Abstract
Studies have shown that Ras homolog enriched in striatum (Rhes) proteins are highly expressed in areas of the central nervous system that have high dopaminergic innervation. In this study, we used Rhes mutant mice (Wild type, Rhes KO, Rhes Heterozygous) of both sexes to explore differences in the effects of Rhes protein levels in basal levels of activity, anxiety, and stereotypy, in relation to sex. Adult male and female mice were evaluated in an open field test for measuring basal levels of activity and anxiety for 5 consecutive days, and they were tested in the apomorphine-induced stereotypy paradigm. Rhes protein levels affected basal levels of activity but it was not found to be related to sex differences. Moreover, a decrease in Rhes protein levels was linked to a nonsignificant anxiolytic effect, mainly in female mice. Finally, a decrease in Rhes protein levels does not affect dopamine D1 and D2 receptor (D1/D2) synergism in female or male mice. Together, these results suggest that Rhes protein levels affect locomotion activity, and have an influence in anxiety depending on sex; Rhes protein levels do not affect D1/D2 synergism in both sexes.
Introduction
Dopamine (DA) is a catecholamine that serves as a major neurotransmitter, mediating diverse behaviors like movement, attention, motivation, and cognition.Citation1 DA receptors are normally divided in D1 and D2 subfamilies. The D1 subfamily is composed of the D1 and D5 subtypes, and the D2 subfamily composed of the D2, D3, and D4 subtypes.Citation2 Under normal conditions, the majority of motor behaviors are mediated by the combined action of the D1 and D2 subtypes rather than the other subtypes.Citation3,Citation4 This combined action of D1 and D2 subtypes is known as D1/D2 receptor synergism.Citation5,Citation6 The D1/D2 receptor synergism has been supported empirically by studies at different levels: behavioral, electrophysiological, and gene expression in the striatum.Citation6–Citation10 However, the mechanism subjacent to this synergism has not been discovered. In order to explain this, it is necessary to elucidate the distribution of D1 and D2 receptors at the level of the striatum, but this has remained theoretically controversial; in effect, information on this topic is complicated. Theories range from complete colocalization to total segregation in striatal neurons.Citation11 Interaction of D1 and D2 receptors subserving synergism could be intercellular, intracellular, or combination of both.
Furthermore, DA receptors are functionally dynamic, altering their sensitivity to agonists in response to changes in the extracellular environment. For instance, following depletion of endogenous DA by means of selective destruction of mesostriatal DA projection with 6-hydroxydopamine (6-OHDA), there is a consequent breakdown in D1/D2 synergism.Citation12,Citation13 This breakdown in D1/D2 synergism can also be generated by treatment with reserpine, a catecholamine depleting agent.Citation14,Citation15 Under both of these conditions, independent stimulation of either D1 or D2 receptors can elicit the full expression of DA-induced behaviors. This breakdown of synergism is invariably associated with profound supersensitivity of both D1 and D2 receptors. It is necessary to consider that the increase in DA receptor sensitivity is not necessarily correlated to an increase in DA receptor density, a phenomenon known as DA receptor dissociation. Moreover, the D1/D2 synergism is independent of D1 and D2 receptor density.Citation15
Ras homolog enriched in striatum (Rhes) protein is a novel striatal specific Ras-like small G protein very similar to Dexras-1.Citation16 Rhes proteins are expressed in different areas of the central nervous system, but mainly in the striatum and olfactory tubercle.Citation16–Citation18 In addition, Rhes mRNA is particularly enriched in brain regions that receive dopaminergic inputs, such as the Striatum and Nucleus Accumbens.Citation17 Rhes mRNA is also found in low to moderate levels in other areas of the nervous system, such as the cerebellum, thalamus, hippocampus, and olfactory bulb during the early stages of postnatal development.Citation17,Citation19 Rhes mRNA in mammalian brains is localized in D1R and D2R bearing striatal projection neurons.Citation20 In rodents, the modulation of Rhes protein expression in the basal ganglia depends mainly on thyroid hormone levels during early stages of development, but depends on dopamine levels and DA innervations during the adult stages.Citation16,Citation19
In general, there is a consensus that Rhes proteins could directly modulate the activation of the heterotrimeric G proteins to which G protein-coupled receptors (GPCRs) are coupled.Citation21,Citation22 Vargiu et al proposed possible explanations for the mechanism of interaction between Rhes and GPCR, and the inhibitory effect of activated D2 receptor on cyclic adenosine monophosphate (cAMP).Citation18 This study also reported that Rhes proteins exert their effects by interacting with G-alpha inhibitory proteins (Gα/i).
