Abstract
Multiple sclerosis (MS) is a debilitating disease of the central nervous system that is most commonly seen in early to middle adulthood, although it can be diagnosed during childhood or later in life. While cognitive impairment can become more prevalent and severe as the disease progresses, signs of cognitive involvement can be apparent in the early stages of the disease. In this review, we discuss the prevalence and types of cognitive impairment seen in early MS, including the specific measures used to identify them, as well as the challenges in characterizing their frequency and progression. In addition to examining the progression of early cognitive involvement over time, we explore the clinical factors associated with early cognitive involvement, including demographics, level of physical disability, disease modifying therapy use, vocational status, and psychological and physical symptoms. Given the prevalence and functional impact these impairments can have for persons with MS, considerations for clinicians are provided, such as the role of early cognitive screenings and the importance of comprehensive neuropsychological assessments.
Introduction
It is estimated that there are 2.8 million people globally living with multiple sclerosis (MS), a chronic neurological condition that is most commonly diagnosed in early to middle adulthood, although it can be seen in children and older adults.Citation1,Citation2 About 85% of persons with MS are initially diagnosed with relapsing remitting MS (RRMS), which is characterized by relapses and stable disability in between.Citation1,Citation2 A diagnosis for RRMS is based on evidence of dissemination in space (ie, distinct location in the central nervous system) and in time, which is met with ≥2 demyelinating episodes and ≥2 lesions.Citation2 Individuals who have had a single episode are considered to have clinically isolated syndrome (CIS).Citation2 Over time, RRMS may evolve to secondary progressive MS (SPMS).Citation2 Another 12% are diagnosed with primary progressive MS (PPMS), who have had at least one year of disability progression without relapses and meet two additional criteria (≥1 periventricular, cortical/juxtacortical, or infratentorial T2- hyperintense lesions, ≥2 spinal cord T2-hyperintense lesions, and/or cerebrospinal fluid-specific oligoclonal bands).Citation1,Citation2
The clinical presentation of MS is heterogeneous, with persons presenting with range of physical and emotional symptoms. A common and often intrusive symptom of MS is cognitive impairment, which can affect up to 70% of the MS population.Citation3 Persons with MS can experience difficulties in several cognitive domains, including processing speed, attention, learning and memory, and executive functioning.Citation3 These impairments can have a negative impact on several aspects of persons’ with MS lives, including their quality of life and completion of everyday activities.Citation4,Citation5 While more severe cognitive impairment is more likely in persons with SPMS,Citation6 signs of cognitive involvement can be present early in the disease process.
Within the first year of diagnosis, about half of persons with MS report having either minimal or mild cognitive difficulties, with greater complaints over the first decade.Citation7 Although uncommon, some persons with MS present with cognitive impairment as their primary symptom.Citation8 Individuals with an aggressive form of cognitive impairment at this early stage can exhibit severe deficits reaching the level of a major neurocognitive disorder.Citation9,Citation10 In addition, cognitive issues may be present preclinically, with one study finding that men who developed MS two years later performed six points lower on an intelligence quotient test compared to a control group.Citation11 As such, there is a strong need to evaluate and monitor cognitive functioning beginning early in the disease so as to detect these issues as soon as possible.
That being said, understanding the prevalence and types of cognitive involvement early in the disease process comes with challenges as there is not a clear definition of what is considered the early stage of MS. The literature is mixed, with studies characterizing early MS by disease duration (either since diagnosis or symptom onset),Citation12–Citation112 level of disability (such as with the Expanded Disability Status Scale; EDSS),Citation113–Citation120 or a combination of both metrics.Citation121–Citation158 Furthermore, the time frame of disease duration for early MS varies greatly. For instance, some studies defined early MS shortly after symptom onset or diagnosis (eg, six months or less),Citation13,Citation15,Citation18,Citation24–Citation26,Citation29,Citation31,Citation32,Citation34,Citation35,Citation37,Citation41,Citation48,Citation54,Citation65,Citation72,Citation94,Citation112,Citation153,Citation156,Citation158 while other studies used broader time frames, such as 10 years or less.Citation33,Citation46,Citation78,Citation89,Citation113,Citation121,Citation129–Citation131 As some studies were investigating the signs of cognitive involvement soon after the onset of initial symptoms, persons with CIS suggestive of MS were included, either comprising the entire early stage groupCitation17,Citation21,Citation26,Citation37,Citation47,Citation48,Citation54,Citation57,Citation58,Citation61–Citation64,Citation66,Citation68,Citation71,Citation79,Citation84,Citation92–Citation94,Citation99,Citation101,Citation103,Citation107,Citation137,Citation142,Citation148,Citation153,Citation156 or as part of a mixed sample with persons with early RRMS.Citation12,Citation18,Citation20,Citation22,Citation28,Citation44,Citation53,Citation56,Citation67,Citation73,Citation75,Citation77,Citation80,Citation82,Citation83,Citation87,Citation105,Citation106,Citation108,Citation109,Citation112,Citation122,Citation143,Citation145,Citation146 In contrast, studies that have examined early MS in relation to late MS have defined the latter as a disease duration of >10Citation23 or >12 years.Citation19,Citation104
Given the need to understand the early signs from a clinical perspective, we aimed to review the current literature on cognitive impairment that occurs early MS in order to answer the following questions: 1) what is the prevalence of cognitive involvement in early MS; 2) what types of cognitive deficits are seen in early MS and how are they measured; 3) how does early MS cognitive involvement evolve over time; and 4) what clinical factors are associated with early MS cognitive involvement and its progression. In order to provide a comprehensive review of the literature in this area, we utilized a broader definition of early MS (ie, 10 years or less disease duration and inclusion of persons with CIS) and discuss how the different criteria may contribute to discrepancies. Finally, we will discuss implications for clinicians, such as screening batteries for early detection and the importance of comprehensive neuropsychological assessments.
Methods
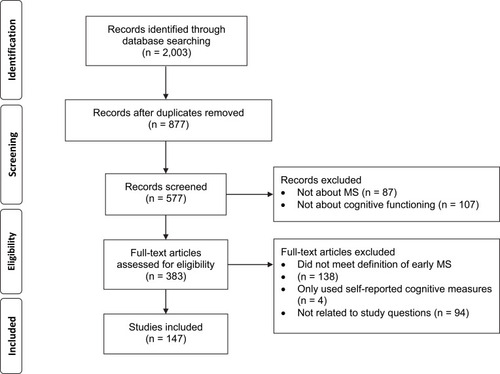
A search was conducted of articles published in English between 1991 and 2020 using PubMed, MEDLINE, and CINAHL using a combination of the search terms cognition, cognitive, neuropsychological, early, multiple sclerosis, and clinically isolated syndrome. Both pediatric-onset and adult-onset cohorts were included. A total of 2003 entries were found (). After removing duplicate entries (n = 1126) and articles where only the abstract was in English (n = 1), literature reviews, protocols, corrections, case studies, working group reports, letters to the editor, and conference abstracts (n = 299) were excluded. The titles and abstracts were then screened, with articles removed if they were not about MS, including animal model-based studies (n = 87), or were not about cognitive functioning (n = 107). The remaining 383 articles were split between the two authors for review. Articles were excluded if they did not fit our definition of early MS (n = 138), only used self-reported cognitive measures (n = 4), or were not related to our study questions (n = 94). If one author was unsure if an article met the inclusion criteria, it was reviewed by the other and a consensus was reached. Due to the breadth of literature that examines neuroimaging and biomarkers with cognitive functioning in MS, it was decided to exclude these articles from the current review in order to focus on neuropsychological indicators. A total of 147 studies were included in this review.
Figure 1 PRISMA flow diagram of article screening and selection for literature review.
Results
Prevalence of Cognitive Impairment in Early MS
Fifty studies provided an estimate on the prevalence of cognitive involvement in early MS, with rates being as high as 61%;Citation82 however, the numbers vary greatly between studies. Besides the variable definitions of early MS, there are several different criteria employed for classifying persons as cognitively impaired. Fischer et alCitation19 identified 20 distinct approaches for defining cognitive impairment in the literature, which could be broadly grouped by 1) the number of impaired measures, 2) a composite score (ie, a z-score calculated by performance on all measures in a domain), and 3) a combination of the first two strategies. Furthermore, different stringencies for impairment have been used, such as the number of required “failed’ measures and the cut-off for impairment (eg, 1.5 standard deviations (SD) below the mean). Fischer et alCitation19 noted that using more liberal criteria could result in the prevalence of cognitive impairment in early MS being overestimated: in their own sample, the rate of cognitive impairment ranged from 0% to 68% using the 20 identified criteria. In addition, it should be kept in mind that healthy controls may demonstrate impairment, and some researchers have adjusted for this in the quantification of impairment in early MS. For instance, Baysal Kıraç et alCitation12 adjusted the number of impaired measures required (ie, four or more measures) based on performance of their healthy controls. Deloire et al,Citation41 on the other hand, calculated the attributable risk for impairment on one or two measures in their early MS cohort based on the percentage of “failures” in their control group.
As demonstrated in , several criteria have been used to define cognitive impairment during the early stage of MS. While the number of “failed” measures varied in those studies, it was often influenced by the assessment battery used. For instance, the Brief International Cognitive Assessment for MS (BICAMS)Citation159 consists of three measures (Symbol Digit Modalities Test (SDMT), five learning trials of the California Verbal Learning Test-Second Edition (CVLT-II), and three learning trials of the Brief Visuospatial Memory Test-Revised (BVMT-R)), and impairment on one measure is considered the threshold.Citation113 Using that criterion, 32.3% of Marstrand et al’ sampleCitation113 were categorized as cognitively impaired; if held to the standard of three impaired measures (ie, the entire battery), the percent of participants categorized as impaired dropped to 4.6%. On the other hand, with a more extensive battery, a higher number of “failed” measures would be more appropriate. For instance, Jønsson et al,Citation27 who had 30 cognitive variables, identified mild impairment if at least five measures were impaired, while severe was defined as 20 or more. They also defined mild, moderate, and severe cognitive impairment by the number of impaired cognitive domains.
