Abstract
Purpose
Antipsychotic monotherapy is often recommended over antipsychotic polypharmacy because of fewer adverse events, reduced treatment complexity, and lower medication cost. This study compared the rate and the duration of antipsychotic monotherapy following initiation of olanzapine or risperidone in the treatment of outpatients with schizophrenia in Japan.
Methods
Outpatients diagnosed with schizophrenia in the Japan Medical Data Center database were identified using International Statistical Classification of Diseases and Related Health Problems, 10th Revision, diagnosis codes. Patients were between 20 and 65 years old, initiated on olanzapine or risperidone therapy between August 2003 and July 2008, and continuously enrolled during the 6 months prior to and the 12 months following the initiation date. Antipsychotic polypharmacy was defined as concurrent use of two or more antipsychotics. The probability of monotherapy during the 12-month follow-up period was assessed using a propensity score-adjusted generalized estimating equation model. Duration of monotherapy was contrasted using a propensity score-adjusted bootstrapping model.
Results
After applying all inclusion and exclusion criteria, the final analytic sample consisted of 332 olanzapine- and 496 risperidone-treated outpatients. At treatment initiation, 61.5% of the olanzapine-treated patients and 45.6% of the risperidone-treated patients received antipsychotic monotherapy (P < 0.001). After correcting for background differences, monotherapy was more common among olanzapine-treated patients (P = 0.001). In addition, olanzapine was used as monotherapy for a longer duration (P = 0.006).
Conclusion
Consistent with prior global research, this retrospective naturalistic study of schizophrenia outpatients in Japan found that olanzapine is more likely to be used as monotherapy and to be used as monotherapy for a longer duration than risperidone.
Introduction
Antipsychotic medications have been a core treatment modality for patients with schizophrenia over the past 40 years, with antipsychotic monotherapy considered the treatment of choice.Citation1 Despite consistent recommendations for antipsychotic monotherapy, antipsychotic polypharmacy, the concomitant use of two or more antipsychotics, is commonplace in the treatment of schizophrenia.Citation2–Citation7 Antipsychotic polypharmacy seems to be increasing over time as additional antipsychotic treatment options become available.Citation8,Citation9
Antipsychotic polypharmacy appears to be used to improve efficacy after partial response or nonresponse to monotherapy,Citation10,Citation11 but these clinical benefits have not been clearly documented.Citation4,Citation7,Citation12,Citation13 However, the disadvantages are clear: antipsychotic polypharmacy has a higher risk of adverse events and potential drug–drug interactions;Citation11 it increases complexity, making assessment of the medication regimen and management of future symptom exacerbations more difficult;Citation14 and it increases antipsychotic treatment costs at a time of growing budget constraints.Citation14–Citation19
The prevalence of antipsychotic polypharmacy has been reported to range between 13% and 70%, depending on the study population, country, year, methodology, and duration.Citation6,Citation7,Citation20–Citation26 A prospective, observational, noninterventional study in the United States (US)Citation27 found that over a 12-month period, 58% of the patients had at least one period of antipsychotic polypharmacy lasting 60 consecutive days or longer. The rate of antipsychotic polypharmacy has been found to be higher in Japan than in the US.Citation28 A Japanese national cross-sectional survey of 9325 inpatients with schizophrenia revealed that 67%–70% were treated with more than one antipsychotic, and 75.6% of those treated with an atypical antipsychotic were also treated with another antipsychotic.Citation25,Citation26 The very high rate of antipsychotic polypharmacy has led the Japanese Ministry of Health, Labour and Welfare to reward physicians who reduce their patients’ atypical antipsychotic polypharmacy.
Prior research has identified multiple factors that predict antipsychotic polypharmacy use. Antipsychotic polypharmacy has been associated with greater illness severity, longer illness duration, comorbid depression, and treatment with typical antipsychotics, as well as the use of certain atypical antipsychotics.Citation4,Citation7,Citation10 The rate and duration of antipsychotic polypharmacy differs among atypical antipsychotics,Citation7 with olanzapine-initiated patients more likely to be treated with monotherapy than those treated with other atypical antipsychotics.Citation16,Citation23,Citation27,Citation29–Citation32 Prior research has shown a higher rate and a longer duration of antipsychotic monotherapy with olanzapine than risperidone in the treatment of patients with schizophrenia in the USCitation27 and in Europe.Citation32 However, it is unclear whether these prior findings can generalize across world geographies, especially to the Japanese health care system, where antipsychotic polypharmacy appears to be highly prevalent.