Previous studies have shown that decreases in mRNA levels occur after manipulations of the dopaminergic system of the basal ganglia in adult animals (eg, DA depletion by 6-OHDA or reserpine treatment), which results in consequent dopamine receptor supersensitivity and breakdown of the D1/D2 receptor synergism.Citation17 Rhes protein levels are also decreased under similar conditions of dopamine receptor supersensitivity and the breakdown of D1/D2 synergism.Citation17
In striatum, there is a relationship among the expression of Rhes proteins, the degree of DA receptor sensitivity, D1/D2 synergism, and the levels of dopamine neurotransmitters. Conditions that result in the breakdown of D1/D2 synergism and DA receptor supersensitivity (ie, DA depletion by 6-OHDA or reserpine treatment) invariably cause a decrease in Rhes mRNA levels in the striatum.Citation17 Possible mechanisms by which Rhes protein levels affect DA receptor signaling have been proposed. Errico et al reported that in mutant mice, a decrease in Rhes proteins results in D1R increased cAMP/ protein kinase A (PKA) signaling mediated by G-olfactory (olf) proteins, and decreased coupling efficiency of D2 receptor to its downstream Gi/o proteins.Citation20 Another study by Harrison et al added that Rhes proteins physically interact with Gα/i and can interfere with adenylyl cyclase (AC) activation by the G-olf coupled D1 receptor in a pertussis toxin (PTX)-sensitive manner.Citation23 Furthermore, Thapliyal et al reported that Rhes proteins inhibit N-type calcium channels, but decrease the ability of ligands for Gi/o-coupled receptors to inhibit these channels, an effect mediated by β-γ subunits released from PTX-sensitive G proteins.Citation24
At a more behavioral level, the striatal Rhes protein has been linked to the inhibition of dopamine mediated behaviors in Rhes knockout (KO) mice. For instance, Rhes KO males denoted enhanced stereotypy after dopamine drug agonism.Citation25 Furthermore, Rhes knockout mice exhibited a higher D2 receptor antagonist-induced catalepsy than Rhes wild type (WT) mice.Citation20
Other behavioral studies by Errico et al reported that Rhes KO mice displayed hyperactivity and increased locomotor response in the novelty-induced exploration task and open field test, respectively.Citation20 However, no differences were found between KO and WT mice in prepulse inhibition on their startle reflex, nor in motor coordination in the acelerating rotarod paradigm test. Furthermore, SKF 81297, a full D1R agonist, produced a higher enhancement of basal levels of activity in Rhes KO compared to their WT counterparts at doses of 1.5 mg/kg and 5 mg/kg.Citation20 On the other hand, D1R agonist SKF 38393 induces less of an increase in grooming behavior in Rhes mutant mice compared to their WT counterparts.Citation25
In addition, the postsynaptic D2R-like antagonist haloperidol induced higher cataleptic behavior in Rhes KO compared to WT mice at all doses (0.25, 0.5, and 1.5 mg/kg). However, it seems that Rhes protein levels do not affect presynaptic D2R functioning as evaluated by locomotor responses to quinpirole at different doses as there were no differences between WT and KO mice. In summary, Errico et al’s behavioral studies concluded that Rhes protein levels act by modulating the motor output associated with postsynaptic dopamine receptors transmission, and that decreasing Rhes protein levels facilitates the motor stimulation induced by a D1R agonist and strengthens the cataleptic response induced by the administration of a D2R antagonist.Citation20
Another preliminary study by Spano et al showed that Rhes KO mice displayed a sex dependent increase in anxiety levels, deficits in motor coordination, while learning and memory processes remained unchanged.Citation26 The present study evaluated whether or not there were sex related differences in the behavioral effects of Rhes protein levels on dopamine mediated behaviors, and assesses in both sexes the hypothesis that Rhes protein levels are causally related to the state of D1/D2 synergism and the degree of D1 and D2 receptor sensitivity.
Materials and methods
Subjects
In the present study, we used Rhes mutant mice of both sexes that had been backcrossed for ten generations on C57/Bl6 background, and bred at the University of New Orleans.Citation26 Mice employed were 2 to 3 months old, and divided in 3 genotypes: WT, KO, and heterozygous (Het). The number of mice totaled 58; 26 females (n ≥ 8, per genotype group), and 32 males (n ≥ 9, per genotype group). The mice were kept in same sex cages, in groups, with free access to food and water. Artificial lighting was provided from 7:00 am to 7:00 pm. The present study followed the guidelines of the NIH regarding the care and use of animals for experimental procedures, and the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of New Orleans. Every effort was performed to minimize animal discomfort and to reduce the number of animals used. For controlling pain levels, mice were deeply anesthetized with intraperitoneal (IP) injections of tribromoethanol (100–150 mg per 10 g of body weight) for the tail biopsy procedures.
Open field (basal levels of activity and anxiety)
To determine whether the presence or absence of Rhes protein levels plays a role in basal levels of activity and anxiety, mice were placed in an open field arena (43.2 cm long by 33 cm wide by 32 cm high) for 30 minutes, for 5 consecutive days. During the test, the distance traveled in the arena field and the time spent in the periphery of the open field (thigmotaxis) were recorded by a digital video camera (Sony Corporation of America, New York, NY) and the video signal was sent directly to a computerized behavior analysis system (SMART System, Harvard Apparatus, Holliston, MA). The time the mice spent in thigmotaxis was used as an index of anxiety. The periphery and center of the arena was defined as: the total area of the arena (1426 cm2) divided in two concentric rectangles (two areas of 713 cm2) with sides parallel to the arena limits. Thus, the total area of the arena was divided into 2 equal parts (center and outside) with the same probability of being occupied.