Table 1 Rates of Impairment in the Literature by Different Criteria
Types of Deficits and the Measures Used to Identify Signs of Cognitive Involvement in Early MS
Eighty studies detailed the specific measures where persons with early MS have been impaired, with deficits in several cognitive domains, including attention, processing speed, working memory, verbal learning and memory, visual learning and memory, executive functioning, language, and to a lesser extent visuospatial ability and theory of mind (Supplementary Table 1). These signs of cognitive involvement were identified either through persons with early MS performing below a specific threshold (eg, 1.5 SD below the normative mean) or in comparison to a matched cohort of healthy controls. Even if persons with early MS do not meet the threshold for being categorized as cognitively impaired, they may still exhibit relative difficulties. For instance, Pitteri et alCitation49 found that their “cognitively normal” group performed worse on measures of verbal learning and memory, attention and processing speed, and executive functioning compared to healthy controls.
Although the measures used varied between studies, two of the most commonly used measures that have detected early cognitive involvement were the SDMT and the Paced Auditory Serial Addition Test (PASAT). Forty-one studies found impairments in early MS using the SDMT, a 90-second measure where the examinee orally matches numbers to corresponding symbols as quickly as possible.Citation160 The SDMT has emerged as a particularly sensitive measure, with one study finding that it correctly classified 75.4% of their sample with cognitive impairment.Citation41 Thirty-four studies identified deficits with either the 3-second or 2-second version of the PASAT, where the examinee attends to and calculates numbers that are presented auditorily via a tape recording.Citation161 Besides issues with complex attention, processing speed, and working memory, persons with early MS have also demonstrated slowed reaction time on attentional measures, although measurement of this metric was less common in the identified studies.
Issues with learning and memory can emerge early in the disease process. Thirty-six studies showed that persons with early MS exhibit difficulty learning and recalling verbal information. The most commonly used measure was the Selective Reminding Test (SRT), which 18 studies found impairments on, particularly on the Long Term Storage, Consistent Long Term Retrieval, and Delayed Recall indices. Besides the SRT, other measures that detected impairments were the CVLT-II (four studies) and the Rey Auditory Verbal Learning Test (RAVLT; six studies). Difficulty learning and remembering visual material was also observed, as noted by 34 studies. The Spatial Recall Test (SPART) was used the most frequently, with issues being identified in 15 studies, particularly on the Delayed Recall index. Impairments were also found in nine studies using the BVMT-R.
While several studies have focused on attention, processing speed, and memory impairments in early MS, executive dysfunction can also occur, as noted by 26 studies. Pitteri et alCitation49 found that 71% of their sample exhibited deficits on a composite executive functioning index, although other studies have found much lower rates of impairment.Citation125 As executive functioning in a broad term, several different processes such as set-shifting (eg, Trail Making Test Part B; TMT-B; eight studies), inhibition (eg, Stroop; 10 studies), and abstraction (eg, Similarities from the Wechsler Adult Intelligence Scale; four studies) can be included in this domain. Verbal fluency has sometimes been included as executive functioning tasks,Citation49 in addition to being a language-based task. Deficits in both phonemic (ie, letter) and semantic (ie, category) fluency have emerged in 15 studies and 16 studies, respectively, as signs of early cognitive involvement. Furthermore, with persons with CIS, both verbal fluency tasks were able to classify individuals with cognitive impairment with 64–82% sensitivity and 66–79% specificity.Citation17
However, because of the heterogeneity of these samples, the types of impairments observed are not consistent across studies. For instance, in their sample of persons with CIS, Viterbo et al noted only 5–7% were impaired on the SDMT and PASAT, while other studiesCitation103,Citation121 did not find differences between healthy controls and persons with early MS on PASAT or SDMT performances. In addition to the different definitions for early MS, another consideration may be in how the measure is utilized and scored, such as in the case of the PASAT where examiners may look at total correct scores, dyads, and/or performance at different points of the test. When Berard et alCitation121 used it to measure cognitive fatigue (eg, performance on the last third of the task compared to the first third), persons with early MS exhibited significantly greater difficulties.
In addition to cognitive involvement becoming more prevalent in late MS,Citation19,Citation58,Citation79,Citation84,Citation88,Citation104 as the disease progresses, some of these cognitive difficulties may become more apparent. For instance, Achiron et alCitation79 found that the percent of persons with MS with cognitive impairment significantly increased after five years of MS onset, while Štecková et alCitation87 noted greater impairments among persons who had had MS for 10 years compared to persons with CIS and persons with MS for five years. In terms of specific deficits, while Caputi et alCitation128 found that persons with early RRMS did not differ from healthy controls in terms of object naming and visual discrimination, deficits were more pronounced in later stages of the disease (ie, SPMS). Processing speed and memory performances have also been shown to be lower in late MS compared to early MS.Citation23,Citation104,Citation157
Evolution of Early Cognitive Impairment
In addition to studies comparing MS cohorts at different stages of the disease, there have been 24 longitudinal studies with persons with early MS. While some studies did not observe a decline on individualCitation121 or a battery of measures,Citation106,Citation131,Citation153 several identified an increase in cognitive impairments over time. For instance, Amato et alCitation133 noted that the number of persons with MS with no cognitive impairment decreased from 74% at baseline to 44% at the 10-year follow-up, with a particular increase in the number with mild cognitive impairment (8% to 34%). Increases in the number of persons with cognitive involvement have also been observed at two years,Citation134,Citation149 three years,Citation115,Citation135 four years,Citation132 five years,Citation47,Citation51,Citation60,Citation112,Citation136 seven years,Citation31,Citation116 and nine yearsCitation158 since the initial neuropsychological evaluation.
Several studies have found that the SDMT is sensitive to progression, with greater impairments noted over time.Citation15,Citation28,Citation60,Citation135,Citation136,Citation143 While declines in attention, processing speed, or memory were detected in several cohorts,Citation31,Citation47,Citation60,Citation112,Citation116,Citation134,Citation158 this was not observed by Wybrecht et al.Citation50 Instead, they found a greater number of persons with MS impaired in executive functioning 10 years later, particularly on the TMT-B, Digit Spans Backwards, and both phonemic and semantic fluency.Citation50 Declines in executive functioning, including verbal fluency, have been noted in other longitudinal early MS cohorts.Citation47,Citation134
While longitudinal cohorts can provide insight into the progression of early MS cognitive impairment, there are potential confounders that may skew results. Some studies have noted improvements on certain measures at the second assessment, which has been attributed to factors such as practice effects, short intervals between the initial and follow-up testing, anxiety at baseline, and initiation of disease modifying therapies (DMT) after the first evaluation.Citation18,Citation27,Citation121 Besides judging the length of time between assessments and using an equivalent alternative form of a measure, clinicians may consider calculating the reliable change to determine if a reduction in performance (or improvement) represents meaningful change.
Clinical Factors Associated with Early Cognitive Involvement and Its Progression
Demographics
When examining their patients’ performances and determining whether there is evidence of cognitive impairment, clinicians should be mindful of factors that may be associated with reduced performance. Although 10 studies did not find a significant relationship between age or gender with cognitive performances in early MS,Citation12,Citation21,Citation37,Citation39,Citation46,Citation47,Citation65,Citation82,Citation125,Citation135 nine noted worse performance with older age or among men.Citation18,Citation24,Citation26,Citation30,Citation44,Citation52,Citation111,Citation133,Citation139 For instance, men with early MS have demonstrated greater impairments in aspects of executive functioning and verbal learning and memory.Citation30,Citation139 One studyCitation78 also found that low free testosterone levels in men were related to more longitudinal changes on the SDMT.
The contributions of race and ethnicity in early MS cognitive involvement have been studied less extensively. While Black and Hispanic persons with MS had lower scores on the oral SDMT compared to White persons with MS, Amezcua et alCitation20 attributed these findings to underlying population differences (eg, sociodemographic factors) rather a more severe disease course. The authors highlighted the importance of using appropriate normative data to identify persons in the early stages of MS who are exhibiting cognitive involvement. Julian et alCitation67 did not find race to be a significant contributor to cognitive performance, but noted a trend with lower scores and identifying as Hispanic.
Seven studies did not demonstrated an association between education level and cognitive performance,Citation39,Citation44,Citation46,Citation47,Citation65,Citation125,Citation135 while 10 showed that persons with fewer years of education have greater levels of impairment.Citation17,Citation18,Citation21,Citation30,Citation37,Citation52,Citation72,Citation111,Citation113,Citation144 For instance, Bonnet et alCitation72 found that persons with early MS who had low educational levels (<12 years or no schooling) were impaired on 13 out of 15 cognitive tests, while persons with high education levels were only impaired on three measures. Persons’ education level has also been utilized as a marker for cognitive reserve. For instance, Barbu et alCitation131 used years of education and performance on the North American Adult Reading Test to calculate cognitive reserve. While they did not find that cognitive reserve predict change in cognitive functioning three years later, their sample did not exhibit significant decline over time. In addition, Planche et alCitation80 hypothesized that their sample’s high education level contributed to the lack of impairment in memory impairments.
Level of Disability
There has been mixed evidence on the connection between level of disability and cognitive impairment. Fifteen studies have found relationships between disability and cognition,Citation16,Citation18,Citation24,Citation26,Citation34,Citation43,Citation54,Citation67,Citation83,Citation105,Citation111,Citation113,Citation114,Citation116,Citation122 with seven studies noting that cognitive performances predicted disease progressionCitation13,Citation24,Citation32,Citation35 or disability predicted cognitive deterioration.Citation45,Citation132,Citation133 In 15 other cohorts, disability and cognitive performance were not significantly associated.Citation12−Citation17−Citation29−Citation37−Citation39−Citation44−Citation46−Citation82−Citation107−Citation109−Citation123–Citation125,Citation135 The discrepancies in these findings may be due to the heterogeneity of the study populations. In the studies that identified a significant relationship between cognitive impairment and disability, three included participants’ EDSS score in their definition of early MS (ie, ≤4Citation122 and ≤5Citation113,Citation132). Comparatively, three of the studies that did not find an association had a narrower EDSS inclusion criterion (ie, ≤2Citation123, ≤2.5Citation125, and ≤3.5Citation135).