The objective of the current retrospective claims database analysis is to assess the rate and duration of antipsychotic polypharmacy among outpatients in Japan who were initiated on olanzapine or risperidone for the treatment of schizophrenia. Olanzapine and risperidone are the two most frequently used atypical antipsychotics for schizophrenia in Japan.Citation33
Methods
Data source
This study utilized the Japan Medical Data Centre Database (JMDC), an employment-based administrative database containing the medical and pharmacy claims from ten different payers (insurance societies). The JMDC included information on approximately 0.6 million employed individuals or their family members who were enrolled between August 2003 and July 2009.
The JMDC consisted of inpatient, outpatient, and pharmacy administrative claims. The medical claims (inpatient and outpatient) included basic demographics, diagnoses, procedures, and fees. Diagnoses were specified with International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10), diagnosis codes.Citation34 For the medical claims, the date of service information was restricted to month and year. Pharmacy claims included the full dispensing date, the days of supply, and dosage information. Drugs were classified using the Anatomical Therapeutic Chemical Classification System codes.Citation35
Sample selection
The initial extract of data from the JMDC database included the administrative claims between August 2003 and July 2009 for patients who had at least one diagnosis of schizophrenia (F20.0–F20.9). This initial data cut included 4861 individuals.
The primary selection criterion for this analysis was the initiation of either olanzapine or risperidone (ie, index drug) before June 30, 2008. Based on each patient’s first pharmacy claim for the index drug, an index date was identified. Initiation was defined as a period of 3 months without a claim for the index drug prior to the index date. Patients were categorized into two mutually exclusive study cohorts based on the most recent initiation: risperidone or olanzapine.
The study period varied for each patient and included the 6 months preceding and the 12 months following the index date. The study period was divided into a baseline period and a treatment period, with the index date marking the first day of the treatment period.
Additional inclusion and exclusion criteria included the following: (1) patients were required to have at least one diagnosis of schizophrenia (F20.0–F20.9) during the study period; (2) patients were excluded from the study if they had ICD-10 diagnoses for any of the following conditions during the study period: organic mental disorders, organic brain disorders, dementia, or Alzheimer’s disease; (3) patients were excluded from the analysis if they did not have at least one claim prior to and one claim after the study period (this was used as a proxy for continuous enrollment); (4) the analysis was restricted to individuals who were between 20 and 65 years of age (the lower cutoff was used to restrict the population to adults and the upper cutoff was used because of potential eligibility for Health Insurance for the Elderly); (5) finally, individuals who were inpatients at the time of the index date were excluded from the analysis.
Outcome measures
Past observational research has differentiated antipsychotic monotherapy from antipsychotic polypharmacy in multiple ways.Citation27 The simplest definition of antipsychotic polypharmacy is the use of two or more antipsychotics on any given day. Unfortunately, this elegant definition also includes cross-tapering and “as needed” use. A more restrictive definition is the concurrent use of two or more antipsychotics for at least 60 days.Citation27 This restrictive definition eliminates cross-tapering and “as needed” use, but it also excludes any short-term polypharmacy.
The primary outcome variable in this study was the proportion of patients treated with monotherapy across each day of the treatment period. The second outcome variable, duration of monotherapy, was defined as the number of days of antipsychotic monotherapy use from the index date to the end of the study. The final outcome variable was defined as the time in days from the index date to the first day of a period of persistent antipsychotic polypharmacy (60+ days). The first two outcome variables use a simple definition of polypharmacy while also incorporating time and the third outcome variable captures only the more restrictive definition of polypharmacy.
Baseline measures
All analyses were adjusted for baseline differences between patients initiated on olanzapine and patients initiated on risperidone. The baseline variables were calculated from information available during the 6 months prior to the index date.
