Abstract
Aim
The impairment experienced by many individuals with depression is closely related to the cognitive symptoms of the disorder. Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive brain stimulation method providing a promising technique for improving cognitive symptoms in treatment-resistant depression (TRD). In the present study, we investigated whether a relationship exists between improvements in frontal lobe dysfunction induced by rTMS and improvement of white matter integrity revealed by diffusion tensor imaging (DTI) in TRD patients receiving rTMS treatment.
Methods
A total of 12 patients with TRD were enrolled in a high-frequency (10 Hz) rTMS study (August 2013–January 2019). Frontal lobe function and depressive symptoms were assessed at baseline and at the endpoint of rTMS treatment. Fractional anisotropy (FA), as a measure of white matter integrity obtained from DTI, was investigated using a region-of-interest (ROI) approach.
Results
rTMS treatment significantly improved depressive symptom scores and some subscales of frontal lobe dysfunction. Category scores in the Word Fluency Test and scores on part 3 of the Color Stroop Test were improved independently of the improvement of depressive symptoms. In the ROI analysis, none of the FA increases in any region were correlated with improvement of any frontal lobe function (n = 12).
Conclusion
Although rTMS resulted in partial improvement of frontal lobe dysfunction as well as white matter integrity, we found no correlation between improved frontal lobe dysfunction and improved white matter integrity in TRD patients.
Introduction
Depression is a serious public health problem with a high global prevalence. Major depressive disorder (MDD) has a 12-month prevalence of 6.6% and a lifetime prevalence of 16.2%, and causes considerable impairment. The impairment experienced by many individuals with depression is closely related to the cognitive symptoms of the disorder.Citation1 Etkin et al.’s results indicated that cognitive dysfunction could have negative predictive value for achieving depressive remission.Citation2 Thus, for individuals with MDD to achieve full, functional recovery, cognitive dysfunction must be addressed. Cognitive functioning is a complex process that can be broken down into multiple domains, and the domains most relevant to MDD are attention, memory and learning, executive function, and psychomotor processing.Citation1 While several neuropsychological studies have demonstrated that cognitive deficits are present across a broad range of cognitive domains in depression, executive function deficits associated with frontal lobe dysfunction are also reported to be prominent among depressed patients.Citation3 Depression leads to a series of molecular and cellular events which include changes in intracellular signaling, gene expression, neuronal function, and cellular architecture within brain regions that control mood and cognition.Citation1 Affected brain regions include the amygdala, prefrontal cortex, hippocampus, thalamus, cingulate cortex, insula, and superior temporal gyrus.Citation1 Individuals with depression tend to translate a negative bias in perception, attention, and memory into conscious thoughts, memories, and actions, often showing greater attention and memory to negative stimuli.Citation1 Indeed, these emotion-laden functions related to negative affect are sometimes referred to as “hot” cognition, to distinguish them from “cold” cognition, or emotion-independent areas including executive function, information processing speed, learning and memory, and attention/concentration.Citation1 One STAR*D study report found that the patients who had not regained normal functioning at remission had a higher depression relapse compared with patients who had recovered normal functioning.Citation1,Citation4 Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive brain stimulation technique that is considered a valuable and promising technique for improving cognitive symptoms in treatment-resistant depression (TRD)Citation5,Citation6 and traumatic brain injuryCitation7 Hayasaka et al reported that 10 daily sessions of 20-Hz left prefrontal rTMS in patients with major depression increased the hippocampal volume on the stimulated side, which could potentially be related to the improvement of cognitive function.Citation8 Nilakantan et al’s study demonstrated that high-frequency transcranial magnetic stimulation selectively modified neural and behavioral hallmarks of age-related memory impairment, indicating effective engagement of memory intervention targets in older adults.Citation9 Shinba et al.’s study demonstrated increase of frontal cerebral blood volume during TMS in depression is related to treatment effectiveness using near-infrared spectroscopy.Citation10
In addition, previous studies have reported that the major brain regions exhibiting white matter abnormalities in depressive patients include the frontal, temporal and limbic lobes.Citation11–Citation15 Diffusion tensor imaging (DTI) with magnetic resonance imaging (MRI) has been widely used to detect white matter microstructural changes.Citation16 The measure of fractional anisotropy (FA) obtained with DTI is a scalar measure ranging from 0 to 1 that indicates the degree of anisotropy in diffusion. However, to date, only a few studies have explored the relationship between changes in FA induced by rTMS and treatment responses.Citation17,Citation18
To the best of our knowledge, no previous reports have examined the relationship between changes in FA values and changes of frontal lobe function induced by rTMS treatment for TRD. Thus, in the present study, we investigated whether a relationship exists between improvements in frontal lobe dysfunction by rTMS and improvement of white matter integrity revealed by DTI in TRD patients undergoing rTMS treatment.