Disease Modifying Therapies
Seven studies have evaluated the impact of starting a DMT on cognitive functioning. Overall improvement in cognitive functioning have been noted with interferon beta-1a three years after initiation.Citation120 In a pediatric cohort,Citation100 persons with MS who had an escalation of their DMT (ie, changing to fingolimod or natalizumab) had better overall cognitive performance than individuals who remained on a first-line DMT. In terms of specific tests, improvements on the SDMT have been found with interferon beta-1a,Citation91,Citation151 interferon beta-1b,Citation91,Citation151 glatiramer acetate,Citation151 and natalizumab,Citation155 with a non-significant increase with the latter in a pediatric cohort with aggressive MS.Citation154 The PASAT-2Citation91 and PASAT-3Citation93 have been noted to improve following treatment with interferon beta-1a and interferon beta-1b, respectively. Interferon beta-1a, interferon beta-1b, and glatiramer acetate have also been associated with improvements on total learning of the CVLT-II and BVMT-R.Citation151 In addition, Mokhber et alCitation91 noted improvements on the SPART Delay with interferon beta-1a, as well as on the SRT Delay and WLG with one version of interferon beta-1a (Avonex).
Vocational Status
Given that the average age of diagnosis is 32,Citation1 employment is a significant concern for many persons with MS. Cognition has been associated with vocational status changes,Citation162 and these issues can be apparent early in the disease course, as noted by two studies. Jongen et alCitation53 showed that persons with MS who wanted to work fewer hours had poorer focused attention and processing speed, while individuals who wanted to change their jobs had worse episodic memory. Furthermore, the number of days that participants worked were positively associated with their working memory, focused attention, and processing speed performances.Citation53 Evidence of early cognitive involvement may also predict later employment. Ruet et alCitation31 demonstrated that baseline processing speed, as well as participants’ decline in cognitive functioning, predicted their vocational status seven years later.
Psychological Symptoms
While depression is prevalent in MS,Citation163 there is mixed evidence on its relationship with cognitive functioning. Ten studies did not find an association between performance and level of depressive symptom severity,Citation29,Citation34,Citation37,Citation46,Citation83,Citation85,Citation113,Citation124,Citation135,Citation141 while 14 studies found a negative impact,Citation16,Citation18,Citation22,Citation30,Citation44,Citation45,Citation69,Citation70,Citation74,Citation98,Citation105,Citation107,Citation125,Citation142 such as higher levels of depression among persons with cognitive impairment or worse performance on verbal fluency, aspects of executive functioning, multitasking, working memory, and processing speed tasks. There is also mixed evidence with quality of life and cognition, with two studies not finding a relationshipCitation82,Citation115 and four studies noting a significant association.Citation22,Citation66,Citation113,Citation125 In addition, baseline memory performance has been found to predict persons’ with MS health-related quality of life seven years later,Citation31 while improvement in cognition was associated with improvement in mental health-related quality of life longitudinally.Citation73
Less research has been done on the impact of anxiety on cognitive performance in early MS. Although six studies did not find a significant relationship,Citation16,Citation30,Citation85,Citation113,Citation142,Citation147 five studiesCitation26,Citation45,Citation74,Citation98,Citation107 showed that greater anxiety symptomatology was associated with worse performance on aspects of executive functioning, working memory, processing speed, immediate structured verbal learning (ie, story memory), and delayed structured verbal memory. Simioni et alCitation125 also found a higher prevalence of anxiety among persons with cognitive involvement.
One studyCitation56 examined the relationship between self-efficacy and cognitive performance, finding that not only was self-efficacy strongly associated with several domains, it predicted 40% of the variability of persons’ with MS performance on Power of Attention and Speed of Memory and 3% on their Reaction Time Variability. While another studyCitation81 found that psychological resilience was correlated with processing speed, it was not significant after adjusting for multiple comparisons or fatigue and mood.
Physical Symptoms
Subjective reports of fatigue have not been found to be consistently related to objective cognitive performance in the wider MS literature.Citation164 This is similar in early MS, with seven studies finding no significant relationship between fatigue and cognitive impairment.Citation12,Citation29,Citation34,Citation46,Citation89,Citation113,Citation135 Seven studies found an association,Citation22,Citation30,Citation68,Citation74,Citation75,Citation125,Citation142 such as higher rates of fatigue among persons with cognitive involvement or worse performance on measures of verbal fluency, verbal memory, aspects of executive functioning, working memory, and processing speed. However, part of the discrepancy may be due to the measures being used. For instance, while Hyncicova et alCitation142 showed that the SDMT was significantly related to the Energy/Fatigue scale on the Short Form-36, it was not associated with the Fatigue Severity Scale.
Two studies investigated the association between pain and cognition in early MS, with oneCitation142 noting a significant relationship between performance on the SDMT and pain ratings in persons with CIS. While the other studyCitation77 found that impaired cognitive functioning contributed to baseline unspecified pain, other factors such as fatigue played a more significant role. Furthermore, the authors did not find that it significantly impacted follow-up unspecified pain or baseline and follow-up neuropathic pain.
Five studies have evaluated the relationship between cognition and difference aspects of eye functioning. One studyCitation55 has investigated pupillary dilation with regards to cognitive functioning in early MS. The authors found that while it was not related to performance on the BICAMS when examining their entire cohort, persons with early MS who had low cognitive scores had smaller pupillary responses compared to controls with low cognitive scores. One studyCitation102 noted that saccadic initiation time was associated with performance on the written version of the SDMT, as was hand functioning, but not with the PASAT. Similarly, Clough et alCitation108 only found a relationship between visually guided latency saccades with late MS and not early RRMS. With regards to anti-saccades, performance on the PASAT has been correlated with latency,Citation103 error proportion,Citation108 and error times,Citation108 while processing speed (ie, SDMT and the Computerized Speed Cognitive Test) has been correlated with error rate.Citation103,Citation110
In terms of sleep, one studyCitation117 found that self-reported daytime sleepiness was negatively correlated with performance on the BICAMS. The authors also found positive associations between cognition and pulmonary and respiratory muscle functioning, suggesting impaired functioning in these areas are related to cognitive involvement.
Discussion
Despite differences in definitions of early MS and impairment, cognitive involvement in common within the first 10 years of MS. During the early stage of MS, persons may exhibit deficits in attention, processing speed, working memory, verbal and visual learning and memory, executive functioning, and verbal fluency. Over time, these impairments can become more significant, with a greater number of individuals exhibiting cognitive involvement. While the SDMT has been shown to be sensitive to both initial cognitive impairmentCitation13,Citation22,Citation26–Citation28,Citation30,Citation31,Citation33,Citation34,Citation36,Citation41,Citation44–Citation46,Citation48,Citation57,Citation59,Citation62,Citation64,Citation65,Citation68,Citation70,Citation76,Citation82,Citation86,Citation94,Citation97,Citation107,Citation109,Citation113,Citation114,Citation118,Citation127,Citation135,Citation140,Citation142–Citation144,Citation148,Citation151,Citation156 and progression,Citation15,Citation28,Citation60,Citation135,Citation136,Citation143 a number of longitudinal early MS cohorts demonstrated declines in executive functioning and verbal fluency,Citation47,Citation50,Citation134 signaling the importance of evaluating and monitoring these domains over time. The evidence of modifying factors is mixed; however, several studies noted a positive influence on cognitive functioning with DMT usageCitation91,Citation93,Citation100,Citation120,Citation151,Citation154,Citation155 and cognitive reserve may help protect against decline.Citation80,Citation131
Given the evidence that cognitive involvement can occur early in MS and its relationship to later changes in functioning, such as vocational status,Citation31 there is a strong need to evaluate persons’ with MS cognition early and monitor over time. It is currently recommended that persons with MS should receive early baseline screening, with annual reassessment with the same measure, and a follow-up comprehensive evaluation if there is evidence of impairment.Citation165 The minimal recommended screening tool is the SDMT or a similar validated measure,Citation165 which coincides with our findings of its sensitivity to early cognitive involvement in MS. That said, as not all persons with MS-related cognitive impairment will demonstrate reductions on the SDMT, clinicians should strongly consider using a short screening battery that assesses several domains. The most commonly used battery is the BICAMS, which includes measures of verbal (CVLT-II) and visual (BVMT-R) learning in addition to the SDMT.Citation159 Besides taking 15 minutes to administer, there is an international standard for validation, allowing it to be used in different countries.Citation166
An alternative battery is the abbreviated Minimal Assessment of Cognitive Function in MS (aMACFIMS), which includes shortened versions of the SDMT, CVLT-II, BVMT-R, and verbal fluency, with both phonemic and semantic fluency in the expanded version.Citation167–Citation170 Deficits in verbal fluency have been noted in several early MS cohorts,Citation12,Citation13,Citation16,Citation17,Citation21,Citation23,Citation27,Citation29,Citation30,Citation38,Citation41,Citation42,Citation57,Citation59,Citation61–Citation66,Citation74,Citation76,Citation82,Citation94,Citation97,Citation101,Citation118,Citation148 and both types have been shown to have good detection of cognitive impairment in persons with CIS,Citation17 which supports its use as a screening battery for persons with early MS. Regardless of which screening battery a clinician chooses, they should be aware that they may not be capturing persons with MS whose deficits are primarily in executive functioning. Although the aMACFIMS was initially developed with an abbreviated executive functioning task (ie, one Card Sort from the D-KEFS),Citation168 it did not significantly contribute to the battery in terms of detecting cognitive impairment and was subsequently removed.Citation169
If there are concerns about a patient’s executive abilities and/or they demonstrate impairments on a screening battery, a comprehensive neuropsychological assessment may be warranted. Besides considering the influences of demographics such as age, education, race/ethnicity, gender, and premorbid functioning on performances, a neuropsychologist can evaluate whether the person’s with MS impairments are influenced by other factors such as comorbidities,Citation171 including psychological and physical symptoms. Two of the most common batteries used in MS are the MACFIMSCitation172 and the Brief Repeatable Battery of Neuropsychological Tests (BRB-N).Citation173 Both of these batteries assess the domains frequently affected in early MS, including attention and processing speed (SDMT in both), working memory (PASAT in both), verbal learning and memory (CVLT-II in the MACFIMS and SRT in the BRB-N), visual learning and memory (BVMT-R in the MACFIMS and 10/36 SPART in the BRB-N), and verbal fluency (Controlled Oral Word Association Test in the MACFIMS and Word List Generation in the BRB-N). The MACFIMS also includes measures of visuospatial functioning (Judgment of Line Orientation) and executive functioning (Delis-Kaplan Executive Function System). As the MACFIMS and BRB-N are considered minimal assessment batteries, clinicians may consider adding ones that evaluate aspects of executive functioning. For instance, a number of studiesCitation34,Citation41,Citation49,Citation71,Citation120 that used the BRB-N also included the Stroop test, which taps into inhibition. However, despite the prevalence of cognitive impairment and its impact, even in early MS, neuropsychological services may be underutilized.Citation174,Citation175 While there are many factors that may influence service utilization, such as cost and accessibility, increased awareness of the deficits observed at the different stages of the disease and how to screen for them may lead to more patients who need these services to receive them.