The Anatomical Therapeutic Chemical Classification System codes from the pharmacy claims were used to create indicator (yes/no) variables for baseline treatment with several relevant classes of drugs. Atypical antipsychotics were defined as olanzapine, risperidone, aripiprazole, blonanserin, clozapine, perospirone, quetiapine, and zotepine. Anticholinergic/antiparkinsonian drugs included amantadine, biperiden, levodopa, carbidopa-levodopa, selegiline, and trihexyphenidyl. Antidepressants included amoxapine, setiptiline, trimipramine, mirtazapine, amitriptyline, imipramine, clomipramine, trazodone, dosulepin, nortriptyline, maprotiline, mianserin, lofepramine, milnacipran, fluvoxamine, sertraline, and paroxetine. Hypnotics/sedatives included amobarbital, secobarbital, barbital, phenobarbital, pentobarbital, passiflora, estazolam, quazepam, zopiclone, triazolam, trichloroethyl, nitrazepam, nimetazepam, haloxazolam, flunitrazepam, flurazepam, brotizolam, bromvalerylurea, lormetazepam, rilmazafone, zolpidem, and chloral hydrate. The final baseline pharmacy variables were an indicator variable for prior use of risperidone and a second indicator variable for prior use of olanzapine.
Baseline comorbidities were identified using the ICD-10 diagnostic codes. Indicator variables for the following comorbid conditions were coded: depression (F32, F33), manic episodes (F30), and diabetes mellitus (E10–E14).
Any physician the patient consulted could have written the initial prescription for the index drug (olanzapine or risperidone). If the physician was identified as a psychiatrist in the administrative claims, the index drug was considered “psychiatrist prescribed.”
Health care utilization variables were created to capture the costs and resources used by patients during the baseline period. Outpatient visits were measured as a count of the number of outpatient visits. Inpatient service utilization was coded as present or absent. Antipsychotic adherence was designated if a patient had filled scripts for any antipsychotic on 80% or more of the days in the baseline period (ie, medication possession ratio ≥0.80). Finally, total health care costs were aggregated for the baseline period, based on the amounts paid by the health plans for medical services and medication prescriptions.
Statistical methods
Univariate comparisons of baseline characteristics between patients initiated on olanzapine or risperidone were conducted using chi-square tests or Fisher’s exact tests for categorical variables and Student’s t-tests or Wilcoxon rank-sum tests for continuous variables. A propensity score predicting the probability of initiating treatment with olanzapine or risperidone was calculated using logistic regression with the baseline variables. Balance between the treatment groups on each baseline characteristic after adjusting for the propensity score was verified using analysis of variance for continuous variables and logistic regression for categorical variables. contains each of the variables used in the propensity score calculation.
Table 1 Baseline characteristics for olanzapine and risperidone initiators
In each of the outcome models, the propensity score was included as a covariate to adjust for background difference. The probability of monotherapy across the 12-month outcome period was estimated using a repeated-measures generalized estimating equation model with a logit link function, an autoregressive error structure, and a binomial error distribution. This model included terms for treatment (olanzapine or risperidone), time (in days), the treatment-by-time interaction, and the propensity score.
Duration of monotherapy was a positively skewed variable; therefore, a nonparametric, propensity score bin bootstrapping resampling approach was used to test difference in duration between olanzapine and risperidone. Finally, survival analyses compared the time to persistent polypharmacy between olanzapine- and risperidone-initiated patients. The time to persistent polypharmacy was compared using the Kaplan-Meier method with a log-rank test and a Cox proportional hazards model that also included the propensity score. Survival curves were constructed from unadjusted Kaplan-Meier estimates. In the survival analyses, patients were censored (no longer eligible to be considered for a “treated with persistent antipsychotic polypharmacy” event) if they discontinued the index drug for a period of 30 or more days or if they completed the full 12-month study period with monotherapy. The significance level was set at α = 0.05 for all hypothesis tests and all analyses were conducted using SAS software (v 9.1.3; SAS Institute Inc, Cary, NC).
