Abstract
Objective
To study the effects of treatment with atypical antipsychotic drugs on brain levels of glutamate plus glutamine in early-stage first-episode schizophrenia.
Participants
Sixteen patients (eight males, eight females; aged 30 ± 11 years) completed the study.
Methods
We used administered 6 months of atypical antipsychotic drugs and used proton magnetic resonance spectroscopy to evaluate the results.
Results
We found that the administration of atypical antipsychotic drugs for 6 months decreased the glutamate plus glutamine/creatine ratio in the frontal lobe. These results suggest that the administration of atypical antipsychotic drugs for at least 6 months decreased glutamatergic neurotransmissions in the frontal lobe in early-stage first-episode schizophrenia, but there was no difference in frontal-lobe levels between patients and control subjects before administration.
Conclusion
Taking these findings into account, the glutamatergic and GABAergic neurons are implicated in early-stage first-episode schizophrenia, but in complex ways.
Introduction
Glutamate’s role in schizophrenia has been investigated in recent years. Abnormal glutamatergic neurotransmission has been reported in human postmortem studies. Proton magnetic resonance spectroscopy (MRS) allows us to examine brain glutamate and glutamine in vivo. Tibbo et alCitation1 reported that the glutamate system might play a role in neuroarchitectural abnormalities seen in schizophrenia and that higher-than-normal glutamatergic metabolites at the early stages of schizophrenia might lead to excitotoxicity, which decreases in the chronic state. Olbrich et alCitation2 also demonstrated that glutamate levels were significantly higher in the left dorsolateral prefrontal cortex in patients with first-episode schizophrenia. In contrast, no difference was found in glutamate plus glutamine between schizophrenia patients and controls in other regions.Citation3,Citation4 Taking these findings into account, brain glutamate levels in schizophrenia seem inconsistent and controversial. The aim of the present study was to investigate the brain levels of glutamate plus glutamine in early-stage first-episode schizophrenia patients. Another aim was to examine the influence of atypical antipsychotic treatment on brain levels of glutamate plus glutamine in the patients. Therefore, we investigated the effects of treatment with atypical antipsychotic drugs for 6 months on brain levels of glutamate plus glutamine in the frontal lobe, left basal ganglia, and the parieto-occipital lobe in early-stage first-episode schizophrenia patients treated with atypical antipsychotics.
Methods
Subjects
A total of 23 patients who fulfilled DSM-IV-TR criteria A, B, D, E, and F, but who were within 6 months of disease onset, were recruited for the study, and all underwent MRS evaluation. After 6 months of follow-up, a diagnosis of schizophrenia was established in 20 of 23 patients. Two patients were excluded from the study, one because of the difficulty of performing MRS given his mental condition, and the other because the image quality was impaired by severe artifacts from dental materials. Thus, 18 patients (nine males, nine females; aged 31 ± 12 years) were finally enrolled in the study. All patients were screened using the Structured Clinical Interview for DSM-IV disorders, and exclusion criteria for all groups included current or past serious medical or neurological illness or dependence on alcohol, or illicit substances. The patients’ psychopathology was assessed with the Positive and Negative Syndrome Scale (PANSS).Citation5 Sixteen (eight males, eight females; 30 ± 11 years) of 18 patients were followed for 6 months. Eighteen healthy volunteers (nine males, nine females; age range 29 ± 11 years) with no current or past psychiatric history were also studied by MRS as sex- and age-matched controls. This study was approved by the Ethics Committee of the University of Occupational and Environmental Health. All participants gave written informed consent to participate in the study.
MRS methodology
All subjects were examined between 4pm and 6pm, by 1H-MRS using a 3T MR system (Signa EXCITE 3T; GE Medical Systems, Waukesha, WI) with a standard quadrature head coil (GE Medical Systems). The regions of interest (ROIs) for 1H-MRS were set for the frontal lobe, the left basal ganglia, and the parieto-occipital lobe (ROI size = 3.0 cm × 3.0 cm × 3.0 cm) using two oriented images (axial image and sagittal image) for each region. We put a voxel in the frontal lobe, the left basal ganglia, and the parieto-occipital lobe. All of these ROIs were placed so as to avoid the lateral ventricle and skull.