While there is a large body of literature investigating early cognitive involvement in MS, there are still limitations with the available data. The first is the lack of a consensus over what is considered “early.” As previously noted, the varying definitions have likely contributed to discrepancies in the types of observed cognitive deficits and their relationships with clinical factors. Although a longer duration like ≤10 years may allow for inclusion of more persons with MS from a clinical sample, it begins to encroach upon some definitions of late MS,Citation23 making it difficult to differentiate between these stages of disease duration. Given that the rate of impairment has been shown to significantly increase after having MS for five years,Citation47,Citation51,Citation79,Citation112 classifying persons with MS with less than five years of disease duration may provide insights into the earliest signs of cognitive involvement.
In addition, the definition used for impairment can affect how many persons with MS are classified as cognitively impairment. The majority of the reviewed studies used at least a cut-off of >1.5 SD, which is a fair stringency.Citation19 Furthermore, the number of impaired measures is also an important factor and is dependent on the size of the battery. Several of the established batteries have a recommended number of impaired measures to consider an individual cognitively impaired: for instance, one for the BICAMSCitation113 and two measures for the aMACFIMSCitation169,Citation170 and MACFIMS.Citation176 With larger batteries, using a cut-off of one or two measures may overestimate the prevalence of cognitive impairment.
Finally, there is a need to evaluate how engagement in different activities and co-occurring conditions may influence cognitive functioning early in the disease process. For instance, research in the broader MS population has shown that cognitive leisure activities, which have been used as a marker for cognitive reserve, is a protective factor for cognitive decline.Citation177 While persons with early MS tend to engage in fewer reserve-building activities, particularly organized sports, job-related exercise, and high and low impact exercise,Citation178 their contributions to cognitive functioning has not been explored at this stage. Furthermore, although there is evidence that there are relationships between diabetes,Citation171 body mass index (BMI),Citation171,Citation179,Citation180 and cholesterol levelCitation179,Citation181,Citation182 with cognition in MS, these studies were not specific to early MS. Understanding if these factors contribute to cognitive deficits early in MS may help with management of patients’ multi-morbidities and general health, and subsequently mediate their impact on cognitive functioning.
Conclusions
Even early in the disease process, many persons with MS can present with signs of cognitive involvement. Although the definitions for early MS and cognitive impairment vary between studies, up to 61% of persons with early MS demonstrate some type of cognitive involvement. Besides impairments in attention, processing speed, and memory, executive dysfunction can occur, which can include reductions in verbal fluency. As cognitive impairment can progress over time and early involvement is associated with later functional difficulties, there is a strong need for early screening and follow-up comprehensive neuropsychological assessments when indicated.
Disclosure
The authors have no conflicts of interest to report.
References
- The Multiple Sclerosis International Federation (MSIF). Part 1: Mapping Multiple Sclerosis Around the World, Key Epidemiology Findings; 2020.
- Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi:10.1016/S1474-4422(17)30470-229275977
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7(12):1139–1151. doi:10.1016/S1474-4422(08)70259-X19007738
- Benito‐Leon J, Morales J, Rivera‐Navarro J. Health‐related quality of life and its relationship to cognitive and emotional functioning in multiple sclerosis patients. Eur j Neurol. 2002;9(5):497–502. doi:10.1046/j.1468-1331.2002.00450.x12220381
- Kalmar JH, Gaudino EA, Moore NB, Halper J, DeLuca J. The relationship between cognitive deficits and everyday functional activities in multiple sclerosis. Neuropsychology. 2008;22(4):442. doi:10.1037/0894-4105.22.4.44218590356
- Planche V, Gibelin M, Cregut D, Pereira B, Clavelou P. Cognitive impairment in a population‐based study of patients with multiple sclerosis: differences between late relapsing− remitting, secondary progressive and primary progressive multiple sclerosis. Eur j Neurol. 2016;23(2):282–289. doi:10.1111/ene.1271525903918
- Kister I, Bacon TE, Chamot E, et al. Natural history of multiple sclerosis symptoms. Int J MS Care. 2013;15(3):146–158. doi:10.7224/1537-2073.2012-05324453777
- Evlice A, Demir T, Kaleağası C, Özcan F, Demirkıran M. Rare onset symptoms in multiple sclerosis. Acta Clin Belg. 2016;71(3):154–157. doi:10.1080/17843286.2016.114767527098603
- Assouad R, Louapre C, Tourbah A, et al. Clinical and MRI characterization of MS patients with a pure and severe cognitive onset. Clin Neurol Neurosurg. 2014;126:55–63. doi:10.1016/j.clineuro.2014.08.01825215443
- Mendes MF, Finkelsztejn A, Gomes S, Fragoso YD. Early and severe cognitive impairment in multiple sclerosis. Dementia Neuropsychol. 2012;6(1):48–52. doi:10.1590/S1980-57642012DN06010008
- Cortese M, Riise T, Bjørnevik K, et al. Preclinical disease activity in multiple sclerosis: a prospective study of cognitive performance prior to first symptom. Ann Neurol. 2016;80(4):616–624. doi:10.1002/ana.2476927554176
- Baysal Kıraç L, Ekmekçi Ö, Yüceyar N, Sağduyu Kocaman A. Assessment of early cognitive impairment in patients with clinically isolated syndromes and multiple sclerosis. Behav Neurol. 2014;2014. doi:10.1155/2014/637694
- Carotenuto A, Moccia M, Costabile T, et al. Associations between cognitive impairment at onset and disability accrual in young people with multiple sclerosis. Sci Rep. 2019;9(1):1–8. doi:10.1038/s41598-019-54153-730626917
- Dujardin K, Donze A, Hautecoeur P. Attention impairment in recently diagnosed multiple sclerosis. Eur j Neurol. 1998;5(1):61–66. doi:10.1046/j.1468-1331.1998.510061.x10210813
- Hankomäki E, Multanen J, Kinnunen E, Hämäläinen P. The progress of cognitive decline in newly diagnosed MS patients. Acta Neurol Scand. 2014;129(3):184–191. doi:10.1111/ane.1216123773012
- Siepman T, Janssens A, de Koning I, Polman C, Boringa J, Hintzen R. The role of disability and depression in cognitive functioning within 2 years after multiple sclerosis diagnosis. J Neurol. 2008;255(6):910. doi:10.1007/s00415-008-0814-x18484237
- Viterbo R, Iaffaldano P, Trojano M. Verbal fluency deficits in clinically isolated syndrome suggestive of multiple sclerosis. J Neurol Sci. 2013;330(1):56–60. doi:10.1016/j.jns.2013.04.00423628466
- Johnen A, Bürkner P-C, Landmeyer NC, et al. Can we predict cognitive decline after initial diagnosis of multiple sclerosis? Results from the German National early MS cohort (KKNMS). J Neurol. 2019;266(2):386–397. doi:10.1007/s00415-018-9142-y30515631
- Fischer M, Kunkel A, Bublak P, et al. How reliable is the classification of cognitive impairment across different criteria in early and late stages of multiple sclerosis? J Neurol Sci. 2014;343(1–2):91–99. doi:10.1016/j.jns.2014.05.04224950898
- Amezcua L, Smith JB, Gonzales EG, Haraszti S, Langer-Gould A. Race, ethnicity, and cognition in persons newly diagnosed with multiple sclerosis. Neurology. 2020;94(14):e1548–e1556. doi:10.1212/WNL.000000000000921032152131
- Diker S, Has AC, Kurne A, Göçmen R, Oğuz KK, Karabudak R. The association of cognitive impairment with gray matter atrophy and cortical lesion load in clinically isolated syndrome. Mult Scler Relat Disord. 2016;10:14–21. doi:10.1016/j.msard.2016.08.00827919482
- Glanz BI, Healy BC, Rintell DJ, Jaffin SK, Bakshi R, Weiner HL. The association between cognitive impairment and quality of life in patients with early multiple sclerosis. J Neurol Sci. 2010;290(1–2):75–79. doi:10.1016/j.jns.2009.11.00419944429
- Brissart H, Morele E, Baumann C, et al. Cognitive impairment among different clinical courses of multiple sclerosis. Neurol Res. 2013;35(8):867–872. doi:10.1179/1743132813Y.000000023223816638
- Moccia M, Lanzillo R, Palladino R, et al. Cognitive impairment at diagnosis predicts 10-year multiple sclerosis progression. Multiple Scl J. 2016;22(5):659–667. doi:10.1177/1352458515599075
- Quintana E, Coll C, Salavedra‐Pont J, et al. Cognitive impairment in early stages of multiple sclerosis is associated with high cerebrospinal fluid levels of chitinase 3‐like 1 and neurofilament light chain. Eur J Neurol. 2018;25(9):1189–1191. doi:10.1111/ene.1368729797629
- Summers M, Swanton J, Fernando K, et al. Cognitive impairment in multiple sclerosis can be predicted by imaging early in the disease. J Neurol Neurosurg Psychiatry. 2008;79(8):955–958. doi:10.1136/jnnp.2007.13868518339729
- Jønsson A, Andresen J, Storr L, Tscherning T, Sørensen PS, Ravnborg M. Cognitive impairment in newly diagnosed multiple sclerosis patients: a 4-year follow-up study. J Neurol Sci. 2006;245(1–2):77–85. doi:10.1016/j.jns.2005.09.01616647089
- Wallach AI, Waltz M, Casper TC, et al. Cognitive processing speed in pediatric-onset multiple sclerosis: baseline characteristics of impairment and prediction of decline. Multiple Scl J. 2019;26(14):1352458519891984. doi:10.1177/1352458519891984