Results
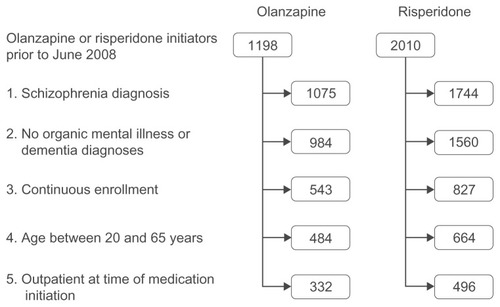
displays a flow diagram for the olanzapine- and risperidone- treatment cohorts following the application of each inclusion and exclusion criteria. Nearly two-thirds (63.7%) of the individuals with schizophrenia in the initial JMDC data had at least one pharmacy claim for olanzapine or risperidone. After applying all inclusion and exclusion criteria, the final analytic sample consisted of 828 individuals – 332 were treated with olanzapine and 496 treated with risperidone. The average age of the patients in the final sample was 36.9 years, and 48.0% were male.
Figure 1 Patient selection. The diagram displays the number of olanzapine and risperidone initiators remaining after each of the inclusion and exclusion criteria were applied. The continuous enrollment criteria required each patient to have at least one claim prior to and following the study period.
provides the baseline demographic characteristics, prior health care resource use, prior medical and psychiatric comorbidities, and prior medication use and univariate comparison between the olanzapine and risperidone cohorts. The risperidone initiators were more likely to have prior claims with diagnoses for diabetes mellitus and prior use of anticholinergic drugs. The olanzapine initiators were more likely to have prior claims with diagnoses for manic episodes and prior antidepressant use. The C-statistic for the logistic regression used to calculate the propensity score from all of the characteristics in was 0.608, indicating that the model could accurately discriminate a randomly selected risperidone-treated individual from a randomly selected olanzapine-treated individual 60.8% of the time.
On the index date, 61.5% of the olanzapine-initiated patients and 45.6% of the risperidone-initiated patients were treated with antipsychotic monotherapy (P < 0.001). For the patients who were treated with antipsychotic polypharmacy, lists the antipsychotics that were used as initial polypharmacy with olanzapine or risperidone.
Table 2 Initial concomitant antipsychotic treatment
displays the percent of olanzapine and risperidone initiated patients who were treated with antipsychotic monotherapy during the 12-month treatment period. gives the results of the generalized estimating equation model, comparing the rates of monotherapy over time after adjusting for baseline differences. Across the 12-month treatment period, olanzapine-treated patients were more likely to be treated with monotherapy than risperidone-treated patients, and the rates of monotherapy increased over time for both treatment groups.
Figure 2 Percentage of patients treated with monotherapy during the 12 months following initiation of olanzapine or risperidone. The generalized estimating equation model showed that, after correcting for baseline characteristics, patients initiated on olanzapine were significantly more likely to be treated with monotherapy across the 12-month treatment period (P = 0.001) and the rate of monotherapy increased over time (P < 0.001).
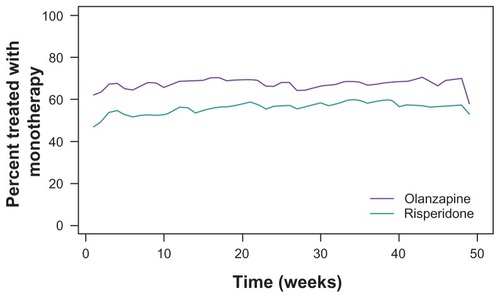
Table 3 Results of generalized estimating equation model predicting probability of monotherapy over the 12-month study
The mean (plus or minus the standard deviation) duration of monotherapy was 116.6 ± 130.6 days for the olanzapine-treated patients and 92.8 ± 123.0 days for the risperidone-treated patients (P = 0.008). The propensity score-adjusted bootstrapping model showed that olanzapine-treated patients were treated with monotherapy for 22.2 (95% confidence interval: 5.0, 39.8) days longer than risperidone-treated patients (P = 0.006).
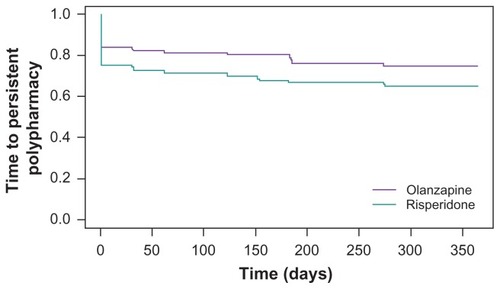
Finally, a time-to-event model was used to contrast the time to persistent polypharmacy for olanzapine- and risperidone-initiated patients. gives the Kaplan- Meier estimates of the proportion of patients remaining on monotherapy across the 12-month study period. In a Cox proportional hazards model, adjusting for background differences using the propensity score, the hazard ratio for antipsychotic polypharmacy use in the olanzapine-treated patients relative to risperidone-treated patients was 0.72 (95% confidence interval: 0.54, 0.97; chi-square = 4.71; P < 0.03). The hazard ratio indicates the odds were 0.72 that an olanzapine-treated patient would utilize polypharmacy before a risperidone-treated patient.