Methods
Subjects
A total of 12 unipolar depression patients (five males and seven females; 52.8 ± 17.8 years old) were recruited from the inpatient and outpatient units at the department of psychiatry at Saga University Hospital, fulfilling not only the diagnostic criteria for major depressive episode (DSM-IV-TR), but also the criteria for TRD between August 2013 and January 2019. Treatment resistance was defined as a failure to respond to at least two different types of antidepressant given for a period longer than 4 weeks at the maximum recommended dose. The diagnoses were made by two experienced psychiatrists, and any patients with other psychiatric axis-I or axis-II disorders, history of epileptic seizures or any other neurological disorder, any kind of metal implants, or any other coarse lesions detected with head MRI were excluded. The content of drugs remained unchanged during rTMS treatment. The Beck Depression Inventory (BDI)Citation19 and 24-item Hamilton Depressive Rating Scale (HAM-D)Citation20 were used to assess clinical symptoms at baseline prior to the first rTMS treatment and at the end of a 6-week treatment period. DTI examination and the Wisconsin Card Sorting Test (WCST), Word Fluency Test (WFT) and Color Stroop Test (CST, 6 × 4 = 24 version), psychological examinations for evaluating frontal lobe function, were performed twice at baseline and at the endpoint of treatment. The rater for the HAM-D and cognitive tests was blind to rTMS intervention.
rTMS Treatment
When the patient was relaxed, a figure-eight-shaped air-cooled magnetic coil (Magstim, Magstim rapid system, MR1000/50, The Magstim Company Ltd, Carmarthenshire, UK) was placed over the left dorsolateral prefrontal cortex (DLPFC). The stimulation location was 5 cm anterior to the scalp position for optimum stimulation of the right interosseous dorsalis muscle in the parasagittal plane.Citation21,Citation22 All patients were naive to rTMS prior to this study. All patients were treated with active rTMS. All patients and their relatives were given detailed information about the study. A total of 12 patients were treated with active rTMS. The treatment was performed by a skilled rTMS operator. Fitzgerald et al reported that there was an overall significant reduction in the Montgomery-Asberg Depression Rating Scale scores for patients with TRD over a 4-week course of the following parameters: 10 Hz, 30 trains of 5 s duration, 1500 stimuli/day, 20 session of stimulation at 100% stimulation intensity (relative to the resting motor threshold; MT).Citation23 Hence, according to their study, in the present study the active stimulation was performed according to the following parameters: 10 Hz, 40 trains of 4 s duration, 1600 stimuli/day, 20 min per session, 30 sessions of stimulation over a 6-week period, at 100% stimulation intensity (relative to the resting MT). The center of the figure-eight coil was held flat over the scalp and kept at a 45° angle with the handle oriented towards the back of the head.
DTI Data Acquisition
DTI scanning was performed using two clinical 3.0-Tesla MRI units (MAGNETOM Trio, A Tim System, Siemens AG, Erlangen, Germany, n = 3 or MAGNETOM Skyra, A Tim System, Siemens AG, Erlangen, Germany, n = 9) with a 12-channel head coil. The magnetic field intensity was identical in the patient before and after rTMS treatment. Diffusion tensor images were acquired using a spin-echo DTI-echo planar imaging sequence. Twelve-direction DTI scans were acquired with a single shot sequence with the following parameters: relaxation time (TR) = 4600 to 5800 ms, excitation time (TE) = 68 to 92 ms, flip angle (degrees) 90, slice thickness 4 mm, 35 to 40 axial slices, acquisition matrix 128 × 128, b-value of 1000 sec/mm2 As soon as the subject was enrolled, the demographic data were collected and related scales were measured. The first DTI scan was then performed as a baseline measure (within 24 h–1 month before the first rTMS treatment). The second DTI scan of patients was performed at the endpoint (after 6 weeks of rTMS treatment, within 24–72 h after the last rTMS session).