We previously obtained brain gamma-aminobutyric acid (GABA) measurements in schizophrenic patients using a MEGA-PRESS sequence 9, and have reported that the reductions in the GABA concentrations were significantly greater in patients at the early stage of schizophrenia than in controls. Citation6 By using a MEGA-PRESS sequence, the spectroscopic measurements of glutamate and glutamine in the same voxel could be obtained. Thus, in the present study, the glutamate and glutamine levels were acquired with the MEGA-PRESS sequence using the following parameters: repetition time (TR) 3 seconds, echo time (TE) 68 milliseconds, and 128 averages. The total acquisition time for each PRESS spectrum was approximately 6 minutes. All spectra were analyzed by an LC model 12 using phantom-generated basis functions for the MEGA-edited spectra. The line width, signal-to-noise ratio, and baseline of each spectrum were checked to ensure the robustness of the data. Eddy-current correction was applied using an unsuppressed water spectrum at the appropriate echo time. The edited spectra were analyzed using LCM-basis functions that were generated from phantom measurements using the MEGA-PRESS sequence with the appropriate acquisition parameters.
Statistical analysis
The unpaired t-test was used to compare differences in the glutamate plus glutamine/creatine (Cr) ratios between the schizophrenia group and the control group. Using nonparametric Wilcoxon and Mann–Whitney tests we analyzed differences in glutamate plus glutamine before and after 6 months’ treatment with atypical antipsychotic drugs. The level of significance was set at P < 0.05.
Results
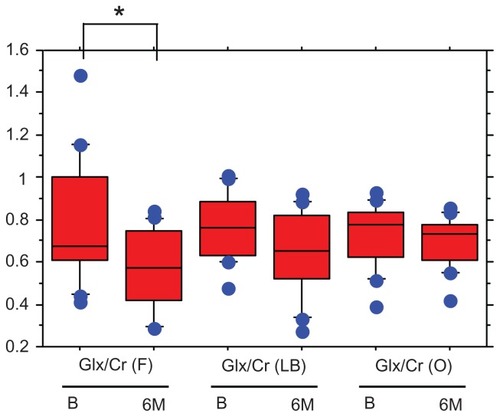
There was no significant difference in age or gender between the healthy controls and the patient groups. The scores of positive (PANSS-P), negative (PANSS-N), general psychopathology (PANSS-G), and total (PANSS-T) in the early schizophrenia patients were 15.9 ± 4.2, 17.2 ± 5.3, 34.2 ± 10.1, and 68.1 ± 17.0 (mean ± standard deviation), respectively. The ratios of glutamate plus glutamine/Cr in the left basal ganglia were significantly higher in the patients than in the controls (0.72 ± 0.16 vs 0.60 ± 0.18, z = −1.930, P = 0.0496), but no differences were found in the frontal lobe (0.7 9 ± 0.28 vs 0.67 ± 0.40, z = −1.226, P = 0.2057) or parieto-occipital lobe (0.71 ± 0.15 vs 0.74 ± 0.13, z = −0.142, P = 0.8868) (). No associations were found between the glutamate plus glutamine/Cr ratios and positive, negative, and general psychopathology scores in PANSS. The administered atypical antipsychotic drugs and mean dosage were as follows: risperidone (n = 5), 2.9 mg; olanzapine (n = 5), 10.1 mg; aripiprazole (n = 4), 16.2 mg; and quetiapine (n = 2), 250 mg. The mean reduction of positive, negative, general psychopathology, and total scores of PANSS after 6 months’ treatment with atypical antipsychotic drugs were 33.3, 26.3, 31.4, and 25.3%, respectively. Six months’ administration of atypical antipsychotic drugs reduced the glutamate plus glutamine/Cr ratio in the frontal lobe (before, 0.78 ± 0.28; after 6 months, 0.57 ± 0.18: z = −2.792, P = 0.052), whereas, those in the left basal ganglia (before, 0.76 ± 0.15; after 6 months, 0.65 ± 0.19: z = −1.448, P = 0.1477), and parieto-occipital lobe (before, 0.73 ± 0.19; after 6 months, 0.69 ± 0.11: z = −1.008, P = 0.3132) were not changed by the medication (). No correlation was observed between the changes in any scores of PANSS and the changes in the glutamate plus glutamine/Cr ratios.