Stereotypy test
We tested whether Rhes protein levels affected D1/D2 synergism and DA receptor sensitivity by measuring stereotypy in response to dopaminergic drug administration. Stereotypy was used since it is a well characterized behavioral measure of D1/D2 synergism.Citation27
Species-specific stereotyped behavior was recorded under each of the four following conditions: (1) stimulation of neither receptor subtype (two saline injections at the scheduled time points), (2) stimulation of both D1 and D2 receptors [injection of saline 30 minutes prior to injection of the mixed D1/D2 agonist apomorphine (3 mg/kg)], (3) stimulation of D2 receptors alone [injection of the D1 antagonist SCH 23390 (0.1 mg/kg) 30 minutes prior to an injection of apomorphine (3 mg/kg)], (4) stimulation of D1 receptors alone [injection of the selective D2 antagonist eticlopride (0.3 mg/kg) 30 minutes prior to injection of apomorphine (3.0 mg/kg)]. All injections were IP, and every mouse was subjected to all conditions using a Latin square design in four sessions, each separated by 72–96 hours. This treatment regimen was used rather than selective agonists in order to eliminate the effects of endogenous DA at the heterotypic receptor. Each mouse was placed in a Plexiglas cylinder (measuring 22 cm high and 10.2 cm in diameter) with a thin layer of wood chip bedding on the floor. After 30 minutes of habituation, the appropriate pretreatment injection was given, followed 30 minutes later by an injection of apomorphine or saline. The testing session was terminated 60 minutes later. Behavior was recorded with a Sony digital video camera for subsequent analysis and rated on a scale of 0 to 5 every 5 minutes during a 30 second observation period. The behavioral analyst was blind to the genotype or pharmacological treatment. This rating scale, which was modified for mice from a similar study in rats, has been previously employed in other mutant mouse studies and is defined as follows: 0 = still; 1 = grooming or normal exploration; 2 = discontinuous unfocused stereotypy (eg, brief episodes of strong sniffing); 3 = continuous unfocused stereotypy behavior (ie, stereotypy directed to multiple objects and/or surfaces); 4 = continuous focused sniffing (ie, sniffing of one object or within a highly circumscribed area); 5 = continuous focused oral stereotypy (ie, licking/chewing of one object or within a highly circumscribed area).Citation15,Citation27 Evaluation of the D1 receptor behavior was described in a previous related work.Citation25 Specifically, grooming in response to the D1 agonist SKF 38393 was observed. Male mice were placed in Plexiglas cylinders for 30 minutes of habituation, then mice received an injection of SKF 38393 (10 mg/kg, IP), and returned to the testing cylinders, and their behavior was recorded for the next hour. The amount of grooming (total seconds spent grooming every 5 minutes during a 60 second observation period) was recorded by an experimenter unaware of genotype.
Statistical analysis
Statistical analysis was performed using Analysis of Variance (ANOVA) factorial with post hoc tests. Significance was set at P value of <0.05. IBM SPSS Statistics (v 19; Armonk, NY) for Windows program was employed for statistical analysis. The numerical values described in the Results section represent the Mean ± Standard Error of the Mean (SE).
Results
The data for all the behavioral experiments were analyzed by a mixed repeated measures design with the IBM SPSS Statistics 19 for Windows. shows a general summary of experimental data for all behavioral tests.
Table 1 Data summary
Basal levels of activity
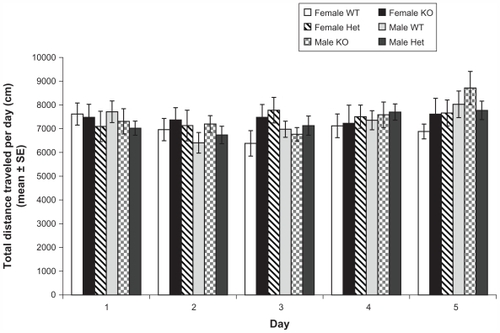
shows locomotion traveled distance across 5 days (30 minutes per day). Mixed repeated measures ANOVA of traveled distance showed significant main effect of days (Wilks’ Lambda: 0.8, F (4, 64) = 5.2, P < 0.01), a significant main effect of genotype (Wilks’ Lambda: 0.8, F (8, 128) = 2.3, P < 0.02), and significant main effect of sex (Wilks’ Lambda: 0.9, F (4, 64) = 2.6, P < 0.05), but a nonsignificant days × genotype × sex interaction (Wilks’ Lambda: 0.9, F (8, 128) = 1.1, P = 0.4). Subsequent analysis of the main effect of days with t student comparisons revealed that the distance traveled on day 1 (7425.7 cm ± 184.6) was significantly different from distances traveled on day 2 (7167.0 cm ± 172.3), day 4 (7296.2 cm ± 165.4), and day 5 (7594.8 cm ± 170.9). The distance traveled on day 3 (6986.9 cm ± 143.2) also differed from the distance traveled on day 5 (7594.8 cm ± 170.9) (P < 0.001, all contrasts). Further analysis of the main effect of genotype with t student comparisons revealed that WT mice (7848.8 cm ± 269.2) displayed higher levels of activity compared to Het mice (7053.7 cm ± 312.6) on day 1 (t (16) = 2.6, P < 0.02); however, WT mice (6489.7 cm ± 236.9) showed lower levels of activity than KO mice (7220.1 cm ± 182.4) on day 3 [t (20) = −2.2, P < 0.05]. On day 5, KO mice (8160.1 cm ± 372.6) exhibited higher levels of activity than Het mice (7280.9 cm ± 156.7) (t (22) = −2.8, P < 0.02). On the other hand, further analysis of the main effect of sex with t student comparisons was unable to reveal significant differences between males and females on any of the five days (P > 0.05). In general, the locomotion tests showed that variation in Rhes protein levels could be related to variation in the levels of activity, but this trend could vary across days of exposition to the open field.
