0.63, F=37.88; β=0.15, P=0.41 for MoCA total scores, β=2.36, P<0.0001 for VSA scores).
Mediational analysis
Logistic regression showed that the presence of PD was an independent predictor of deficits in affective ToM (β=−0.27, P<0.0001), but also in global cognitive functioning (β=−1.88, P=0.001). Disease (PD) was also a significant predictor of VSA (β=−2.77, P<0.0001), EF (β=−2.89, P<0.0001), AT (β=−1.31, P<0.0001), and MEM (β=−1.52, P<0.0001), but not of L and O (P>0.05). Since MEM, L and O were not predictive of affective ToM abilities, they were not included in the next analyses.
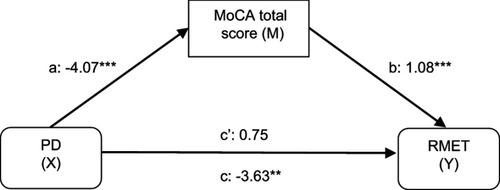
To begin with, a simple mediation analysis was conducted to test the impact of disease (PD) on affective ToM abilities (assessed with a visual affective ToM task – RMET) through the mediator effect of global cognitive status (MoCA total scores). The number of correct answers in RMET was entered as the dependent variable (Y), the presence of PD as the independent variable (X), MoCA total score as the mediator (M) and the BPRS-E sub-score for depression-anxiety symptoms, age and gender as covariates. As shown in , lower MoCA total scores were significantly related to the presence of PD (a=−4.07; P<0.0001), and deficits in MoCA total scores were subsequently related to lower RMET scores (b=1.08; P<0.0001). The 95% bias-corrected CI based on 10,000 bootstrap samples revealed that the indirect effect of PD on ToM through cognitive status was significant (effect estimate: −4.38, 95% CI: −6.28; −2.67). The total effect of PD on ToM (c=−3.63; P=0.001, 95% CI: −5.74; −1.51) turned statistically non-significant when MoCA total score was entered into the model (total direct effect: c’=0.75; P=0.53, 95% CI: −1.60; 3.11) and thus, full mediation can be assumed.
Figure 1 The mediating effect of global cognitive status on affective theory of mind in Parkinson’s disease, controlling for depression-anxiety symptomatology and gender.
Abbreviations: PD, Parkinson’s disease; MoCA, montreal cognitive assessment; RMET, Reading the Mind in the Eyes test.
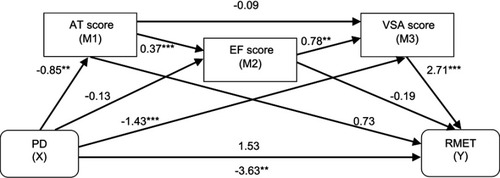
Questioning which cognitive domain might be driving this mediation, another model was constructed, this time using the three cognitive domains that had a significant influence on affective ToM performance in PD patients (as shown by the regression analyses): VSA, AT and EF. Because it has been suggested that causality might exist between these potential mediators, a serial model was deployed. The mediators were entered as follows: AT was considered as first mediator (M1), exerting an influence on EF (second mediator, M2), which in turn affected VSA (third mediator, M3). Similar to the simple mediation model, the number of correct answers in RMET was introduced as the dependent variable (Y), the presence of PD as the independent variable (X), BPRS-E scores for depression-anxiety, age and gender as covariates. As indicated in , the long-way mediation (PD → AT scores → EF scores → VSA scores → RMET scores) was significant (indirect effect: −0.66, 95% CI: −1.41; −0.12), as was the specific path PD → VSA scores → RMET scores (indirect effect: −3.88, 95% CI: −5.77; −2.19). However, the specific indirect effects through AT (indirect effect: −0.62; 95% CI: −1.56; 0.14) and EF (indirect effect: 0.03; 95% CI: −0.29; 0.31) were not significant, as they contained 0. The specific indirect effect of PD on affective ToM through both AT and EF (PD → AT scores → EF scores → RMET scores) was also insignificant (indirect effect: 0.06; 95% CI: −0.54; 0.64), as were the specific paths PD → AT scores → VSA scores → RMET scores (indirect effect: −0.20; 95% CI: −0.28; 0.82), and PD → EF scores → VSA scores → RMET scores (indirect effect: −0.28; 95% CI: −0.74; 0.04). The total effect of the model was significant (total effect: c=−3.63; P=0.001, 95% CI: −5.74; −1.51). As for the simple mediation model, the direct effect of PD on affective ToM also became statistically insignificant (direct effect: c’=1.53, P=0.12, 95% CI: −0.39; 3.44) when AT, EF and VSA scores were introduced into de model (hence, full mediation can be inferred). Furthermore, 41% of the total effect of attention and executive functions on affective ToM was mediated by visuospatial abilities (R2=0.41, F=12.74, P<0.0001).