Discussion
Consistent with prior observational research in Japan, antipsychotic polypharmacy was common in this naturalistic sample of outpatients with schizophrenia. A univariate analysis showed that the patients treated with olanzapine (61.5%) were more likely to be initiated on antipsychotic monotherapy than those treated with risperidone (45.6%, P < 0.001). When adjusting for background characteristics and examining the probability of antipsychotic monotherapy across the full 12-month study period, the patients treated with olanzapine were significantly more likely to be treated with monotherapy (odds ratio [OR] = 1.62) and the rate of monotherapy increased over time for both treatment groups. Additionally, the duration of antipsychotic monotherapy was significantly longer for olanzapine- than risperidone-treated patients. In Japan, where antipsychotic polypharmacy is particularly common in the treatment of schizophrenia, olanzapine treatment was associated with a significantly greater rate and a longer duration of monotherapy than risperidone treatment.
Greater use of antipsychotic monotherapy for patients using olanzapine than risperidone has previously been found in naturalistic studies outside of Japan.Citation27,Citation32 In a large observational study in the US, olanzapine was found to be significantly (OR = 1.36) more likely to be used as monotherapy than risperidone and for a significantly longer duration (21.5 days longer; 252.1 vs 230.6 days).Citation27 The difference in duration found in the current study (22.5 days longer) was very similar. In the Pan-European Schizophrenia Outpatient Health Outcomes study, the odds of monotherapy for olanzapine treatment relative to risperidone were 1.56, remarkably similar to the current study (OR = 1.62), with significantly more days of monotherapy for olanzapine- than risperidone-treated patients (272 vs 261 days).Citation32 The findings in the current Japanese study replicate the findings previously reported in the US and Europe. However, the duration of monotherapy was shorter in this Japanese study (116.6 and 92.8 days for olanzapine and risperidone, respectively), which is consistent with prior research reporting more frequent use of polypharmacy in Japan.Citation36
The finding that olanzapine was associated with greater antipsychotic monotherapy use was not unanticipated, considering that antipsychotic polypharmacy may be implemented to bolster medication efficacy.Citation10,Citation11 A meta-analysis of 78 studies with 13,558 participants found olanzapine to confer significantly greater efficacy than other atypicals, including risperidone, in the treatment of schizophrenia.Citation37 The results of this meta-analysis were rather robust with regard to the effects of industry sponsorship, study quality, dosages, and trial duration. Greater effectiveness of olanzapine than risperidone in the treatment of schizophrenia offers a potential explanation of the current findings. However, the reasons physicians chose to use antipsychotic monotherapy or polypharmacy for each patient could not be specifically examined in this claims database study. Physicians may prefer to use risperidone as polypharmacy because of other properties of the medications or for patients presenting with a specific set of symptoms.
Limitations
Administrative claims data are collected for reimbursement rather than clinical purposes, thus the accuracy and level of detail are limited. Many conditions are not coded in claims and some comorbid conditions might have been underdiagnosed. The percentage of patients with claims for diabetes mellitus was higher than the 8.6% prevalence rate reported for patients with schizophrenia in Japan from past chart review research.Citation38 Diabetes mellitus diagnoses may be coded on a claim any time a blood test (ie, HbA1c) is conducted, regardless of the status of the results, leading to the potential for overdiagnosis.
Retrospective analyses of administrative claims data allow for the unobtrusive observation of usual clinical care at the cost of experimental control. Patients were not randomized to olanzapine or risperidone. Although propensity scores were used to adjust for multiple background variables, differences between olanzapine- and risperidone-initiated patients on variables not collected in this database could still bias the results. For instance, physician characteristics and preferences were not available to be included in the propensity score model. Finally, because the administrative data contained claims only for employees or their families, the sample was younger and might not be representative of the Japanese population as a whole.