Figure 1 Profile of locomotor activity in female and male wild type (WT), knockout (KO), and heterozygous (Het) mice (day X sex X genotype) in the open field.
Abbreviations: WT, wild type; KO, knockout; Het, heterozygous; SE, standard error.
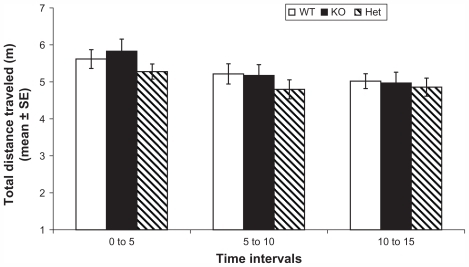
shows locomotion-traveled distance in the first 15 minutes of day 1. Mixed repeated measures design of traveled distance showed a significant effect of time (Wilks’ Lambda: 0.8, F (2, 55) = 8.8, P < 0.001), a nonsignificant effect of genotype (Wilks’ Lambda: 0.9, F (4, 110) = 0.7, P = 0.6), a nonsignificant effect of sex (Wilks’ Lambda: 0.9, F (2, 55) = 0.3, P = 0.7), and a significant time × genotype× sex interaction (Wilks’ Lambda: 0.8, F (4, 110) = 2.8, P = 0.03). Subsequent analysis of the effect of time with t student comparisons did not find a significant difference between groups. Compared to Spano et al’s results, our results did not show significant differences between genotypes; however, Rhes Het mice exhibited lower levels of activity compared to WT mice in the first 10 minutes, and Spano et al’s results reported that KO mice showed lower levels of activity than their WT counterparts in the first 5 minutes (however, the main effects in that were not significant).Citation26 Taking together this study’s and Spano et al’s previous results, it could be interpreted as Rhes protein level reduction is related to some decrease in basal levels of activity. Also, differences between both studies could be explained by differences in sampling.
Figure 2 Profile of locomotor activity in WT, KO, and Het mice during the first 15 minutes of day 1 in the open field.
Abbreviations: WT, wild type; KO, knockout; Het, heterozygous; SE, standard error.
Anxiety
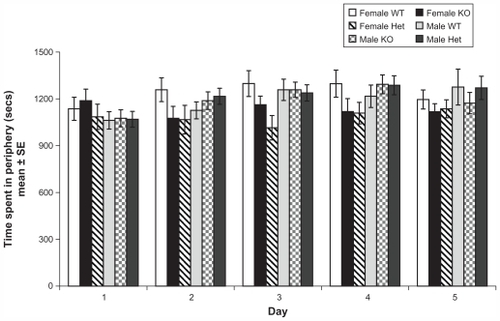
shows time spent in thigmotaxis across 5 days (30 minutes per day). Mixed repeated measures design for thigmotaxis time showed a significant main effect of days (Wilks’ Lambda: 0.7, F (4, 64) = 5.6, P < 0.01), a nonsignificant main effect of genotype (Wilks’ Lambda: 0.8, F (8, 128) = 1.7, P = 0.09), a nonsignificant main effect of sex (Wilks’ Lambda: 0.9, F (4, 64) = 0.9, P = 0.4), and a nonsignificant day × genotype × sex interaction (Wilks’ Lambda: 0.8, F (8, 128) = 1.6, P = 0.1). Subsequent analysis of the main effect of days with t student comparisons revealed that thigmotaxis in day 1 (1105.9 sec ± 27.3) was significantly different from the rest of the days: day 2 (1198.3 sec ± 26.2), day 3 (1162.2 sec ± 26.9), day 4 (1230.6 sec ± 29.4), and day 5 (1205.5 sec ± 28.3) (P < 0.001, all contrasts). Despite the fact that the genotype main effect was not significant, there was a marked trend (P = 0.095) that a decrease in Rhes protein levels was related to lower levels of anxiety (lower time in thigmotaxis) (WT: 1198.5 sec ± 42.8, KO: 1133.0 sec ± 46.4, Het: 1136.8 sec ± 41.8).