Figure 2 The mediating effect of three cognitive domains (attention, executive functions and visuospatial abilities) in the relationship between Parkinson’s disease and affective theory of mind.
Abbreviations: PD, Parkinson’s disease; AT, attention; EF, executive functions; VSA, visuospatial abilities; RMET, Reading the Mind in the Eyes test.
Discussion
This study had three main objectives. The first one was to assess affective ToM abilities (using a visual ToM task) and cognitive performance in a sample of PD patients (using a HC group for comparison, and also analyzing performances taking into account the stage of PD). The second objective focused on exploring the relationship between affective ToM abilities and cognitive status (independent of PD presence), whereas the third objective centered on examining the role of PD on affective ToM abilities, considering the mediator effect of cognitive performance (global cognitive status and specific cognitive domains).
Consistent with previous research,Citation17,Citation21 our results revealed that affective ToM abilities were preserved in mild PD (patients in stages 1, 1.5 and 2 according to H&Y criteria having a similar performance to HC). By contrast, moderate PD patients (stages 2.5 and 3) presented significantly worse affective ToM skills than participants from both the HC and the early (mild) PD sample, affective ToM impairment in PD appearing to develop as the disease progressed (a fact also suggested by the significant negative correlation between RMET scores and PD duration). Since only patients with mild-moderate motor symptomatology (according to H&Y staging criteria) were included in this study, it is probable that additional decline in affective ToM abilities may ensue, along with patients’ motor and cognitive progressive deterioration. These findings are in accordance to other studies suggesting that affective ToM deficits appear during the later stages of PD and seem to worsen throughout the progression of PD.Citation14,Citation15,Citation53 It must be noted that impaired affective ToM abilities (as assessed with a visual affective ToM task) were not consequences of pure visual deficits, as all participants had a satisfactory or corrected vision.
Similar to other studies,Citation2,Citation13,Citation23 we found that PD was associated with abnormalities in visuospatial perception, which appeared early during the course of the disease (mild and moderate PD patients), followed by executive dysfunctions and attention/memory deficits (moderate PD patients). As expected, cognitive performance appeared to deteriorate throughout PD progression, moderate PD patients revealing significantly lower MoCA total scores than both mild PD patients and HC.
While some authors mentioned the influence of psychiatric symptoms, particularly those related to emotional disturbances (depression and anxiety) on ToM performance in PD patients,Citation27 others suggested that ToM abilities are not related to affective symptomatology in PD.Citation11 More recent data proposed that affective ToM dysfunctions in PD patients could be related to severity of apathy (assessed with the Apathy Evaluation Scale) and disturbances in behavior (assessed with the Frontal behavioral inventory).Citation15 The influence of psychosis on ToM has been also demonstrated in patients suffering from schizophrenia and other related psychotic disorders (during the acute state and also during remission).Citation28 The BPRS was used in this study to assess all possible psychiatric symptoms that could have a negative impact on affective ToM abilities. However, our participants scored higher only in items related to depression-anxiety symptomatology and therefore, a BPRS-E sub-score for depression-anxiety was calculated for each sample. Although this score did not correlate significantly with RMET scores, which is consistent with other findings,Citation27 BPRS-E scores for depression-anxiety correlated negatively with MoCA total scores. Because it has been demonstrated that depression-anxiety symptoms may have a negative impact on cognitive functions in PD,Citation52 the regression and mediational analyses were conducted while controlling for the presence and severity of these symptoms, so they cannot influence the results of our study.