Region Of Interest (ROI) Determination
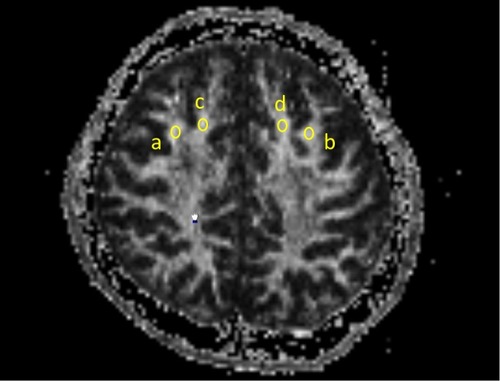
Oval regions of interest (ROIs) of identical size (20 mm2) were used to measure FA values. The same ROI was used for both measures, and all ROIs were placed on axial slices. Bilateral superior gyri and middle frontal gyri ROIs were placed halfway between the precentral sulcus and anterior boundary of the brain, on the most inferior slice where both gyri were visible as separate structures. The researcher who drew the ROIs was blind to the subjects’ clinical measurements. We measured the average FA values in each ROI ().
Figure 1 Oval regions of interest (ROIs) of identical size (20 mm2) were used to measure fractional anisotropy (FA) values. The same ROI was used for both measures, and all ROIs were placed on axial slices. Bilateral superior gyri and middle frontal gyri ROIs were placed halfway between the precentral sulcus and anterior boundary of the brain, on the most inferior slice where both gyri were visible as separate structures. a: the right middle frontal gyrus, b: the left middle frontal gyrus, c: the right superior frontal gyrus, d: the left superior frontal gyrus.
Statistical Analysis
Paired t-tests were conducted to analyze the pre- and post-treatment difference on HAM-D, BDI and frontal lobe function scores.
DTI analysis was performed with an ROI approach.
For the ROI approach, a one-sample paired t-test was used for data analysis between pre- and post-treatment. Correlation analysis was applied to evaluate the relationship between the average FA values and depressive scores with Bonferroni correction. These analyses were implemented using IBM SPSS Statistics (SPSS Inc., Chicago, Illinois, USA, Version 18.0).
Results
Participant Characteristics
A total of 12 participants completed the study. All participants voluntarily agreed to take part in the present study. The age range was 28 to 74 years, with an average age of 52.8 years (SD = 17.8 years). All participants were Japanese. Four of 12 subjects were rTMS-responders (responder: reduction of 50% from baseline HAM-D scores). All subjects had MRI scans conducted before and after rTMS treatments. Participants’ characteristics by group are reported in .
Table 1 Participant Characteristics (N = 12)
Changes In Depressive Symptoms And Frontal Lobe Function Induced By rTMS
The mean HAM-D score before rTMS was 20.3 ± 6.9 (n = 12) and the mean BDI score was 26.3 ± 10.7 (n = 12). The corresponding scores after rTMS were 13.2 ± 9.0 and 19.5 ± 12.0, respectively. Paired t-tests analyzing the pre- and post-treatment difference revealed significant decreases in HAM-D and BDI scores (p = 0.0004 and p = 0.03, respectively) ().
Table 2 Changes In Depressive Symptoms And Frontal Lobe Function Induced By rTMS
rTMS treatment significantly improved Category scores in WFT (p = 0.04, n = 12) and part 3 of CST scores (p = 0.04, n = 12). In rTMS-responders (n = 4) or rTMS-non-responders (n = 8), rTMS treatment did not significantly improve any frontal lobe function ().
Changes In FA Values Induced By rTMS
ROI Approach
The ROI approach (n = 12) revealed that white matter FA in the right superior frontal gyri was significantly increased by rTMS (p = 0.004). In rTMS-responders (n = 4), the ROI approach did not reveal significantly increased white matter FA in the left and right middle and superior frontal gyri by rTMS. In rTMS-non-responders (n = 8), the ROI approach revealed that rTMS significantly increased white matter FA in the right superior frontal gyrus ().
Table 3 Changes In FA Values Induced By rTMS
Correlation Between Changes In FA Values And Frontal Lobe Function As Well As Changes In FA Values And Depressive Symptoms
In the ROI approach, none of the FA increases in any region were correlated with increases in HAM-D, BDI scores or the improvement of any frontal lobe function (n = 12) (). The amount of changes in FA values and frontal lobe function was calculated by post-pre comparison.