Conclusion
Consistent with prior global research, this retrospective naturalistic study of outpatients with schizophrenia in Japan found that olanzapine is more likely than risperidone to be used as monotherapy and for a longer duration. Antipsychotic monotherapy is consistently recommended over polypharmacy because of reduced complexity, reduced risk of adverse events, reduced risk of drug interactions, and reduced total cost of drug therapy.
Acknowledgments
Eli Lilly and Company, Indianapolis, IN, provided funding for this study. Michael Stensland of Agile Outcomes Research Inc, Rochester, MN, and Susan Dennett of Strategic Health Outcomes Inc, Carmel, IN, provided technical writing support.
Disclosure
Wenyu Ye, Yuka Tanji, Jennifer Flynn, and Michihiro Takahashi are full-time employees of Eli Lilly Japan KK, Kobe, Japan. Haya Ascher-Svanum and Robert Conley are full-time employees of Eli Lilly and Company. All authors are minor stockholders in Eli Lilly and Company.
References
- LehmanAFLiebermanJADixonLBPractice guideline for the treatment of patients with schizophrenia, 2nd edAm J Psychiatry2004161Suppl 2115000267
- RittmannsbergerHMeiseUSchauflingerKHorvathEDonatHHinterhuberHPolypharmacy in psychiatric treatment: patterns of psychotropic drug use in Austrian psychiatric clinicsEur Psychiatry1999141334010572323
- WilliamsCLJohnstoneBMKestersonJGJavorKASchmetzerADEvaluation of antipsychotic and concomitant medication use patterns in patients with schizophreniaMed Care1999374 Suppl LillyAS818610217396
- MillierASarlonEAzorinJMRelapse according to antipsychotic treatment in schizophrenic patients: a propensity-adjusted analysisBMC Psychiatry2011112421314943
- SchumacherJEMakelaEHGriffinHRMultiple antipsychotic medication prescribing patternsAnn Pharmacother2003377–895195512841799
- TappAWoodAESecrestLErdmannJCubberleyLKilziehNCombination antipsychotic therapy in clinical practicePsychiatr Serv2003541555912509667
- GangulyRKotzanJAMillerLSKennedyKMartinBCPrevalence, trends, and factors associated with antipsychotic polypharmacy among Medicaid-eligible schizophrenia patients, 1998–2000J Clin Psychiatry200465101377138815491242
- GhaemiSN“All the worse for the fishes”: conceptual and historical background of polypharmacy in psychiatryGhaemiSNPolypharmacy in PsychiatryNew York (NY)Marcel Dekker2002134
- OepenGPolypharamcy in schizophreniaGhaemiSNPolypharmacy in PsychiatryNew York (NY)Marcel Dekker2002101132
- CorrellCUAntipsychotic polypharmacy, part 1: shotgun approach or targeted cotreatment?J Clin Psychiatry200869467467518507487
- BarnesTRPatonCAntipsychotic polypharmacy in schizophrenia: benefits and risksCNS Drugs201125538339921476610
- FreudenreichOGoffDCAntipsychotic combination therapy in schizophrenia: a review of efficacy and risks of current combinationsActa Psychiatr Scand2002106532333012366465
- AdvokatCDixonDSchneiderJComatyJEJrComparison of risperidone and olanzapine as used under “real-world” conditions in a state psychiatric hospitalProg Neuropsychopharmacol Biol Psychiatry200428348749515093956
- MillerALCraigCSCombination antipsychotics: pros, cons, and questionsSchizophr Bull200228110510912047009
- LoosbrockDLZhaoZJohnstoneBMMorrisLSAntipsychotic medication use patterns and associated costs of care for individuals with schizophreniaJ Ment Health Policy Econ200362677514578539
- JaffeABLevineJAntipsychotic medication coprescribing in a large state hospital systemPharmacoepidemiol Drug Saf2003121414812616846
- ZhuBAscher-SvanumHFariesDECorrellCUKaneJMCost of antipsychotic polypharmacy in the treatment of schizophreniaBMC Psychiatry200881918394168
- RupnowMFGreenspanAKosik-GonzalezCZhuYGarawabiGStahlSMUse and cost of polypharmacy in schizophrenia: data from a randomized double-blind study of risperidone and quetiapineValue Health20058399