Certain affective ToM tasks depend more heavily on the visual processing of information (eg, attributing emotions to faces), other tasks that assess affective mental states rely on written stories, whereas the ones that explore the higher-order ToM abilities appear to rely mostly on executive processing (eg, story-based tasks, tasks involving inferring and associating information and also hypothesizing).Citation54 Some authors suggest that impairments in mindreading tasks are associated to executive dysfunctions, such as difficulties in understanding and taking others’ perspective, or deficiencies in merging different sources of information (past events and knowledge about others), arguing for shared mechanisms between ToM and executive functions.Citation55 Functional neuroimaging studies indicate that the frontal lobes, along with several other brain regions, might be involved in ToM impairments. The frontal lobes are involved in many cognitive and non-cognitive processes, such as executive functions, attention, memory, language, motor functionality, processes underlying affect, personality, social and moral perception and reasoning.Citation56 It has been suggested that damage occurring in the frontal lobes not only affects cognitive and motor functions, but also social behavior, self-awareness, personality.Citation57 Lesions in the orbitofrontal and ventromedial areas were observed to be responsible personality disturbances, appearance of indifferent behavior, deficiencies in social judgment, self-regulation deficits, impaired affective response, impaired pragmatics, incapacity of associating social circumstances with personal affective indicators.Citation58 Therefore, the orbitofrontal cortex and the dorsomedial prefrontal cortex have been connected to ToM abilities and behavioral self-awareness. Studies have revealed that while damages of the orbitofrontal cortex appear to associate with impairments in affective ToM, injuries/lesions of the dorsomedial prefrontal cortex have been associated with the cognitive feature of self-reference, as well as with a reliable assessment of one’s personal behavior.Citation59 Since cognitive deficits observed in PD patients were also found to associate with frontal lobe dysfunction,Citation60 the possibility that cognitive deficits might explain ToM impairments in these patients appears plausible. The ability to ascribe emotions to facial expressions is considered to be an elaborate cognitive process, relying on the functional integrity of several cognitive domains, such as executive functions, visuospatial perception, attention, memory, and language.Citation61 As cognitive decline appears to be frequently associated with PD, occurring even during the early stages of the disease,Citation2,Citation16,Citation41 the role of cognitive impairment in affective and cognitive ToM processing in PD has been brought into question. Data on the relationship between cognitive deficits and impaired ToM in PD are heterogeneous, certain authors arguing that no connection has been found between these processes,Citation11,Citation13,Citation17 whereas others supporting the hypothesis that cognitive decline (especially executive and visuospatial dysfunctions) might be related to affective and cognitive ToM abilities.Citation14,Citation15,Citation31 Complex assessments of ToM abilities, concerning both the affective and the cognitive facets of ToM, revealed significant associations between ToM performance and executive functions in PD (using the Faux pas detection test,Citation62 the Advanced Test of Theory of Mind,Citation15 the intention attribution test,Citation63 and other story-based tasks). However, studies using less cognitive demanding tests, such as the RMET, have provided mixed results, showing significant correlations between ToM abilities and executive functions only in the more advanced stages of PD.Citation63–Citation65 Impaired RMET performance has been also associated with dysfunctions in visuospatial abilities and deficient non-verbal reasoning.Citation32,Citation66 The results of this research support the theory that in patients with PD cognitive deficits are predictive of affective ToM impairments. However, it must be taken into account that the whole sample of PD was considered in the mediational analysis, and that the impact of PD severity on affective ToM impairments through cognitive performance was not examined.