Table 4 Correlation Between Changes In FA Values And Frontal Lobe Function As Well As Changes In FA Values And Depressive Symptoms (N=12)
Discussion
Paired t-tests analyzing the pre- and post-treatment difference revealed significant decreases in HAM-D and BDI scores (p = 0.0004 and p = 0.03, respectively) (). rTMS treatment significantly improved Category scores in WFT (p = 0.04, n = 12) and part 3 of CST scores (p = 0.04, n = 12). In rTMS-responders (n = 4) or rTMS-non-responders (n = 8), rTMS treatment did not significantly improve any frontal lobe function (). The ROI approach (n = 12) revealed that white matter FA in the right superior frontal gyri was significantly increased by rTMS (p = 0.004). In rTMS-responders (n = 4), the ROI approach did not reveal significantly increased white matter FA in any region by rTMS. In rTMS-non-responders (n = 8), the ROI approach revealed that rTMS significantly increased white matter FA in the right superior frontal gyrus (). In the ROI approach, none of the FA increases in any region were correlated with increases in HAM-D, BDI scores or the improvement of any frontal lobe function (n = 12) ().
Previous systematic reviews reported that rTMS can induce significant cognitive improvement.Citation24,Citation25 Fitzgerald et al demonstrated significant overall improvements in verbal fluency, independently of the type (high frequency/low frequency) of rTMS received.Citation26 Boggio et al reported trends towards improvement in WCST and CST scores in patients with depression and Parkinson’s disease.Citation27 The DLPFC is a crucial brain region for neurocognitive performance (including attention, memory, executive functions, psychomotor speed and social cognition), and is neuroanatomically connected to fronto-subcortical brain areas, the dysfunctions of which are heavily involved in many neuropsychiatric diseases.Citation28 Pallanti et al reported that low-frequency rTMS over right DLPFC in the treatment of TRD improved performance in the Corsi test (p < 0.02) and phonemic verbal fluency (p = 0.065), both of which can be used to detect right hemisphere cognitive dysfunction.Citation29 These improvements were independent of depressive symptom variation. In the present study, rTMS treatment significantly improved depression symptoms and Category scores in WFT and part 3 of CST scores of frontal lobe dysfunction. These measures improved independently of the improvement of depressive symptoms.
To date, only a few studies have explored the relationship between the white matter microstructure changes induced by rTMS and treatment responses.Citation17,Citation18 In Kozel et al.’s study, four TRD patients treated with active stimulation were compared with four matched patients with sham stimulation.Citation17 They concluded that rTMS resulted in no damage to white matter on the side of stimulation. Peng et al reported significantly reduced FA using a voxel-based analysis method in the left middle frontal gyrus in TRD patients.Citation18 This reduction of FA was significantly improved by active rTMS treatment, but not by sham stimulation.
To the best of our knowledge, only a few studies reported an association between white matter FA with cognitive function in MDD using DTI analysis.Citation30,Citation31 Alves et al reported that FA in the right posterior cingulate cluster (PCC) (but not left PCC) was significantly positively correlated with performance in a verbal naming task, and showed a non-significant trend towards a correlation with verbal fluency and episodic memory performance.Citation30 Rizk et al found that structural connectivity of the white matter network of the caudal anterior cingulate cortex was correlated with the magnitude of Stroop interference in healthy volunteers, but not in MDD patients.Citation31
To the best of our knowledge, no previous reports have examined the relationship between changes in FA values and changes of frontal lobe function induced by rTMS treatment for TRD. The present study revealed that none of the FA increases in any region were correlated with the improvement of any frontal lobe function (n = 12). Stämpfli et al.’s study suggested that a new fiber density (FD) measure may be more sensitive to subtle changes in white matter microstructure compared with FA in schizophrenia.Citation32 Therefore, it may be useful for future studies to use FD instead of FA, to examine this issue in more depth.
Limitations
The main limitation of the present study was the lack of a control condition using a sham rTMS procedure. In addition, our sample size was relatively small. It has to be pointed out the possibility that we might be able to get some positive correlation by increasing the number of the subjects. The negative correlation might be caused only by this small sample size. It may be useful for future replication studies to confirm the present findings in more depth, using a larger patient sample and including a sham rTMS procedure. The learning effect on the cognitive tests appeared to be robust, because the tests were repeated in relatively short periods (6 weeks), despite the lack of a control group. We did not conduct structured clinical interviews, such as the Structured Clinical Interview for DSM-IV-TR or the Mini International Neuropsychiatric Interview. DTI scanning was performed using two clinical 3.0-Tesla MRI units (MAGNETOM Trio or MAGNETOM Skyra).