- Faiss J, Dähne D, Baum K, et al. Reduced magnetisation transfer ratio in cognitively impaired patients at the very early stage of multiple sclerosis: a prospective, multicenter, cross-sectional study. BMJ Open. 2014;4:4. doi:10.1136/bmjopen-2013-004409
- DiGiuseppe G, Blair M, Morrow SA. Prevalence of cognitive impairment in newly diagnosed relapsing-remitting multiple sclerosis. Int J MS Care. 2018;20(4):153–157. doi:10.7224/1537-2073.2017-02930150898
- Ruet A, Deloire M, Hamel D, Ouallet J-C, Petry K, Brochet B. Cognitive impairment, health-related quality of life and vocational status at early stages of multiple sclerosis: a 7-year longitudinal study. J Neurol. 2013;260(3):776–784. doi:10.1007/s00415-012-6705-123081755
- Pitteri M, Romualdi C, Magliozzi R, Monaco S, Calabrese M. Cognitive impairment predicts disability progression and cortical thinning in MS: an 8-year study. Multiple Scl J. 2017;23(6):848–854. doi:10.1177/1352458516665496
- Berrigan LI, LeFevre J-A, Rees LM, Berard J, Freedman MS, Walker LA. Cognition in early relapsing-remitting multiple sclerosis: consequences may be relative to working memory. J Int Neuropsychol Soc. 2013;19(8):938. doi:10.1017/S135561771300069623866100
- Deloire M, Salort E, Bonnet M, et al. Cognitive impairment as marker of diffuse brain abnormalities in early relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76(4):519–526. doi:10.1136/jnnp.2004.04587215774439
- Deloire M, Ruet A, Hamel D, Bonnet M, Brochet B. Early cognitive impairment in multiple sclerosis predicts disability outcome several years later. Multiple Scl J. 2010;16(5):581–587. doi:10.1177/1352458510362819
- Bodini B, Cercignani M, Khaleeli Z, et al. Corpus callosum damage predicts disability progression and cognitive dysfunction in primary‐progressive MS after five years. Hum Brain Mapp. 2013;34(5):1163–1172. doi:10.1002/hbm.2149922328451
- Feuillet L, Reuter F, Audoin B, et al. Early cognitive impairment in patients with clinically isolated syndrome suggestive of multiple sclerosis. Multiple Scl J. 2007;13(1):124–127. doi:10.1177/1352458506071196
- López-Góngora M, Escartín A, Martínez-Horta S, et al. Neurophysiological evidence of compensatory brain mechanisms in early-stage multiple sclerosis. PLoS One. 2015;10(8):e0136786. doi:10.1371/journal.pone.013678626322632
- Nourbakhsh B, Nunan-Saah J, Maghzi A-H, et al. Longitudinal associations between MRI and cognitive changes in very early MS. Mult Scler Relat Disord. 2016;5:47–52. doi:10.1016/j.msard.2015.10.01026856943
- Okada K, Kobata M, Sennari Y, et al. Levels of nitric oxide metabolites in cerebrospinal fluid correlate with cognitive impairment in early stage multiple sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(10):892–893. doi:10.1136/jnnp-2017-31558528446603
- Deloire MS, Bonnet M, Salort E, et al. How to detect cognitive dysfunction at early stages of multiple sclerosis? Multiple Scl J. 2006;12(4):445–452. doi:10.1191/1352458506ms1289oa
- Louapre C, Perlbarg V, García‐Lorenzo D, et al. Brain networks disconnection in early multiple sclerosis cognitive deficits: an anatomofunctional study. Hum Brain Mapp. 2014;35(9):4706–4717. doi:10.1002/hbm.2250524687771
- Schulz D, Kopp B, Kunkel A, Faiss JH. Cognition in the early stage of multiple sclerosis. J Neurol. 2006;253(8):1002–1010. doi:10.1007/s00415-006-0145-816609812
- Glanz B, Holland C, Gauthier S, et al. Cognitive dysfunction in patients with clinically isolated syndromes or newly diagnosed multiple sclerosis. Multiple Scl J. 2007;13(8):1004–1010. doi:10.1177/1352458507077943
- Penny S, Khaleeli Z, Cipolotti L, Thompson A, Ron M. Early imaging predicts later cognitive impairment in primary progressive multiple sclerosis. Neurology. 2010;74(7):545–552. doi:10.1212/WNL.0b013e3181cff6a620157157
- Morelli ME, Baldini S, Sartori A, et al. Early putamen hypertrophy and ongoing hippocampus atrophy predict cognitive performance in the first ten years of relapsing-remitting multiple sclerosis. Neurol Sci. 2020;1–12.33231810
- Reuter F, Zaaraoui W, Crespy L, et al. Frequency of cognitive impairment dramatically increases during the first 5 years of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2011;82(10):1157–1159. doi:10.1136/jnnp.2010.21374420971755
- Planche V, Ruet A, Coupé P, et al. Hippocampal microstructural damage correlates with memory impairment in clinically isolated syndrome suggestive of multiple sclerosis. Multiple Scl J. 2017;23(9):1214–1224. doi:10.1177/1352458516675750
- Pitteri M, Ziccardi S, Dapor C, Guandalini M, Calabrese M. Lost in classification: lower cognitive functioning in apparently cognitive normal newly diagnosed RRMS patients. Brain Sci. 2019;9(11):321. doi:10.3390/brainsci9110321
- Wybrecht D, Reuter F, Pariollaud F, et al. New brain lesions with no impact on physical disability can impact cognition in early multiple sclerosis: a ten-year longitudinal study. PLoS One. 2017;12(11):e0184650. doi:10.1371/journal.pone.018465029149177
- Zaaraoui W, Reuter F, Rico A, et al. Occurrence of neuronal dysfunction during the first 5 years of multiple sclerosis is associated with cognitive deterioration. J Neurol. 2011;258(5):811–819. doi:10.1007/s00415-010-5845-421132325
- Dekker I, Eijlers A, Popescu V, et al. Predicting clinical progression in multiple sclerosis after 6 and 12 years. Eur j Neurol. 2019;26(6):893–902. doi:10.1111/ene.1390430629788
- Jongen PJ, Wesnes K, van Geel B, et al. Relationship between working hours and power of attention, memory, fatigue, depression and self-efficacy one year after diagnosis of clinically isolated syndrome and relapsing remitting multiple sclerosis. PLoS One. 2014;9(5):e96444. doi:10.1371/journal.pone.009644424787714
- Forn C, Rocca MA, Valsasina P, et al. Functional magnetic resonance imaging correlates of cognitive performance in patients with a clinically isolated syndrome suggestive of multiple sclerosis at presentation: an activation and connectivity study. Multiple Scl J. 2012;18(2):153–163. doi:10.1177/1352458511417744
- de Rodez Benavent SA, Nygaard GO, Harbo HF, et al. Fatigue and cognition: pupillary responses to problem‐solving in early multiple sclerosis patients. Brain Behav. 2017;7(7):e00717. doi:10.1002/brb3.71728729927
- Jongen PJ, Wesnes K, van Geel B, et al. Does self-efficacy affect cognitive performance in persons with clinically isolated syndrome and early relapsing remitting multiple sclerosis? Mult Scler Int. 2015;2015. doi:10.1155/2015/960282
- Labiano-Fontcuberta A, Martínez-Ginés ML, Aladro Y, et al. A comparison study of cognitive deficits in radiologically and clinically isolated syndromes. Multiple Scl J. 2016;22(2):250–253. doi:10.1177/1352458515591072
- Ruano L, Portaccio E, Goretti B, et al. Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Multiple Scl J. 2017;23(9):1258–1267. doi:10.1177/1352458516674367
- Caceres F, Vanotti S, Benedict R, Group RW. Cognitive and neuropsychiatric disorders among multiple sclerosis patients from Latin America: results of the RELACCEM study. Mult Scler Relat Disord. 2014;3(3):335–340. doi:10.1016/j.msard.2013.10.00725876470
- Glanz BI, Healy BC, Hviid LE, Chitnis T, Weiner HL. Cognitive deterioration in patients with early multiple sclerosis: a 5-year study. J Neurol Neurosurg Psychiatry. 2012;83(1):38–43. doi:10.1136/jnnp.2010.23783421746743
- Anhoque CF, Biccas-Neto L, Domingues S, Teixeira A, Domingues R. Cognitive impairment and optic nerve axonal loss in patients with clinically isolated syndrome. Clin Neurol Neurosurg. 2013;115(7):1032–1035. doi:10.1016/j.clineuro.2012.10.02523182176
- Reuter F, Zaaraoui W, Crespy L, et al. Cognitive impairment at the onset of multiple sclerosis: relationship to lesion location. Multiple Scl J. 2011;17(6):755–758. doi:10.1177/1352458511398265
- Anhoque CF, Biccas Neto L, Domingues SCA, Teixeira AL, Domingues RB. Cognitive impairment in patients with clinically isolated syndrome. Dementia Neuropsychol. 2012;6(4):266–269. doi:10.1590/S1980-57642012DN06040011
- Potagas C, Giogkaraki E, Koutsis G, et al. Cognitive impairment in different MS subtypes and clinically isolated syndromes. J Neurol Sci. 2008;267(1–2):100–106. doi:10.1016/j.jns.2007.10.00217997417
- Achiron A, Barak Y. Cognitive impairment in probable multiple sclerosis. J Neurol Neurosurg Psychiatry. 2003;74(4):443–446. doi:10.1136/jnnp.74.4.44312640060
- Anhoque CF, Biccas-Neto L, Domingues SCA, Teixeira AL, Domingues RB. Cognitive impairment is correlated with reduced quality of life in patients with clinically isolated syndrome. Arq Neuropsiquiatr. 2013;71(2):74–77. doi:10.1590/S0004-282X201300500000423295369
- Julian L, Serafin D, Charvet L, et al. Cognitive impairment occurs in children and adolescents with multiple sclerosis: results from a United States network. J Child Neurol. 2013;28(1):102–107. doi:10.1177/088307381246481623155206
- Pokryszko-Dragan A, Dziadkowiak E, Zagrajek M, et al. Cognitive performance, fatigue and event-related potentials in patients with clinically isolated syndrome. Clin Neurol Neurosurg. 2016;149:68–74. doi:10.1016/j.clineuro.2016.07.02227484631
- Glukhovsky L, Kurz D, Brandstadter R, et al. Depression and cognitive function in early multiple sclerosis: multitasking is more sensitive than traditional assessments. Multiple Scl J. 2020;1352458520958359.