In our study, three of the six cognitive domains that were examined with MoCA had a significant influence on affective ToM performance: visuospatial abilities, attention, and executive functions. Theories that associate cognitive impairments with abnormalities of the frontal-basal ganglia neural circuits (central to executive functions), suggest that perceptual and visual impairments (visuospatial deficits) can be attributed to slow processing of information, which can affect executive functions in PD.Citation67,Citation68 More precisely, previous studies opine that attention and executive dysfunctions should be considered primary impairments that will eventually lead to other cognitive deficits, such as memory, learning, verbal fluency, and visuospatial deficiencies. However, in order to properly function, executive processes require, among other cognitive domains, the integrity of attention.Citation69,Citation70 To test their potential mediator effect in the relationship of PD and affective ToM, MoCA sub-scores for attention, executive functions and visuospatial abilities were entered simultaneously in a serial mediation model (in that order, as was suggested by the above-mentioned literature data). These three cognitive domains working combined fully mediated the relationship between PD and affective ToM in our study: PD was responsible for attention decline, which consequently led to executive dysfunctions, which in turn generated visuospatial impairments, which ultimately impacted affective ToM abilities in a negative manner. Moreover, PD seemed to have a direct negative impact on affective ToM via visuospatial impairments, without attention and executive functions being involved. Attention and executive functions did not act as independent mediators, but rather appeared to remain part of an extended causal sequence that ultimately involved visuospatial capacities. RMET is considered to be an advanced affective ToM task that involves the attribution of mental states to facial expressions and complex facial emotion representation from photographs,Citation71 thus requiring the correct perception of the picture for the processing of this information. Hence, it was to be expected that visuospatial abilities should play the most important mediating role in the relationship between PD and affective ToM. However, neither attention, nor executive functions remained significant predictors of affective ToM deficits when visuospatial abilities were controlled for. Furthermore, global cognitive performance lost its predictive value for affective ToM deficits when visuospatial abilities were accounted for in the regression analysis. This indicates that affective ToM, which is a complex process, should not be considered only a cognitive deficit and that there might be other factors that could influence affective ToM performance in PD, other than cognition alone. The contribution of global cognitive status and visuospatial abilities on affective ToM performance in PD was speculated in previous studies (using MMSE as a scale for measuring cognitive performance, the line orientation test for examining visuospatial abilities and RMET for evaluating affective ToM).Citation32 Using another assessment scale for cognitive performance, our results also support the theory that visuospatial deficiencies might play a direct role in affective ToM decline (when assessed using a visual affective ToM task) in PD, also having an important contribution in the relationship between executive dysfunctions and affective ToM impairments (assessed with a visual ToM task) in PD patients.
Another point that must be considered refers to our study limitations. First of all, only the affective component of ToM was assessed using a visual ToM task. This limits our ability to generalize the conclusions to affective ToM performance at large. Utilizing more complex tasks that examine both the affective and cognitive aspects of ToM might have enabled us to explore the role of cognitive performance and of specific cognitive domains in ToM performance. The cross-sectional design of our study limits the sustenance of a definite model; longitudinal data could have confirmed if the associations revealed by this study remained stable across time. The relatively small sample size and the use of only one cognitive assessment scale also restrict us to generalize these results. Moreover, the results must be interpreted taking into account the large presence of MCI in the PD sample and the fact that the mean performance of the PD sample in RMET was near the floor effect. Because cognitive functions were assessed in our study with MoCA, which is a screening tool, in order to provide more robust conclusions, the connection between cognitive deficits and ToM impairments should be further explored in future studies using other tests that assess in detail specific cognitive domains (eg, batteries of specific tests for examining visuospatial abilities, executive functions, attention and memory) and also using classical ToM tasks, for which the construct validity is more well established.
Conclusion
The results of our study suggest that affective ToM performance is preserved in the early stages of PD, appearing to decline with the progression of the disease. Patients with moderate PD exhibit impaired affective ToM abilities. These deficiencies are not influenced by the presence of psychiatric symptomatology, such as anxiety-depression symptoms.