Figure 3 Profile of anxiety behavior in female and male WT, KO, and Het mice in the open field.
Abbreviations: WT, wild type; KO, knockout; Het, heterozygous; SE, standard error.
Stereotypy
shows stereotypy scores averaged across the 60 minute observation period. Mixed repeated measures design of stereotypy scores showed significant main effects of treatment (Wilks’ Lambda: 0.1, F (3, 51) = 240,3, P < 0.001), genotype (Wilks’ Lambda: 0.7, F (6, 102) = 4.1, P < 0.001) and sex (Wilks’ Lambda: 0.8, F (3, 51) = 3.1, P = 0.04), but a nonsignificant treatment × genotype × sex interaction (Wilks’ Lambda: 0.9, F (6, 102) = 0.7, P = 0.6).
Figure 4 Profile of stereotypy behavior in female and male WT, KO, and Het mice.
Abbreviations: WT, wild type; KO, knockout; Het, heterozygous; SE, standard error.
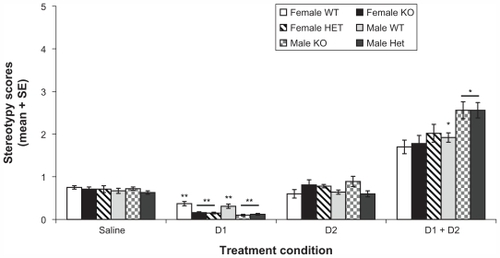
Concomitant analysis of the main effect of treatment with t student comparisons revealed that D1 + D2 treatment (2.0 ± 0.1) revealed significantly higher stereotypy scores compared to the rest of the treatments [saline (0.7 ± 0.0), D1 (0.2 ± 0.0), and D2 (0.6 ± 0.1)]; moreover, t student’s comparisons also revealed that D1 treatment (0.2 ± 0.0) was significantly different from saline (0.7 ± 0.0) and D2 treatment (0.6 ± 0.1) (P < 0.001, all contrasts). This suggests that D1 + D2 treatment varied significantly from saline, D1, and D2 treatment conditions, signifying that Rhes protein levels do not influence D1/D2 synergism.
Subsequent analysis of the main effect of genotype with t student comparisons revealed that under D1 agonism treatment conditions, the WT group had (0.3 ± 0.0) higher stereotypy scores compared to the KO (0.1 ± 0.0) and Het groups (0.1 ± 0.0) (P < 0.001, all contrasts); furthermore, t students comparisons also revealed that under D1 + D2 treatment condition the WT group (1.7 ± 0.1) showed lower levels of stereotypy compared to KO (2.2 ± 0.2) and Het (2.2 ± 0.2) groups, but these differences did not reach significant levels (P = 0.06 and P = 0.11, respectively). Furthermore, the analysis of the main effect of sex with t student comparisons revealed that under D1 + D2 agonism treatment, the male group (2.2 + 0.1) showed higher levels of stereotypy compared to the female group (1.7 ± 0.1) (P = 0.05).
In synthesis some trends were evident in the effects of treatments: under D1 treatment condition, both KO and Het mice showed significantly lower stereotypy scores compared to WT mice. Furthermore, under D1 + D2 treatment, KO and Het mice showed a higher (nonsignificant) response than WT mice.
Discussion
The present results of the effects of Rhes protein levels in basal levels of activity are in partial agreement with previous similar behavioral studies. For instance, Spano et al reported in the open field that KO mice showed lower levels of activity in the first 5 minutes of the open field (15 minute test) compared to WT mice.Citation26 In our study, the open field test was performed for 30 minutes for 5 consecutive days, however, we included an additional analysis of the data as performed by Spano et al (15 minutes of day 1, ); this analysis denoted that Het mice showed lower levels of activity than their WT counterpart, but the tendency was not significant. It is important to consider that Spano et al’s study reported a significant post hoc effect despite the absence of a main significant effect.Citation26 However, both studies agreed that a decrease in Rhes protein levels could be associated with a decrease in basal levels of activity.