Figure 1 The ratios of Glx/Cr in the three brain regions.
Abbreviations: Glx, glutamate plus glutamine; Cr, creatine; F, frontal lobe; LB, left basal ganglia; O, parieto-occipital lobe; HC, healthy control; SC, early-stage first-episode schizophrenia.
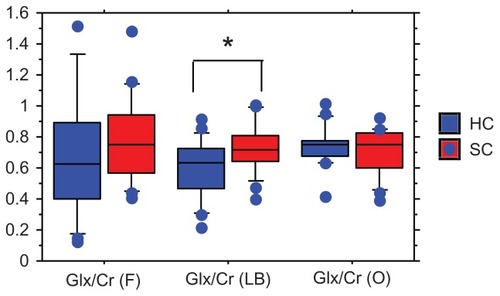
Figure 2 The changes in Glx/Cr before and 6 months after treatment with atypical antipsychotic drugs.
Abbreviations: Glx, glutamate plus glutamine; Cr, creatine; F, frontal lobe; LB, left basal ganglia; O, parieto-occipital lobe; B, before treatment with atypical antipsychotic drugs; 6M, 6 months after treatment with atypical antipsychotic drugs.
Discussion
We found that the glutamate plus glutamine/Cr ratio in the left basal ganglia was significantly higher in early-stage first-episode schizophrenia patients than in control subjects. No differences were observed in the frontal lobe and parieto-occipital lobe between the two groups. Findings regarding glutamatergic metabolites in schizophrenia have been contro-versial. Stanley et alCitation7 and Ohrmann et alCitation8 reported no change in prefrontal glutamate levels in treatment-naïve first-episode schizophrenia patients. Theberge et alCitation9 found increased glutamine in the left anterior cingulate cortex and thalamus of treatment-naïve first-episode schizophrenia patients. The results in the present study were basically in accordance with the results of Stanley et al,Citation7 Ohrmann et al,Citation8 Bustillo et al,Citation3 and Reid et al.Citation4 Recently, great attention has been paid to the basal ganglia, which is an important area of interaction between GABAergic and glutamatergic neurons.Citation10,Citation11 De la Fuente-Sandoval et alCitation11 first reported that the glutamate/Cr ratio in the dorsal caudate nucleus was higher in drug-naïve schizophrenia patients than in control subjects. The present study’s findings of higher glutamate plus glutamine/Cr ratios in the left basal ganglia did not contradict those of De la Fuente-Sandoval et al.Citation11 To the best of our knowledge, this is the first report demonstrating that treatment with atypical antipsychotic drugs for at least 6 months decreased the glutamate plus glutamine/Cr ratio in the frontal cortex in early-stage first-episode schizophrenia. However, no correlation was observed between the changes in any scores in PANSS and the changes in glutamate plus glutamine/Cr. Theberge et alCitation9 reported that brain glutamate and glutamine levels were significantly decreased in the anterior cingulate cortex, which belongs to the frontal lobe, in patients with chronic medicated schizophrenia. The authors speculated that the findings might indicate a decrease in the number of glutamatergic synapses in this region. On the other hand, we reported that the brain glutamate plus glutamine/Cr ratio was significantly increased, whereas GABA/Cr was significantly reduced in the left basal ganglia in patients with early-stage first-episode schizophrenia,Citation6 and treatment with atypical antipsychotic drugs did not change brain GABA levels.Citation12 It has been reported that glutamatergic and GABAergic neurons might be disturbed in schizophrenia patients, and that these types of neurons interact.Citation13 Taking these findings into account, glutamatergic neurotransmission might be different in different brain regions and the situation becomes more complicated depending on the stage of schizophrenia. Converging experimental and clinical evidence suggests that dysfunction of proper GABAergic inhibition and consequently imbalance between excitation and inhibition in the cerebral cortex underlies at least part of the pathophysiological process of schizophrenia.Citation14 The finding that the brain levels of glutamate plus glutamine at early-stage first-episode schizophrenia were significantly increased in the left basal ganglia, and subsequently decreased in the frontal lobe, but not in the left basal ganglia, suggests that the concentrations and changes in brain GABA and glutamate plus glutamine might vary in different brain regions. In future works, we will measure glutamate and glutamine separately, and in absolute amounts rather than as a Cr ratio, and also use drug-naïve schizophrenia patients as subjects.