Conclusion
To our knowledge, this is the first study to investigate the relationship between rTMS-induced improvements of frontal lobe dysfunction and white matter integrity in TRD patients. The present results suggest that rTMS is effective for treating TRD, and that it is associated with significant improvements on several subscales of frontal lobe dysfunction. Category scores in WFT and part 3 of the CST scores improved independently of the improvement of depressive symptoms. Importantly, we found no correlation between the improvement of frontal lobe dysfunction by rTMS and the improvement of white matter integrity by rTMS in TRD patients.
Statement Of Ethics
Before enrollment, all subjects were fully informed about the study and gave written informed consent before taking part. The experimental protocols were carried out in accordance with the Declaration of Helsinki and were approved by the Saga University Hospital ethics committee. The registration ID of the clinical trial was 2012-05-02.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgment
Dr. Tateishi was supported by the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number 17K10307).
Disclosure
The authors report no conflicts of interest in this work.
References
- Culpepper L, Lam RW, McIntyre RS. Cognitive impairment in patients with depression: awareness, assessment, and management. J Clin Psychiatry. 2017;78(9):1383–1394. doi:10.4088/JCP.tk16043ah5c29345866
- Etkin A, Patenaude B, Song YJ, et al. A cognitive-emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial. Neuropsychopharmacology. 2015;40(6):1332–1342. doi:10.1038/npp.2014.33325547711
- Fossati P, Ergis AM, Allilaire JF. Executive functioning in unipolar depression: a review. Encephale. 2002;28(2):97–107.
- Ishak WW, Greenberg JM, Cohen RM. Predicting relapse in major depressive disorder using patient-reported outcomes of depressive symptom severity, functioning, and quality of life in the Individual Burden of Illness Index for Depression (IBI-D). J Affect Disord. 2013;151(1):59–65. doi:10.1016/j.jad.2013.05.04823790554
- Serafini G, Pompili M, Belvederi Murri M, et al. The effects of repetitive transcranial magnetic stimulation on cognitive performance in treatment-resistant depression. A Systematic Review. Neuropsychobiology. 2015;71(3):125–139. doi:10.1159/00038135125925699
- Boes AD, Kelly MS, Trapp NT, Stern AP, Press DZ, Pascual-Leone A. Noninvasive brain stimulation: challenges and opportunities for a new clinical specialty. J Neuropsychiatry Clin Neurosci. 2018;30(3):173–179. doi:10.1176/appi.neuropsych.1711026229685065
- Neville IS, Hayashi CY, El Hajj SA, Zaninotto AL, Sabino JP, Sousa LM Jr. Repetitive transcranial magnetic stimulation (rTMS) for the cognitive rehabilitation of traumatic brain injury (TBI) victims: study protocol for a randomized controlled trial. Trials. 2015;16:440. doi:10.1186/s13063-015-0944-226438108
- Hayasaka S, Nakamura M, Noda Y, et al. Lateralized hippocampal volume increase following high-frequency left prefrontal repetitive transcranial magnetic stimulation in patients with major depression. Psychiatry Clin Neurosci. 2017;71(11):747–758. doi:10.1111/pcn.2017.71.issue-1128631869
- Nilakantan AS, Mesulam MM, Weintraub S, Karp EL, VanHaerents S, Voss JL. Network-targeted stimulation engages neurobehavioral hallmarks of age-related memory decline. Neurology. 2019;92(20):e2349–e2354. doi:10.1212/WNL.000000000000750230996057
- Shinba T, Kariya N, Matsuda S. Increase of frontal cerebral blood volume during transcranial magnetic stimulation in depression is related to treatment effectiveness: A pilot study with near-infrared spectroscopy. Psychiatry Clin Neurosci. 2018;72(8):602–610. doi:10.1111/pcn.2018.72.issue-829774621
- Bae JN, MacFall JR, Krishnan KRR, Payne ME, Steffens DC, Taylor WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60:1356–1363. doi:10.1016/j.biopsych.2006.03.05216876144
- Li CX, Sun XL, Zou K, et al. Voxel based analysis of DTI in depression patients. Int J MRI. 2007;1:43–48.