Another study by Errico et al explored basal levels of activity in Rhes mutant mice. They reported that KO mice expressed higher rather than lower levels of activity compared to WT mice in the last 20 minutes of the open field test (30 minute session).Citation20 In the present study, KO mice also showed higher levels of activity than WT mice but only on the third day of the test. In this sense, both studies agree that a reduction in Rhes protein levels is related to an increase in basal levels of activity. The hyperactivity of the Rhes mutant mice can be explained by the control exerted by Rhes protein levels on G-olf protein levels, which are expressed higher in striatum of Rhes KO mice compared to their WT counterparts (KO mice expressed 114% to 124% of WT mice levels), according to Errico et al.Citation20
The present study found a marked but not significant association between a decrease in Rhes protein levels and anxiolysis; KO and Het mice showed lower levels of anxiety compared to their WT counterparts, a tendency that was more marked in female mice (shown in ). Our results in anxiety differ from those obtained by Spano et al.Citation26 Those authors found higher levels of anxiety in the female KO mice compared to their WT counterpart, while our study found the opposite tendency: KO mice displayed significantly lower levels of anxiety. This discrepancy could be explained by differences in the number of subjects employed and the type of anxiety test. Spano et al’s study used the elevated plus maze test rather than the open field test. As a reference, other mutant models of anxiety, such as the Galanin R1 receptor (GAL-R1), have also found inconsistent results between the elevated plus maze and open field tests.Citation28 In general, despite the apparent discrepancies between our study and Spano et al’s study, one commonality is that female KO mice differ from WT and Het mice on anxiety levels, an effect that should be explored further.Citation26
Different authors have pointed out relationships between DA receptor dynamics and anxiety states. For instance, different authors have reported that lower doses of apomorphine (an agonist of D1 and D2 receptors) have sedating, relaxing and anxiolytic effects.Citation29–Citation33 Moreover, imaging studies (PET) have shown marked relationships between central D2 receptor density, DA synthesis capacity in the striatum, and neuroticism anxiety (an intense feeling of anxiety, disproportionate to the trigger and can actually interfere with an individual’s ability to function normally).Citation34,Citation35 In general, other studies have proposed a relevant role of DA in the genesis and treatment of anxiety states.Citation36 A possible explanation that can be drawn from the study is that because Rhes protein levels influence DA receptor supersensitivity, then an increase in DA receptor sensitivity is related to an anxiolytic effect, mainly in female mice.Citation17 Moreover, a clinical study reported that dopamine receptor density could affect anxiety conditions.Citation37 In effect, that study reached a consensus that sex differences of neuroticism-anxiety related to variations in D2 receptor density exist; it reported a sex specific (male but not female) association between lower neuroticism-anxiety and the expression of the A1 + allele of the DRD2 Taq 1 polymorphism (lower levels of anxiety correlated to lower levels of D2 receptor density). Then, if there were associations between dopamine receptor density and anxiety, even sex specific relationships, then the existence of relationships between dopamine receptor sensitivity and anxiety should also be possible.
Our present results in the stereotypy test suggest a sex difference in the response to apomorphine; males showed higher levels of stereotypy response compared to females. Other similar studies in rodents have found different results; for instance, it has been reported that females display higher levels of apomorphine and cocaine-induced stereotypy than males.Citation38,Citation39 A possible explanation for this difference is due to levels of estrogen in females.Citation40 Female rats in estrus (high estrogen level) display low levels of stereotypy; furthermore, males treated with estrogen display a lower level of stereotypy compared to their nontreated counterparts.Citation40
The ovarian hormones effect on stimulants response is complex, and research results are contradictory.Citation41 Some studies found no effect of ovariectomy in amphetamine-induced stereotypy.Citation38,Citation42 However, other studies have found larger effects of cocaine and amphetamine in stereotypy under the estrus phase.Citation43,Citation44
The decrease in Rhes protein levels does not seem to affect D1/D2 synergism, because D1 agonism and D2 agonism treatments induced lower stereotypic responses compared to D1 + D2 agonism treatment in KO and Het mice. If D1/D2 synergism were broken, then D1 or D2 agonism treatments should generate a higher stereotypic response, similar to D1 + D2 agonism treatment. Furthermore, KO and Het mice showed lower stereotypic responses under D1 treatment compared to their WT counterparts, suggesting that Rhes protein levels are facilitatory of D1 mediated behaviors. This is also supported by previous results published by our group reporting that Rhes protein levels appear to facilitate D1-specific grooming behaviors in males.Citation25 Our results suggest that Rhes protein levels are inhibitory of behaviors mediated by D1 + D2 receptor co-stimulation. A possible explanation for the difference between the facilitatory effect of Rhes proteins on D1 agonism behavior and the inhibitory effect on D1 + D2 agonism behavior could be linked to a previous result by Errico et al. They reported that the mutant mice model that decreases Rhes protein levels results in a D1R increased cAMP/PKA signaling which is mediated by G-olf proteins, and a decreased coupling efficiency of the D2 receptor to its downstream Gi/o proteins.Citation20 Our previous work with a group of male mice reported a significant difference between KO and Het mice compared to WT mice under D1 + D2 treatment. In the present study, female groups did not show the same trend as male groups, and this could explain the differences in trends when both sexes were combined.Citation25
The present results suggest that Rhes proteins do not seem to be indispensable for DA D1/D2 receptor synergism in both sexes. However, Rhes proteins exert intricate effects on D1 and D2 receptor sensitivity. Specifically, Rhes proteins suppress behaviors generated by combined agonism of D1 and D2 receptors, mainly in male mice (). The modulatory effects on D1 receptors are more intricate as well. Although it has been shown previously to be inhibitory to GαS activation and Gα-olf expression, its presence is necessary for full expression of the D1-mediated grooming behavior. Despite the fact that Rhes protein levels do not seem to affect DA synergism, they do affect the sensitivity of the response to DA receptor activation. The increased response to D1 + D2 receptor co-activation by apomorphine in male but not in female KO mice suggests that Rhes proteins are repressive of this response and a potential sex difference. Other studies performed in Rhes mutant mice suggest a repressive role for Rhes proteins in DA-mediated events. For example, Rhes KO mice show an increased locomotor response to the D1 agonist SKF 81297, and increased Gα-olf levels and Glutamate R1 phosphorylation.Citation20 In a heterologous expression system, Rhes protein levels were shown to inhibit the activation of GαS-coupled receptors at a point proximal to G protein activation.Citation18 The results for D2 receptor activation are less clear, with KO mice showing reduced D2-mediated G protein activation and increased sensitivity to D2 antagonists. Citation20 Our results indicate that for stereotypy, a behavior elicited only by concomitant stimulation of D1 and D2 receptors, Rhes proteins are inhibitory mainly in male mice rather than female mice.