Conclusion
In conclusion, it is possible that the administration of atypical antipsychotic drugs for at least 6 months decreased glutamatergic neurotransmissions in the frontal lobe in early-stage first-episode schizophrenia patients.
Acknowledgment
We thank Mr Toru Sato for his generous help in measuring the MRS.
Disclosure
The authors report no conflicts of interest in this work.
References
- TibboPHanstockCAllenP3-T proton MRS investigation of glutamate and glutamine in adolescents at high genetic risk for schizophreniaAm J Psychiatry20041611116111815169703
- OlbrichHMValeriusGRuschNFrontolimbic glutamate alterations in first episode schizophrenia: evidence from a magnetic resonance spectroscopy studyWorld J Biol Psychiatry20089596317853298
- BustilloJRRowlandLMMullinsP1H-MRS at 4 tesla in minimally treated early schizophreniaMol Psychiatry20101562963619918243
- ReidMAStoeckelLEWhiteDMAssessments of function and biochemistry of the anterior cingulate cortex in schizophreniaBiol Psychiatry20106862563320570244
- KaySRFiszbeinAOplerLAThe positive and negative syndrome scale (PANSS) for schizophreniaSchizophr Bull1987132612763616518
- GotoNYoshimuraRUedaNReduction of brain gamma-aminobutylic acid (GABA) concentrations in early-stage schizophrenia patients: 3T proton MRS studySchizophr Res200911219219319464152
- StanleyJAWilliamsonPCDrostDJAn in vivo proton magnetic resonance spectroscopy study of schizophrenia patientsSchizophr Bull1996225976098938914
- OhrmannPSiegmundASuslowTCognitive impairment and in vivo metabolites in first-episode neuroleptic-naïve and chronic medicated schizophrenic patients: a proton magnetic resonance spectroscopy studyJ Psychiatr Res20074162563416949099
- ThebergeJAl-SemaanYWilliamsonPCGlutamate and glutamine in anterior cinglate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRSAm J Psychiatry20031602231223314638596
- BenesFMAmygdalocortical circuitry in schizophrenia: from circuits to moleculesNeuropsychopharmacology20103523925719727065
- De la Fuente-SandovalCFavillaRAlvaradoPGlutamate increase in the associative striatum in schizophrenia: a longitudinal magnetic resonance spectroscopy preliminary studyGac Med Mex2009145109113 Spanish19518017
- GotoNYoshimuraRKakedaSNo alteration of brain GABA after 6 months of treatment with atypical antipsychotic drugs in early-stage first-episode schizophreniaProg Neuropsychopharmacol Biol Psychiatry2010341480148320727934
- LewisSSynaptic neurotransmission: a closer look at GABA(B) receptorsNat Rew201011664
- LevittPEaglesonKLPowellEMRegulation of neocortical interneuron development and the implications for neurodevelopmental disordersTrends Neurosci20042740040615219739
- MescherMMerkleHKirshJGarwoodMSimultaneous in vivo spectral editing and water suppressionNMR Biomed1998112662729802468
- ProvencherSWEstimation of metabolite concentrations from localized in vivo proton NMR spectraMagn Reson Med1993306726798139448