Cognitive performance appears to mediate the relationship between PD and affective ToM through the combined effect of attention, executive functions and visuospatial abilities. Attention deficits negatively impact on executive functions, which adversely influence visuospatial abilities, which in turn lead to affective ToM deficiencies. Although attention and executive functions do not act as individual mediators, they are part of an extended causal chain that ultimately involves visuospatial abilities. Visuospatial skills may have a direct involvement in affective ToM impairments displayed by PD patients, as assessed with a visual task.
Author contributions
Ana-Maria Romosan, Liana Dehelean and Radu-Stefan Romosan share first authorship. Ana-Maria Romosan, Liana Dehelean, and Radu-Stefan Romosan: study design; acquisition, analysis and interpretation of data; article drafting; critical revision of the manuscript for intellectual content. Minodora Andor and Ana Cristina Bredicean: significant contribution to data acquisition, analysis and interpretation; critical revision of the manuscript for intellectual content. Mihaela Adriana Simu: study design and supervision; data analysis and interpretation; critical revision and approval of the final version of the manuscript. All authors approved the final version of this article for publication and assume responsibility for the accuracy and integrity of all aspects regarding this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi:10.1136/jnnp.55.3.1811564476
- Aarsland D, Brønnick K, Larsen JP, Tysnes OB, Alves G; Norwegian ParkWest Study G. Cognitive impairment in incident, untreated Parkinson disease: The Norwegian ParkWest Study. Neurology. 2009;72:1121–1126. doi:10.1212/WNL.0b013e3181a9fad119020293
- Aarsland D, Brønnick K, Alves G, et al. The spectrum of neuropsychiatric symptoms in patients with early untreated Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2009;80:928. doi:10.1136/jnnp.2008.16695919608786
- Rodríguez-Ferreiro J, Cuetos F, Herrera E, Menéndez M, Ribacoba R. Cognitive impairment in Parkinson’s disease without dementia. Mov Disord. 2010;25:2136–2141. doi:10.1002/mds.v25:1320725913
- Ricciardi L, Visco-Comandini F, Erro R, et al. Facial emotion recognition and expression in Parkinson’s disease: an emotional mirror mechanism. PLoS One. 2017;12(1):e0169110. doi:10.1371/journal.pone.016911028068393
- Gray HM, Tickle-Degnen L. A meta-analysis of performance on emotion recognition tasks in Parkinson’s disease. Neuropsychol. 2010;24:176–191. doi:10.1037/a0018104
- Ariatti A, Benuzzi F, Nichelli P. Recognition of emotions from visual and prosodic cues in Parkinson’s disease. Neurol Sci. 2008;29:219–227. doi:10.1007/s10072-008-0971-918810595
- Schröder C, Nikolova Z, Dengler R. Changes of emotional prosody in Parkinson’s disease. J Neurol Sci. 2010;289(1):32‐35. doi:10.1016/j.jns.2009.08.038
- Buxton SL, MacDonald L, Tippett LJ. Impaired recognition of prosody and subtle emotional facial expressions in Parkinson’s disease. Behav Neurosci. 2013;127(2):193–203. doi:10.1037/a003201323565934
- Frith CD, Frith U. Interacting minds – a biological basis. Science. 1999;286:1692–1695. doi:10.1126/science.286.5441.96410576727
- Bodden ME, Mollenhauer B, Trenkwalder C, et al. Affective and cognitive theory of mind in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2010;16(7):466–470. doi:10.1016/j.parkreldis.2009.07.00120538499
- Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–178. doi:10.1038/nrn105612612630