- Lim KO, Helpen JA. Neuropsychiatric applications of DTI: a review. NMR Biomed. 2002;15:587–593. doi:10.1002/nbm.78912489105
- Liu X, Wang YZ, Liu HH, Liu ZN, Zhou WB. Diffusion tensor imaging and resting state functional magnetic resonance imaging on young patients with major depressive disorder. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35(1):25–31. doi:10.3969/j.issn.1672-7347.2010.01.00420130361
- Yang Q, Huang XB, Hong N, Yu X. White matter microstructural abnormalities in late-life depression. Int Psychogeriatr. 2007;19(4):757–766. doi:10.1017/S104161020700487517346365
- Scheinost D, Holmes SE, DellaGioia N, et al. Multimodal investigation of network level effects using intrinsic functional connectivity, anatomical covariance, and structure-to-function correlations in unmedicated major depressive disorder. Neuropsychopharmacology. 2018;43(5):1119–1127. doi:10.1038/npp.2017.22928944772
- Kozel FA, Johnson KA, Nahas Z, et al. Fractional anisotropy changes after several weeks of daily left high-frequency repetitive transcranial magnetic stimulation of the prefrontal cortex to treat major depression. J ECT. 2011;27(1):5–10. doi:10.1097/YCT.0b013e3181e6317d20559144
- Peng H, Zheng H, Li L, et al. High-frequency rTMS treatment increases white matter FA in the left middle frontal gyrus in young patients with treatment-resistant depression. J Affect Disord. 2012;136(3):249–257. doi:10.1016/j.jad.2011.12.00622217432
- Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7(0):151–169.4412100
- Pan S, Liu ZW, Shi S, et al. Hamilton rating scale for depression-24 (HAM-D24) as a novel predictor for diabetic microvascular complications in type 2 diabetes mellitus patients. Psychiatry Res. 2017;258:177–183. doi:10.1016/j.psychres.2017.07.05028774662
- Avery D, Holtzheimer P, Fawaz W, et al. Repetitive transcranial magnetic stimulation (rTMS) is clinically effective in treatment-resistant major depression. Biol Psychiatry. 2004;55:718.
- Fitzgerald PB. Repetitive transcranial magnetic stimulation treatment for depression: lots of promise but still lots of questions. Brain Stimul. 2009;2(4):185–187. doi:10.1016/j.brs.2009.08.00520633418
- Fitzgerald PB, Hoy K, McQueen S, et al. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology. 2009;34(5):1255–1262. doi:10.1038/npp.2008.23319145228
- Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd ed Arlington, VA: American Psychiatric Association; 2010.
- Guse B, Falkai P, Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm (Vienna). 2010;117(1):105–122. doi:10.1007/s00702-009-0333-719859782
- Fitzgerald PB, Hoy K, Daskalakis ZJ, Kulkarni J. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26(3):229–234. doi:10.1002/da.v26:319105212
- Boggio PS, Fregni F, Bermpohl F, et al. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson’s disease and concurrent depression. Mov Disord. 2005;20(9):1178–1184. doi:10.1002/(ISSN)1531-825715895421
- Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51(4):356–367. doi:10.1016/j.jaac.2012.01.00822449642
- Pallanti S, Di Rollo A, Antonini S, Cauli G, Hollander E, Quercioli L. Low-frequency rTMS over right dorsolateral prefrontal cortex in the treatment of resistant depression: cognitive improvement is independent from clinical response, resting motor threshold is related to clinical response. Neuropsychobiology. 2012;65(4):227–235. doi:10.1159/00033699922653158
- Alves GS, Karakaya T, Fußer F, et al. Association of microstructural white matter abnormalities with cognitive dysfunction in geriatric patients with major depression. Psychiatry Res. 2012;203(2–3):194–200. doi:10.1016/j.pscychresns.2011.12.00622959814
- Rizk MM, Rubin-Falcone H, Keilp J, et al. White matter correlates of impaired attention control in major depressive disorder and healthy volunteers. J Affect Disord. 2017;222:103–111. doi:10.1016/j.jad.2017.06.06628688263
- Stämpfli P, Sommer S, Manoliu A, et al. Subtle white matter alterations in schizophrenia identified with a new measure of fiber density. Sci Rep. 2019;9(1):4636. doi:10.1038/s41598-019-40070-230874571