To elucidate the effects of Rhes on activation of D1 and D2 receptors independently, we tested behavior induced by each receptor subtype alone. Agonism of D1 receptors did not induce stereotypy, but the scores on the rating scale were reduced in KO and Het mice compared to their WT counterparts in both sexes. This effect is probably linked to an enhanced sensitivity to the D2 antagonist eticlopride, which was administered prior to apomorphine in the D1 group, and is in agreement with the findings of Errico et al, who reported increased catalepsy to haloperidol treatment in KO mice.Citation20 Activation of D2 receptors did not induce significant differences in stereotypy among experimental groups for both sexes.
As in all experiments using mice in which a gene is knocked-out from the single cell stage, interpretations of the present results require caution. This model of mutant mice is limited by potential genetic compensatory responses that could lead to anatomical and physiological compensations during development. Subsequent studies could explore the present findings with a knockout model in which the knocking out condition is restricted in time, for example, to adulthood (temporal conditional knockout), or restricted in space; for instance, limited to the striatum.
Conclusion
In conclusion, the present research shows that changes in Rhes protein levels affect basal levels of activity and stereotypy response under DA receptor agonism conditions; furthermore, a decrease in Rhes protein levels could be linked to anxiolysis in females. A decrease in Rhes protein levels does not affect D1/D2 synergism in female and males.
Acknowledgments
The authors thank Dr G J LaHoste for research support and lab facilities. This study was supported by NIH grant RR016816, and also by SNI funding (SENACYT-PANAMA) awarded to GQ.
Disclosure
The authors report no conflicts of interest in this work.
References
- Goldman-RakicPSSelemonLDNew frontiers in basal ganglia research. IntroductionTrends Neurosci19901372412441695397
- SibleyDRMonsmaFJJrMolecular biology of dopamine receptorsTrends Pharmacol Sci199213261691561715
- LaHosteGJHenryBLMarshallJFDopamine D1 receptors synergize with D2, but not D3 or D4, receptors in the striatum without the involvement of action potentialsJ Neurosci200020176666667110964971
- O’SullivanGJKinsellaAGrandyDKTigheOCrokeDTWaddingtonJLEthological resolution of behavioral topography and D2-like vs D1-like agonist responses in congenic D4 dopamine receptor “knockouts”: identification of D4:D1-like interactionsSynapse200659210711816320306
- BraunARChaseTNObligatory D-1/D-2 receptor interaction in the generation of dopamine agonist related behaviorsEur J Pharmacol19861312–33013063493161
- LahosteGJMarshallJFDopamine receptor interactions in the brainStoneTWCNS Neurotransmitters and Neuromodulators: DopamineBoca Raton, FLCRC Press1996107119
- BordiFMellerEEnhanced behavioral stereotypies elicited by intrastriatal injection D1 and D2 dopamine agonists in intact ratsBrain Res198950422762832574622
- WaltersJRBergstromDACarlsonJHChaseTNBraunARD1 dopamine receptor activation required for postsynaptic expression of D2 agonist effectsScience198723648027197222953072
- WhiteFJBednarzLMWachtelSRHjorthSBroodersonRJIs stimulation of both D1 and D2 receptors necessary for the expression of dopamine-mediated behaviors?Pharmacol Biochem Behav19883011891932902644
- LaHosteGJYuJMarshallJFStriatal Fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivityProc Natl Acad Sci U S A19939016745174558102797
- DengYPLeiWLReinerADifferential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labelingJ Chem Neuroanat2006322–410111616914290
- ArntJHyperactivity induced by stimulation of separate dopamine D-1 and D-2 receptors in rats with bilateral 6-OHDA lesionsLife Sci19853787177233927097
- ArntJHyttelJDifferential inhibition by dopamine D-1 and D-2 antagonists of circling behaviour induced by dopamine agonists in rats with unilateral 6-hydroxydopamine lesionsEur J Pharmacol198410223493546148252
- HuXTWachtelSRGallowayMPWhiteFJLesions of the nigrostriatal dopamine projection increase the inhibitory effects of D1 and D2 dopamine agonists on caudate-putamen neurons and relieve D2 receptors from the necessity of D1 receptor stimulationJ Neurosci1990107231823291973947