- Saltzman J, Strauss E, Hunter M, Archibald S. Theory of mind and executive functions in normal human aging and Parkinson’s disease. J Int Neuropsychol Soc. 2000;6(7):781–788. doi:10.1017/S135561770067705611105468
- Peron J, Vicente S, Leray E, et al. Are dopaminergic pathways involved in theory of mind? A study in Parkinson’s disease. Neuropsychol. 2009;47(2):406–414. doi:10.1016/j.neuropsychologia.2008.09.008
- Santangelo G, Vitale C, Trojano L, et al. Neuropsychological correlates of theory of mind in patients with early Parkinson’s disease. Mov Disord. 2012;27(1):98–105. doi:10.1002/mds.2403521915910
- Tsuruya N, Kobayakawa M, Kawamura M. Is “reading mind in the eyes” impaired in Parkinson’s disease? Parkinsonism Relat Disord. 2011;17:246–248. doi:10.1016/j.parkreldis.2010.09.00120889365
- Roca M, Torralva T, Gleichgerrcht E, et al. Impairments in social cognition in early medicated and unmedicated Parkinson disease. Cogn Behav Neurol. 2010;23(3):152–158. doi:10.1097/WNN.0b013e3181f20cdd20829664
- Rossetto F, Castelli I, Baglio F, et al. Cognitive and affective theory of mind in mild cognitive impairment and Parkinson’s disease: preliminary evidence from the italian version of the yoni task. Dev Neuropsychol. 2018;43(8):764–780. doi:10.1080/87565641.2018.152917530299987
- Kawamura M, Koyama S. Social cognitive impairment in Parkinson’s disease. J Neurol. 2007;254:49–53. doi:10.1007/s00415-007-0648-y
- Euteneuer F, Schaefer F, Stuermer R, et al. Dissociation of decision making under ambiguity and decision making under risk in patients with Parkinson’s disease: a neuropsychological and psychophysiological study. Neuropsychologia. 2009;47:2882–2890. doi:10.1016/j.neuropsychologia.2008.11.02719545579
- Mimura M, Oeda R, Kawamura M. Impaired decision-making in Parkinson’s disease. Parkinsonism Relat Disord. 2006;12(3):169–175. doi:10.1016/j.parkreldis.2005.12.00316563850
- Peron J, Le Jeune F, Haegelen C, et al. Subthalamic nucleus stimulation affects theory of mind network: a PET study in Parkinson’s disease. PLoS One. 2010;5:e9919. doi:10.1371/journal.pone.000991920360963
- Pardini M, Nichelli PF. Age-related decline in mentalizing skills across adult life span. Exp Aging Res. 2009;35:98–106. doi:10.1080/0361073080254525919173104
- Chaudhuri KR, Odin P, Antonini A, Martinez-Martin P. Parkinson’s disease: the non-motor issues. Parkinsonism Relat Disord. 2011;17:717–723. doi:10.1016/j.parkreldis.2011.02.01821741874
- Petrovic M, Stefanova E, Ziropadja L, Stojkovic T, Kostic V. Neuropsychiatric symptoms in Serbian patients with Parkinson’s disease. J Neurol Sci. 2016;367:342–346. doi:10.1016/j.jns.2016.06.02727423616
- De la Riva P, Smith K, Xie SX, Weintraub D. Course of psychiatric symptoms and global cognition in early Parkinson disease. Neurol. 2014;83:1096–1103. doi:10.1212/WNL.0000000000000801
- Mengelberg A, Siegert RJ. Is theory-of-mind impaired in Parkinson’s disease? Cogn Neuropsychiatry. 2003;8:191–209. doi:10.1080/1354680024400029216571560
- Marjoram D, Gardner C, Burns J, Miller P, Lawrie S, Johnstone E. Symptomatology and social inference: a theory of mind study of schizophrenia and psychotic affective disorder. Cogn Neuropsychiatry. 2005;10(5):347–359. doi:10.1080/1354680044400009216571466
- Bora E, Berk M. Theory of mind in major depressive disorder: a meta-analysis. J Affect Disord. 2016;191:49–55. doi:10.1016/j.jad.2015.11.02326655114
- Hezel DM, McNally RJ. Theory of mind impairments in social anxiety disorder. Behav Ther. 2014;45(4):530–540. doi:10.1016/j.beth.2014.02.01024912465