- Landrø NI, Celius EG, Sletvold H. Depressive symptoms account for deficient information processing speed but not for impaired working memory in early phase multiple sclerosis (MS). J Neurol Sci. 2004;217(2):211–216. doi:10.1016/j.jns.2003.10.01214706226
- Iaffaldano P, Viterbo RG, Goretti B, Portaccio E, Amato MP, Trojano M. Emotional and neutral verbal memory impairment in Multiple Sclerosis. J Neurol Sci. 2014;341(1–2):28–31. doi:10.1016/j.jns.2014.03.03824713509
- Bonnet MC, Deloire MS, Salort E, et al. Evidence of cognitive compensation associated with educational level in early relapsing–remitting multiple sclerosis. J Neurol Sci. 2006;251(1–2):23–28. doi:10.1016/j.jns.2006.08.00217097108
- Nourbakhsh B, Julian L, Waubant E. Fatigue and depression predict quality of life in patients with early multiple sclerosis: a longitudinal study. Eur j Neurol. 2016;23(9):1482–1486. doi:10.1111/ene.1310227416110
- Holland AA, Graves D, Greenberg BM, Harder LL. Fatigue, emotional functioning, and executive dysfunction in pediatric multiple sclerosis. Child Neuropsychol. 2014;20(1):71–85. doi:10.1080/09297049.2012.74888823216329
- Håkansson I, Johansson L, Dahle C, Vrethem M, Ernerudh J. Fatigue scores correlate with other self-assessment data, but not with clinical and biomarker parameters, in CIS and RRMS. Mult Scler Relat Disord. 2019;36:101424. doi:10.1016/j.msard.2019.10142431586802
- Reuter F, Del Cul A, Audoin B, et al. Intact subliminal processing and delayed conscious access in multiple sclerosis. Neuropsychologia. 2007;45(12):2683–2691. doi:10.1016/j.neuropsychologia.2007.04.01017517425
- Heitmann H, Haller B, Tiemann L, et al. Longitudinal prevalence and determinants of pain in multiple sclerosis: results from the German National Multiple Sclerosis Cohort study. Pain. 2020;161(4):787–796. doi:10.1097/j.pain.000000000000176732197038
- Bove R, Musallam A, Healy B, et al. Low testosterone is associated with disability in men with multiple sclerosis. Multiple Scl J. 2014;20(12):1584–1592. doi:10.1177/1352458514527864
- Achiron A, Chapman J, Magalashvili D, et al. Modeling of cognitive impairment by disease duration in multiple sclerosis: a cross-sectional study. PLoS One. 2013;8(8):e71058. doi:10.1371/journal.pone.007105823936485
- Planche V, Ruet A, Charré‐Morin J, Deloire M, Brochet B, Tourdias T. Pattern separation performance is decreased in patients with early multiple sclerosis. Brain Behav. 2017;7(8):e00739. doi:10.1002/brb3.73928828205
- Klineova S, Brandstadter R, Fabian MT, et al. Psychological resilience is linked to motor strength and gait endurance in early multiple sclerosis. Multiple Scl J. 2020;26(9):1111–1120. doi:10.1177/1352458519852725
- Lanzillo R, Chiodi A, Carotenuto A, et al. Quality of life and cognitive functions in early onset multiple sclerosis. Eur j Paediatric Neurol. 2016;20(1):158–163. doi:10.1016/j.ejpn.2015.08.005
- von Bismarck O, Dankowski T, Ambrosius B, et al. Treatment choices and neuropsychological symptoms of a large cohort of early MS. Neurol Neuroimmunol Neuroinflammation. 2018;5:3. doi:10.1212/NXI.0000000000000446
- Dackovic J, Pekmezovic T, Mesaros S, et al. The Rao’s Brief Repeatable Battery in the study of cognition in different multiple sclerosis phenotypes: application of normative data in a Serbian population. Neurol Sci. 2016;37(9):1475–1481. doi:10.1007/s10072-016-2610-127207679
- Skorve E, Lundervold AJ, Torkildsen Ø, Myhr K-M. The Norwegian translation of the brief international cognitive assessment for multiple sclerosis (BICAMS). Mult Scler Relat Disord. 2019;36:101408. doi:10.1016/j.msard.2019.10140831610403
- Landmeyer NC, Dzionsko I, Brockhoff L, et al. The agony of choice? Preserved affective decision making in early multiple sclerosis. Front Neurol. 2020;11:914. doi:10.3389/fneur.2020.0091432982932
- Štecková T, Hluštík P, Sládková V, Odstrčil F, Mareš J, Kaňovský P. Thalamic atrophy and cognitive impairment in clinically isolated syndrome and multiple sclerosis. J Neurol Sci. 2014;342(1–2):62–68. doi:10.1016/j.jns.2014.04.02624819917
- Meijer KA, Eijlers AJ, Geurts JJ, Schoonheim MM. Staging of cortical and deep grey matter functional connectivity changes in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2018;89(2):205–210. doi:10.1136/jnnp-2017-31632928986469
- Wilting J, Rolfsnes HO, Zimmermann H, et al. Structural correlates for fatigue in early relapsing remitting multiple sclerosis. Eur Radiol. 2016;26(2):515–523. doi:10.1007/s00330-015-3857-226026721
- Simioni S, Amarù F, Bonnier G, et al. MP2RAGE provides new clinically-compatible correlates of mild cognitive deficits in relapsing-remitting multiple sclerosis. J Neurol. 2014;261(8):1606–1613. doi:10.1007/s00415-014-7398-424912471
- Mokhber N, Azarpazhooh A, Orouji E, et al. Cognitive dysfunction in patients with multiple sclerosis treated with different types of interferon beta: a randomized clinical trial. J Neurol Sci. 2014;342(1–2):16–20. doi:10.1016/j.jns.2014.01.03824841321
- Tortorella C, Romano R, Direnzo V, et al. Load-dependent dysfunction of the putamen during attentional processing in patients with clinically isolated syndrome suggestive of multiple sclerosis. Multiple Scl J. 2013;19(9):1153–1160. doi:10.1177/1352458512473671
- Penner I-K, Stemper B, Calabrese P, et al. Effects of interferon beta-1b on cognitive performance in patients with a first event suggestive of multiple sclerosis. Multiple Scl j. 2012;18(10):1466–1471. doi:10.1177/1352458512442438
- Audoin B, Zaaraoui W, Reuter F, et al. Atrophy mainly affects the limbic system and the deep grey matter at the first stage of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2010;81(6):690–695. doi:10.1136/jnnp.2009.18874820392976
- Staffen W, Mair A, Zauner H, et al. Cognitive function and fMRI in patients with multiple sclerosis: evidence for compensatory cortical activation during an attention task. Brain. 2002;125(6):1275–1282. doi:10.1093/brain/awf12512023316
- Cirillo S, Rocca MA, Ghezzi A, et al. Abnormal cerebellar functional MRI connectivity in patients with paediatric multiple sclerosis. Multiple Scl J. 2016;22(3):292–301. doi:10.1177/1352458515592191
- Mathiesen HK, Jonsson A, Tscherning T, et al. Correlation of global N-acetyl aspartate with cognitive impairment in multiple sclerosis. Arch Neurol. 2006;63(4):533–536. doi:10.1001/archneur.63.4.53316606765
- Leavitt VM, Brandstadter R, Fabian M, et al. Dissociable cognitive patterns related to depression and anxiety in multiple sclerosis. Multiple Scl J. 2020;26(10):1247–1255. doi:10.1177/1352458519860319
- Shaheen HA, Sayed SS, Daker LI, AbdelAziz HE, Taha MA. Does vitamin D deficiency predict early conversion of clinically isolated syndrome? A preliminary Egyptian study. Int J Neurosci. 2018;128(10):946–951. doi:10.1080/00207454.2018.144695429493311
- Johnen A, Elpers C, Riepl E, et al. Early effective treatment may protect from cognitive decline in paediatric multiple sclerosis. Eur J Paediatric Neurol. 2019;23(6):783–791. doi:10.1016/j.ejpn.2019.08.007
- Kocer B, Unal T, Nazliel B, et al. Evaluating sub-clinical cognitive dysfunction and event-related potentials (P300) in clinically isolated syndrome. Neurol Sci. 2008;29(6):435–444. doi:10.1007/s10072-008-1020-419002651
- Nygaard GO, Sigrid A, Harbo HF, et al. Eye and hand motor interactions with the Symbol Digit Modalities Test in early multiple sclerosis. Mult Scler Relat Disord. 2015;4(6):585–589. doi:10.1016/j.msard.2015.08.00326590666
- Gajamange S, Shelton A, Clough M, White O, Fielding J, Kolbe S. Functional correlates of cognitive dysfunction in clinically isolated syndromes. PLoS One. 2019;14(7):e0219590. doi:10.1371/journal.pone.021959031314815
- Köhler W, Fischer M, Bublak P, et al. Information processing deficits as a driving force for memory impairment in MS: a cross-sectional study of memory functions and MRI in early and late stage MS. Mult Scler Relat Disord. 2017;18:119–127. doi:10.1016/j.msard.2017.09.02629141793
- Engel S, Graetz C, Salmen A, et al. Is APOE ε4 associated with cognitive performance in early MS? Neurol Neuroimmunol Neuroinflammation. 2020;7:4. doi:10.1212/NXI.0000000000000728
- Charvet L, O’donnell E, Belman A, et al. Longitudinal evaluation of cognitive functioning in pediatric multiple sclerosis: report from the US Pediatric Multiple Sclerosis Network. Multiple Scl J. 2014;20(11):1502–1510. doi:10.1177/1352458514527862
- Guenter W, Bieliński M, Bonek R, Borkowska A. neurochemical changes in the brain and neuropsychiatric symptoms in clinically isolated syndrome. J Clin Med. 2020;9(12):3909. doi:10.3390/jcm9123909
- Clough M, Millist L, Lizak N, et al. Ocular motor measures of cognitive dysfunction in multiple sclerosis I: inhibitory control. J Neurol. 2015;262(5):1130–1137. doi:10.1007/s00415-015-7645-325851743
- Clough M, Mitchell L, Millist L, et al. Ocular motor measures of cognitive dysfunction in multiple sclerosis II: working memory. J Neurol. 2015;262(5):1138–1147. doi:10.1007/s00415-015-7644-425851742
- Moroso A, Ruet A, Lamargue-Hamel D, et al. Preliminary evidence of the cerebellar role on cognitive performances in clinically isolated syndrome. J Neurol Sci. 2018;385:1–6. doi:10.1016/j.jns.2017.11.03729406885
- El Ayoubi NK, Ghassan S, Said M, Allam J, Darwish H, Khoury SJ. Retinal measures correlate with cognitive and physical disability in early multiple sclerosis. J Neurol. 2016;263(11):2287–2295. doi:10.1007/s00415-016-8271-427544501
- Koubiyr I, Deloire M, Brochet B, et al. Structural constraints of functional connectivity drive cognitive impairment in the early stages of multiple sclerosis. Multiple Scl J. 2020;1352458520971807.