- LaHosteGJMarshallJFDopamine supersensitivity and D1/D2 synergism are unrelated to changes in striatal receptor densitySynapse199212114261357762
- FalkJDVargiuPFoyePERhes: A striatal-specific Ras homolog related to Dexras1J Neurosci Res199957678278810467249
- HarrisonLMLaHosteGJRhes, the Ras homolog enriched in striatum, is reduced under conditions of dopamine supersensitivityNeuroscience2006137248349216352400
- VargiuPDe AbajoRGarcia-RaneaJAThe small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptorsOncogene200423255956814724584
- HarrisonLMLahosteGJRuskinDNOntogeny and dopaminergic regulation in brain of ras homolog enriched in striatum (Rhes)Brain Res20081245162518929545
- ErricoFSantiniEMigliariniSThe GTP-binding protein Rhes modulates dopamine signalling in striatal medium spiny neuronsMol Cell Neurosci200837233534518035555
- CismowskiMJMaCRibasCActivation of heterotrimeric G-protein signaling by a ras-related protein. Implications for signal integrationJ Biol Chem200027531234212342410840027
- GrahamTEProssnitzERDorinRIDexras1/AGS-1 inhibits signal transduction from the Gi-coupled formyl peptide receptor to Erk-1/2 MAP kinasesJ Biol Chem200227713108761088211751935
- HarrisonLMHeYRhes and AGS1/Dexras1 affect signaling by dopamine D1 receptors through adenylyl cyclaseJ Neurosci Res201189687488221374700
- ThapliyalABannisterRAHanksCAdamsBAThe monomeric G proteins AGS1 and Rhes selectively influence Galphai-dependent signaling to modulate N-type (CaV2.2) calcium channelsAm J Physiol Cell Physiol20082955C1417142618815223
- QuinteroGCSpanoDLahosteGJHarrisonLMThe Ras homolog Rhes affects dopamine D1 and D2 receptor-mediated behavior in miceNeuroreport200819161563156618845937
- SpanoDBranchiIRosicaARhes is involved in striatal functionMol Cell Biol200424135788579615199135
- NolanEBHarrisonLMLahosteGJRuskinDNBehavioral synergism between D(1) and D(2) dopamine receptors in mice does not depend on gap junctionsSynapse200761527928717318881
- HolmesAKinneyJWWrennCCGalanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-mazeNeuropsychopharmacology20032861031104412700679
- AngristBThompsonHShopsinBGershonSClinical studies with dopamine-receptor stimulantsPsychopharmacologia19754432732801208762
- CorsiniGUDel ZompoMManconiSPiccardiMPOnaliPLMangoniAEvidence for dopamine receptors in the human brain mediating sedation and sleepLife Sci197720916131618875637
- DouglasCJDelirium tremensNew York Medical Journal190072
- DouglasCJA prompt emergency hypnoticNew York Medical Journal19171061032
- LalSDe la VegaCEApomorphine and psychopathologyJ Neurol Neurosurg Psychiatry19753877227261099172
- KestlerLPMalhotraAKFinchCAdlerCBreierAThe relation between dopamine D2 receptor density and personality: preliminary evidence from the NEO personality inventory-revisedNeuropsychiatry Neuropsychol Behav Neurol2000131485210645736
- LaaksoAWalliusEKajanderJPersonality traits and striatal dopamine synthesis capacity in healthy subjectsAm J Psychiatry2003160590491012727694
- GoldbergHLFinnertyRJThe comparative efficacy of buspirone and diazepam in the treatment of anxietyAm J Psychiatry1979136911841187382878
- WackerJReuterMHennigJStemmlerGSexually dimorphic link between dopamine D2 receptor gene and neuroticism-anxietyNeuroreport200516661161415812318
- SavageauMMBeattyWWGonadectomy and sex differences in the behavioral responses to amphetamine and apomorphine of ratsPharmacol Biochem Behav198114117217193328
- WalkerQDCabassaJKaplanKASex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomyNeuropsychopharmacology200125111813011377925
- MillerJCSex differences in dopaminergic and cholinergic activity and function in the nigrostriatal system of the ratPsychoneuroendocrinology1983822252366353470
- Van HartesveldtCJoyceJNEffects of estrogen on the basal gangliaNeurosci Biobehav Rev19861011142871534
- CampDMRobinsonTESusceptibility to sensitization. II. The influence of gonadal hormones on enduring changes in brain monoamines and behavior produced by the repeated administration of D-amphetamine or restraint stressBehav Brain Res198830169882458742
- Quinones-JenabVHoASchlussmanSDFranckJKreekMJEstrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer ratsBehav Brain Res19991011152010342395
- BeckerJBChaJHEstrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysisBehav Brain Res19893521171252818831