- Eddy CM, Beck SR, Mitchell IJ, Praamstra P, Pall HS. Theory of mind deficits in Parkinson’s disease: a product of executive dysfunction? Neuropsychol. 2013;27(1):37–47. doi:10.1037/a0031302
- McKinlay A, Albicini M, Kavanagh PS. The effect of cognitive status and visuospatial performance on affective theory of mind in Parkinson’s disease. Neuropsychiatr Dis Treat. 2013;9:1071. doi:10.2147/NDT.S4910424019747
- Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations the Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Mov Disord. 2004;19(9):1020–1028. doi:10.1002/mds.2021315372591
- Teng EL, Chui HC. The modified mini mental state (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318.3611032
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42(2):241–251. doi:10.1111/1469-7610.0071511280420
- Khorashad BS, Baron-Cohen S, Roshan GM, et al. The “Reading the Mind in the Eyes” test: investigation of psychometric properties and test–retest reliability of the persian version. J Autism Dev Disord. 2015;45(9):2651–2666. doi:10.1007/s10803-015-2427-425832800
- Voracek M, Dressler SG. Lack of correlation between digit ratio (2D: 4D) and Baron-Cohen’s “Reading the Mind in the Eyes” test, empathy, systemising, and autism-spectrum quotients in a general population sample. Pers Individ Diff. 2006;41:1481–1491. doi:10.1016/j.paid.2006.06.009
- Dehning S, Girma E, Gasperi S, Meyer S, Tesfaye M, Siebeck M. Comparative cross-sectional study of empathy among first year and final year medical students in Jimma University, Ethiopia: steady state of the heart and opening of the eyes. BMC Med Educ. 2012;12(1):34. doi:10.1186/1472-6920-12-3422624580
- Prevost M, Carrier ME, Chowne G, Zelkowitz P, Joseph L, Gold I. The Reading the Mind in the Eyes test: validation of a French version and exploration of cultural variations in a multi-ethnic city. Cogn Neuropsychiatry. 2014;19(3):189–204. doi:10.1080/13546805.2013.82385923937473
- Wakabayashi A, Katsumata A. The Motion Picture Mind-Reading Test: measuring individual differences of social cognitive ability in a young adult population in Japan. J Individ Differ. 2011;32:55–64. doi:10.1027/1614-0001/a000034
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x15817019
- Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717–1725.21060094
- Lukoff D, Nuechterlein H, Ventura J. Manual for the expanded brief psychiatric rating scale. Schizophr Bull. 1986;12:594–602.
- Dingemans PMaJ, Linszen DH, Lenior ME, Smeets RMW. Component structure of the expanded Brief Psychiatric Rating Scale (BPRS-E). Psychopharmacol. 1995;122:263–267. doi:10.1007/BF02246547
- Burlingame GM, Seaman S, Johnson JE, et al. Sensitivity to change of the Brief Psychiatric Rating Scale – Extended (BPRS-E): an item and subscale analysis. Psychol Serv. 2006;3:77–87. doi:10.1037/1541-1559.3.2.77
- Ruggeri M, Koeter M, Schene A, et al. Factor solution of the BPRS-expanded version in schizophrenic outpatients living in five European countries. Schizophr Res. 2005;75:107–117. doi:10.1016/j.schres.2004.05.01715820329
- Kopelowicz A, Ventura J, Liberman RP, Mintz J. Consistency of brief psychiatric rating scale factor structure across a broad spectrum of schizophrenia patients. Psychopathology. 2008;41:77–84. doi:10.1159/00011155118033976
- Thomas A, Donnell AJ, Young TR. Factor structure and differential validity of the expanded Brief Psychiatric Rating Scale. Assessment. 2004;11(2):177–187. doi:10.1177/107319110326289315171466
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: Guilford Press; 2013.
- Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behav Res. 2007;42:185–227. doi:10.1080/0027317070134131626821081
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi:10.3758/BF0320655315641418
- Starkstein SE, Preziosi TJ, Berthier ML, Bolduc PL, Mayberg HS, Robinson RG. Depression and cognitive impairment in Parkinson’s disease. Brain. 1989;112(5):1141–1153. doi:10.1093/brain/112.5.11412804609
- Bora E, Walterfang M, Velakoulis D. Theory of mind in Parkinson’s disease: a meta-analysis. Behav Brain Res. 2015;292:515–520. doi:10.1016/j.bbr.2015.07.01226166188
- Stone VE, Gerrans P. What’s domain-specific about theory of mind? Social Neurosci. 2006;1(3–4):309–319. doi:10.1080/17470910601029221
- Apperly IA, Samson D, Humphreys GW. Studies of adults can inform accounts of theory of mind development. Dev Psychol. 2009;45(1):190. doi:10.1037/a001409819210001
- Chayer C, Freedman M. Frontal lobe functions. Curr Neurol Neurosci Rep. 2001;1(6):547–552. doi:10.1007/s11910-001-0060-411898568
- Stuss DT, Picton TW, Alexander MP. Consciousness, Self-awareness, and the Frontal Lobes. Arlington: American Psychiatric Publishing, Inc; 2001.
- Nauta WJ. Connections of the frontal lobe with the limbic system In: Laitinen LV, Livingston KE, editors. Surgical Approaches in Psychiatry. Lancaster: Medical and Technical Publishing Co. Ltd; 1973:303–314.
- Jonker FA, Wattjes MP, Scherder EJ. Impaired behavioural self-awareness and affective theory of mind deficits following prefrontal cortex damage. Neuropsychiatry. 2017;7(5):750–758. doi:10.4172/Neuropsychiatry
- Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1996;244(1):2–8. doi:10.1007/PL00007725
- Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. doi:10.1146/annurev.psych.60.110707.16351418771388
- Yu RL, Rm W, Chiu MJ, Tai CH, Lin CH, Hua MS. Advanced theory of mind in patients at early stage of Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(1):21–24. doi:10.1016/j.parkreldis.2011.08.00321868278
- Enrici I, Mitkova A, Castelli L, Lanotte M, Lopiano L, Adenzato M. Deep brain stimulation of the subthalamic nucleus does not negatively affect social cognitive abilities of patients with Parkinson’s disease. Sci Rep. 2017;7(1):9413. doi:10.1038/s41598-017-09737-628842656
- Narme P, Mouras H, Roussel M, Duru C, Krystkowiak P, Godefroy O. Emotional and cognitive social processes are impaired in Parkinson’s disease and are related to behavioral disorders. Neuropsychology. 2013;27(2):182–192. doi:10.1037/a003152223527646
- Poletti M, Vergallo A, Ulivi M, Sonnoli A, Bonuccelli U. Affective theory of mind in patients with Parkinson’s disease. Psychiatry Clin Neurosci. 2013;67(4):273–276. doi:10.1111/pcn.2013.67.issue-423683159
- Roca M, Manes F, Chade A, et al. The relationship between executive functions and fluid intelligence in Parkinson’s disease. Psychol Med. 2012;42(11):2445–2452. doi:10.1017/S003329171200045122440401
- Crucian GP, Okun MS. Visual-spatial ability in Parkinson’s disease. Front Biosci. 2003;8:992–997.
- Brown LA, Cooper SA, Doan JB, et al. Impaired foveal processing and cognitive visual deficits in PD warrant further studies. Parkinsonism Relat Disord. 2006;12(6):376–381. doi:10.1016/j.parkreldis.2006.03.00416720099
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson’s disease: relationship to frontostriatal circuitry. Cogn Behav Neurol. 2003;16(4):193–210. doi:10.1097/00146965-200312000-0000114665819
- Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD. Visual dysfunction in Parkinson disease without dementia. Neurol. 2005;65(12):1907–1913. doi:10.1212/01.wnl.0000191565.11065.11
- Baron-Cohen S, Bowen DC, Holt RJ, et al. The “reading the mind in the eyes” test: complete absence of typical sex difference in ~400 men and women with autism. PLoS One. 2015;10(8):e0136521. doi:10.1371/journal.pone.013652126313946