- Marstrand L, Østerberg O, Walsted T, Skov AC, Schreiber KI, Sellebjerg F. Brief international cognitive assessment for multiple sclerosis (BICAMS): a danish validation study of sensitivity in early stages of MS. Mult Scler Relat Disord. 2020;37:101458. doi:10.1016/j.msard.2019.10145831683230
- Kern KC, Gold SM, Lee B, et al. Thalamic–hippocampal–prefrontal disruption in relapsing–remitting multiple sclerosis. NeuroImage. 2015;8:440–447. doi:10.1016/j.nicl.2014.12.01526106524
- Cohen M, Brochet B, Clavelou P, et al. Cognition and quality of life in clinically isolated syndrome patients starting a disease modifying therapy in the QUALICIS study may not predict treatment response at one year. J Neurol Sci. 2017;382:73–78. doi:10.1016/j.jns.2017.09.03029111024
- Haase C, Tinnefeld M, Daum I, Ganz R, Haupts M, Faustmann P. Cognitive, but not mood dysfunction develops in multiple sclerosis during 7 years of follow-up. Eur Neurol. 2004;52(2):92–95. doi:10.1159/00007993715273430
- Hashim NA, Ismail NA, Emad EM. Evolving relationship between respiratory functions & impairment in sleep and cognition in patients with multiple sclerosis. Mult Scler Relat Disord. 2020;46:102514. doi:10.1016/j.msard.2020.10251432992131
- Eva N, Adam K, Tomáš N, Martin V, Eva M, Jan L. Spatial navigation in early multiple sclerosis: a neglected cognitive marker of the disease? J Neurol. 2020;1–13.
- Brenk A, Laun K, Haase C. Short-term cognitive training improves mental efficiency and mood in patients with multiple sclerosis. Eur Neurol. 2008;60(6):304–309. doi:10.1159/00015788518824859
- Patti F, Amato M, Bastianello S, et al. Effects of immunomodulatory treatment with subcutaneous interferon beta-1a on cognitive decline in mildly disabled patients with relapsing—remitting multiple sclerosis. Multiple Scl J. 2010;16(1):68–77. doi:10.1177/1352458509350309
- Berard JA, Smith AM, Walker LA. A longitudinal evaluation of cognitive fatigue on a task of sustained attention in early relapsing-remitting multiple sclerosis. Int J MS Care. 2018;20(2):55–61. doi:10.7224/1537-2073.2016-10629670491
- Said M, El Ayoubi NK, Hannoun S, et al. The Bayesian risk estimate at onset (BREMSO) correlates with cognitive and physical disability in patients with early multiple sclerosis. Mult Scler Relat Disord. 2018;26:96–102. doi:10.1016/j.msard.2018.09.00330243236
- Kraemer M, Herold M, Uekermann J, et al. Perception of affective prosody in patients at an early stage of relapsing–remitting multiple sclerosis. J Neuropsychol. 2013;7(1):91–106. doi:10.1111/j.1748-6653.2012.02037.x23126275
- Kraemer M, Herold M, Uekermann J, et al. Theory of mind and empathy in patients at an early stage of relapsing remitting multiple sclerosis. Clin Neurol Neurosurg. 2013;115(7):1016–1022. doi:10.1016/j.clineuro.2012.10.02723199520
- Simioni S, Ruffieux C, Bruggimann L, Annoni J, Schluep M. Cognition, mood and fatigue in patients in the early stage of multiple sclerosis. Swiss Med Wkly. 2007;137(35–36):496–501.17990136
- Roca M, Torralva T, Meli F, et al. Cognitive deficits in multiple sclerosis correlate with changes in fronto-subcortical tracts. Multiple Scl J. 2008;14(3):364–369. doi:10.1177/1352458507084270
- Olivares T, Nieto A, Sánchez M, Wollmann T, Hernández M, Barroso J. Pattern of neuropsychological impairment in the early phase of relapsing-remitting multiple sclerosis. Multiple Scl J. 2005;11(2):191–197. doi:10.1191/1352458505ms1139oa
- Caputi N, Matrella A, Totaro R, et al. Object decision and multiple sclerosis: a preliminary study. Funct Neurol. 2017;32(2):69. doi:10.11138/FNeur/2017.32.2.06928676139
- Amato M, Bartolozzi M, Zipoli V, et al. Neocortical volume decrease in relapsing–remitting MS patients with mild cognitive impairment. Neurology. 2004;63(1):89–93. doi:10.1212/01.WNL.0000129544.79539.D515249616
- Zivadinov R, De Masi R, Nasuelli D, et al. MRI techniques and cognitive impairment in the early phase of relapsing-remitting multiple sclerosis. Neuroradiology. 2001;43(4):272–278. doi:10.1007/s00234000050011338408
- Barbu RM, Berard JA, Gresham LM, Walker LA. Longitudinal stability of cognition in early-phase relapsing-remitting multiple sclerosis: does cognitive reserve play a role? Int J MS Care. 2018;20(4):173–179. doi:10.7224/1537-2073.2016-07330150901
- Amato MP, Ponziani G, Pracucci G, Bracco L, Siracusa G, Amaducci L. Cognitive impairment in early-onset multiple sclerosis: pattern, predictors, and impact on everyday life in a 4-year follow-up. Arch Neurol. 1995;52(2):168–172. doi:10.1001/archneur.1995.005402600720197848126
- Amato MP, Ponziani G, Siracusa G, Sorbi S. Cognitive dysfunction in early-onset multiple sclerosis: a reappraisal after 10 years. Arch Neurol. 2001;58(10):1602–1606. doi:10.1001/archneur.58.10.160211594918
- Rojas JI, Murphy G, Sanchez F, et al. Thalamus volume change and cognitive impairment in early relapsing–remitting multiple sclerosis patients. Neuroradiol J. 2018;31(4):350–355. doi:10.1177/197140091878197729869576
- Amato MP, Portaccio E, Goretti B, et al. Relevance of cognitive deterioration in early relapsing-remitting MS: a 3-year follow-up study. Multiple Scl J. 2010;16(12):1474–1482. doi:10.1177/1352458510380089
- Summers M, Fisniku L, Anderson V, Miller D, Cipolotti L, Ron M. Cognitive impairment in relapsing—remitting multiple sclerosis can be predicted by imaging performed several years earlier. Multiple Scl J. 2008;14(2):197–204. doi:10.1177/1352458507082353
- Panou T, Mastorodemos V, Papadaki E, Simos PG, Plaitakis A. Early signs of memory impairment among multiple sclerosis patients with clinically isolated syndrome. Behav Neurol. 2012;25(4):311–326. doi:10.1155/2012/10547122713377
- Rimkus C, Junqueira T, Lyra KP, et al. Corpus callosum microstructural changes correlate with cognitive dysfunction in early stages of relapsing-remitting multiple sclerosis: axial and radial diffusivities approach. Mult Scler Int. 2011;2011.