- StahlSMAntipsychotic polypharmacy: squandering precious resources?J Clin Psychiatry2002632939411874226
- ProcyshynRMKennedyNBTseGThompsonBAntipsychotic polypharmacy: a survey of discharge prescriptions from a tertiary care psychiatric institutionCan J Psychiatry200146433433911387789
- ClarkREBartelsSJMellmanTAPeacockWJRecent trends in antipsychotic combination therapy of schizophrenia and schizoaffective disorders: implications for state mental health policySchizophr Bull2002281758412047024
- CovellNHJacksonCTEvansACEssockSMAntipsychotic prescribing practices in Connecticut’s public mental health system: rates of changing medications and prescribing stylesSchizophr Bull2002281172912047017
- JerrellJMCost-effectiveness of risperidone, olanzapine, and conventional antipsychotic medicationsSchizophr Bull200228458960512795493
- CentorrinoFGorenJLHennenJSalvatorePKelleherJPBaldessariniRJMultiple versus single antipsychotic agents for hospitalized psychiatric patients: case-control study of risks versus benefitsAm J Psychiatry2004161470070615056517
- YoshiTKurosawaMSugimuraKCurrent status of the psychopharmacological treatment of schizophrenic patients in Japan: from 2005 nationwide survey of 9 psychiatric hospitals in the psychiatric clinical pharmacy societyJpn J Clin Psychopharmacol20071017211731
- YoshiTUnoJNakagawaMSurvey of the prescription for psychotherapy in Japanese inpatients with schizophrenia in 2006Jpn J Clin Psychopharmacol20101315351545
- FariesDAscher-SvanumHZhuBCorrellCKaneJAntipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychoticsBMC Psychiatry200552615921508
- BitterIChouJCUngvariGSPrescribing for inpatients with schizophrenia: an international multi-center comparative studyPharmacopsychiatry200336414314912905100
- CorrellCUKaneJMO’SheaDRaziKMalhotraAKAntipsychotic polypharmacy in the treatment of schizophreniaSchizophr Res2003601 Suppl 1S37
- WangPFZhaoZComparison of olanzapine versus quetiapine in the treatment of hospitalized patients with schizophreniaValue Health20036355
- CorrellCUFredericksonAMKaneJMManuPDoes antipsychotic polypharmacy increase the risk for metabolic syndrome?Schizophr Res2007891–39110017070017
- NovickDAscher-SvanumHBrugnoliRBertschJHongJHaroJMAntipsychotic monotherapy treatment with atypicals in outpatients with schizophrenia: results from a naturalistic observational studySchizophr Bull201137Suppl 1S316317
- YoshimuraROkamotoTNakamuraJPrescription pattern of antipsychotic drugs for schizophrenic inpatients in Japan: research on East Asia Psychotropic Prescription Pattern-Antipsychotics studyPsychiatry Clin Neurosci200660677877917109719
- World Health OrganizationInternational Statistical Classification of Diseases and Related Health Problems 10th Revision version for 20072nd ed2007 Available from: http://apps.who.int/classifications/apps/icd/icd10online2007/Accessed January 20, 2011
- World Health OrganizationCollaborating Centre for Drug Statistics MethodologyWorld Health Organization Anatomical Therapeutic Chemical (ATC) classification index including defined daily doses (DDDs)Oslo, NorwayWorld Health Organization Collaborating Centre for Drug Statistics Methodology Available from: http://www.whocc.no/atc_ddd_index/Accessed January 20, 2011
- ShinfukuNTanCHPharmacotherapy for schizophrenic inpatients in East Asia: changes and challengesInt Rev Psychiatry200820546046819012132
- LeuchtSKomossaKRummel-KlugeCA meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophreniaAm J Psychiatry2009166215216319015230
- OkumuraYItoHKobayashiMMayaharaKMatsumotoYHirakawaJPrevalence of diabetes and antipsychotic prescription patterns in patients with schizophrenia: a nationwide retrospective cohort studySchizophr Res20101191–314515220304611