- Prokopova B, Hlavacova N, Vlcek M, et al. Early cognitive impairment along with decreased stress-induced BDNF in male and female patients with newly diagnosed multiple sclerosis. J Neuroimmunol. 2017;302:34–40. doi:10.1016/j.jneuroim.2016.11.00727979325
- Wuerfel E, Weddige A, Hagmayer Y, et al. Cognitive deficits including executive functioning in relation to clinical parameters in paediatric MS patients. PLoS One. 2018;13(3):e0194873. doi:10.1371/journal.pone.019487329566099
- Haase CG, Tinnefeld M, Lienemann M, Ganz RE, Faustmann PM. Depression and cognitive impairment in disability-free early multiple sclerosis. Behav Neurol. 2003;14(1–2):39–45. doi:10.1155/2003/84376012719637
- Hyncicova E, Kalina A, Vyhnalek M, et al. Health-related quality of life, neuropsychiatric symptoms and structural brain changes in clinically isolated syndrome. PLoS One. 2018;13(7):e0200254. doi:10.1371/journal.pone.020025429979757
- Guevara C, Villa E, Diaz V. et al. Inclusion of the Symbol Digit Modalities Test in a revised assessment of ‘no evidence of disease activity-4 (NEDA-4)’in Latin-American patients with multiple sclerosis. Mult Scler Relat Disord;2020 102076. doi:10.1016/j.msard.2020.10207632361478
- Schmidt SL, da Silva MS, Schmidt JJ, et al. Neuropsychiatric assessments in patients with multiple sclerosis in early phases and with low disability. Neuropsychiatr Dis Treat. 2018;14:1665. doi:10.2147/NDT.S16348029950848
- Lima FS, Simioni S, Bruggimann L, et al. Perceived behavioral changes in early multiple sclerosis. Behav Neurol. 2007;18(2):81–90. doi:10.1155/2007/67407517538194
- Simioni S, Ruffieux C, Kleeberg J, Bruggimann L, Annoni J-M, Schluep M. Preserved decision making ability in early multiple sclerosis. J Neurol. 2008;255(11):1762–1769. doi:10.1007/s00415-008-0025-519009335
- Hayter AL, Salkovskis PM, Silber E, Morris RG. The impact of health anxiety in patients with relapsing remitting multiple sclerosis: misperception, misattribution and quality of life. Br J Clin Psychol. 2016;55(4):371–386. doi:10.1111/bjc.1210626806805
- Hynčicová E, Vyhnálek M, Kalina A, et al. Cognitive impairment and structural brain changes in patients with clinically isolated syndrome at high risk for multiple sclerosis. J Neurol. 2017;264(3):482–493. doi:10.1007/s00415-016-8368-928028623
- Zivadinov R, Sepcic J, Nasuelli D, et al. A longitudinal study of brain atrophy and cognitive disturbances in the early phase of relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2001;70(6):773–780. doi:10.1136/jnnp.70.6.77311385012
- Crivelli L, Farez MF, González CD, et al. Alerting network dysfunction in early multiple sclerosis. J Int Neuropsychol Soc. 2012;18(4):757. doi:10.1017/S135561771200041022621916
- Cinar BP, Kösehasanoğulları G, Yigit P, Ozakbas S. Cognitive dysfunction in patients with multiple sclerosis treated with first-line disease-modifying therapy: a multi-center, controlled study using the BICAMS battery. Neurol Sci. 2017;38(2):337–342. doi:10.1007/s10072-016-2775-727885448
- Tabrizi YM, Mazhari S, Nazari MA, Zangiabadi N, Sheibani V, Azarang S. Compromised motor imagery ability in individuals with multiple sclerosis and mild physical disability: an ERP study. Clin Neurol Neurosurg. 2013;115(9):1738–1744. doi:10.1016/j.clineuro.2013.04.00223639730
- Uher T, Blahova-Dusankova J, Horakova D, et al. Longitudinal MRI and neuropsychological assessment of patients with clinically isolated syndrome. J Neurol. 2014;261(9):1735–1744. doi:10.1007/s00415-014-7413-924952618
- Margoni M, Rinaldi F, Riccardi A, Franciotta S, Perini P, Gallo P. No evidence of disease activity including cognition (NEDA-3 plus) in naïve pediatric multiple sclerosis patients treated with natalizumab. J Neurol. 2020;267(1):100–105. doi:10.1007/s00415-019-09554-z31562558
- Perumal J, Fox RJ, Balabanov R, et al. Outcomes of natalizumab treatment within 3 years of relapsing-remitting multiple sclerosis diagnosis: a prespecified 2-year interim analysis of STRIVE. BMC Neurol. 2019;19(1):116. doi:10.1186/s12883-019-1337-z31176355
- Jakob GB, Remšak T, Jazbec SŠ, Ledinek AH, Rot U. Step initiation interferes with working memory in nondisabled patients with the earliest multiple sclerosis–a dual-task study. Gait Posture. 2017;51:201–207. doi:10.1016/j.gaitpost.2016.10.01627816048
- Hänninen K, Viitala M, Paavilainen T, et al. Thalamic atrophy without whole brain atrophy is associated with absence of 2-year NEDA in multiple sclerosis. Front Neurol. 2019;10:459. doi:10.3389/fneur.2019.0045931130911
- Friedova L, Motyl J, Srpova B, et al. The weak association between neurofilament levels at multiple sclerosis onset and cognitive performance after 9 years. Mult Scler Relat Disord. 2020;46:102534. doi:10.1016/j.msard.2020.10253433032055
- Langdon D, Amato M, Boringa J, et al. Recommendations for a brief international cognitive assessment for multiple sclerosis (BICAMS). Multiple Scl J. 2012;18(6):891–898. doi:10.1177/1352458511431076
- Smith A. Symbol Digit Modalities Test: Manual. Los Angeles, CA: Western Psychological Services; 1982.
- Gronwall D. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44(2):367–373. doi:10.2466/pms.1977.44.2.367866038
- Clemens L, Langdon D. How does cognition relate to employment in multiple sclerosis? A systematic review. Mult Scler Relat Disord. 2018;26:183–191. doi:10.1016/j.msard.2018.09.01830268039
- Boeschoten RE, Braamse AM, Beekman AT, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331–341. doi:10.1016/j.jns.2016.11.06728017241
- Arnett PA, Strober LB. Cognitive and neurobehavioral features in multiple sclerosis. Expert Rev Neurother. 2011;11(3):411–424. doi:10.1586/ern.11.1221375446
- Kalb R, Beier M, Benedict RH, et al. Recommendations for cognitive screening and management in multiple sclerosis care. Multiple Scl J. 2018;24(13):1665–1680. doi:10.1177/1352458518803785
- Benedict RHB, Amato MP, Boringa J, et al. Brief International Cognitive Assessment for MS (BICAMS): international standards for validation. BMC Neurol. 2012;12(1):55. doi:10.1186/1471-2377-12-5522799620
- Gromisch ES, Zemon V, Benedict RHB, et al. Using a highly abbreviated California Verbal Learning Test-II to detect verbal memory deficits. Multiple Scl J. 2013;19:498–501. doi:10.1177/1352458512454347
- Gromisch ES, Zemon V, Holtzer R, et al. Assessing the criterion validity of four highly abbreviated measures from the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS). Clin Neuropsychol. 2016;30(7):1032–1049. doi:10.1080/13854046.2016.118959727279357
- Gromisch ES, Portnoy JG, Foley FW. Comparison of the abbreviated minimal assessment of cognitive function in multiple sclerosis (aMACFIMS) and the brief international cognitive assessment for multiple sclerosis (BICAMS). J Neurol Sci. 2018;388:70–75. doi:10.1016/j.jns.2018.03.01229627034
- Gromisch ES, Foley FW. Can semantic fluency be used as an alternative or additional measure in the abbreviated Minimal Assessment of Cognitive Function in Multiple Sclerosis (aMACFIMS)? J Neurol Sci. 2020;410:116640. doi:10.1016/j.jns.2019.11664031884353
- Marrie RA, Patel R, Figley CR, et al. Diabetes and anxiety adversely affect cognition in multiple sclerosis. Mult Scler Relat Disord. 2019;27:164–170. doi:10.1016/j.msard.2018.10.01830384203
- Benedict RHB, Fischer JS, Archibald CJ, et al. Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol. 2002;16:381–397. doi:10.1076/clin.16.3.381.1385912607150
- Rao S. A manual for the brief repeatable battery of neuropsychological tests in multiple sclerosis. Milwaukee. 1990;1696.
- Foley FW, Benedict RHB, Gromisch ES, DeLuca J. The need for screening, assessment, and treatment for cognitive dysfunction in multiple sclerosis: results of a multidisciplinary CMSC consensus conference, September 24, 2010. Int J MS Care. 2012;14(2):58–64. doi:10.7224/1537-2073-14.2.5824453735
- Gromisch ES, Kulas JF, Altalib H. et al. Neuropsychological assessments and psychotherapeutic services in Veterans with multiple sclerosis: rates of utilization and their associations with socio-demographics and clinical characteristics using Veterans Health Administration-based data. Mult Scler Relat Disord;2020 102220. doi:10.1016/j.msard.2020.10222032480347
- Benedict RHB, Cookfair D, Gavett R, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc. 2006;12(4):549–558. doi:10.1017/S135561770606072316981607
- Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve and cognitive reserve in multiple sclerosis: what you’ve got and how you use it. Neurology. 2013;80(24):2186–2193. doi:10.1212/WNL.0b013e318296e98b23667062
- Schwartz CE, Ayandeh A, Ramanathan M, et al. Reserve-building activities in multiple sclerosis patients and healthy controls: a descriptive study. BMC Neurol. 2015;15:135. doi:10.1186/s12883-015-0395-026264858
- Andaloro A, Russo M, Pastura C, et al. Is there a correlation between dyslipidemia and cognitive impairment in patients with multiple sclerosis? Int J Neurosci. 2020;1–6.
- Owji M, Ashraf-Ganjouei A, Sahraian MA, Bidadian M, Ghadiri F, Moghadasi AN. The relationship between cognitive function and body mass index in multiple sclerosis patients. Mult Scler Relat Disord. 2019;32:37–40. doi:10.1016/j.msard.2019.04.02431030017
- Noori H, Gheini MR, Rezaeimanesh N, et al. The correlation between dyslipidemia and cognitive impairment in multiple sclerosis patients. Mult Scler Relat Disord. 2019;36:101415. doi:10.1016/j.msard.2019.10141531586799
- Hernández-Ledesma AL, Rodríguez-Méndez AJ, Gallardo-Vidal LS, et al. Lipid profile: causal relationship on cognitive performance in multiple sclerosis? Mol Biol Rep. 2020;47(12):9667–9676. doi:10.1007/s11033-020-06011-333259011
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group (2020). Preferred Reporting Items for Systematic Reviews and Meta Analyses: The PRISMA Statement PLoS Med6(7): e1000097. doi:10.1371/journal.pmed.1000097
