Abstract
Background
Depression is the common mental disorder in the world. However, the pathophysiology mechanism underlying depression remains elusive. It has been reported that aberrant expression of miR-144 is closely related to depression. This study was to investigate whether and how miR-144 involves in depressive-like behaviors in a chronic unpredictable mild stress (CUMS) animal model.
Methods
A rat model of CUMS was established, and qRT-PCR was performed to detect the expression of miR-144 in the hippocampus of a depressed rat. The lentiviral vector carried miR-144 (LV-miR-144) was injected into the hippocampus of the CUMS rat to investigate the effects of miR-144 on the behaviors and PTP1B/TrkB/BDNF signal transduction in the hippocampus of the rat. The interaction between miR-144 and PTP1B was investigated by biological analyses and dual-luciferase reporter assay.
Results
The results showed that CUMS rats had typical depressive behaviors, and the expression of miR-144 in the hippocampus of CUMS rats was significantly lower than that of the control group. In addition, PTP1B protein expression was significantly up-regulated, while the expression of pTrkB and BDNF protein was significantly down-regulated in the hippocampus of CUMS rats. Moreover, PTP1B was a direct target of miR-144, and miR-144 could activate the downstream TrkB/BDNF signaling pathway by inhibiting the expression of PTP1B in primary hippocampus neurons.
Conclusion
MiR-144 played an anti-depressive role in hippocampus dysfunction by inhibiting PTP1B and activating the TrkB/BDNF signaling pathway in the hippocampus of CUMS rats.
Keywords:
Introduction
Depression is a common clinical mental disease, mainly manifested as depressed mood, loss of interest or pleasure in regular activities, and decreased energy.Citation1 Currently, about 20% of the world’s population suffers from depression, and the World Health Organization predicts that depression will be the world’s leading burden of disease by 2030.Citation2 The pathogenesis of depression is very complex, involving a variety of dysfunctions, such as neuroendocrine dysfunction, neuronal plasticity injury, and inflammatory response.Citation3,Citation4 The hippocampus is a functional brain region involved in pattern recognition, emotion regulation, spatial learning, and memory.Citation5 Stress is an important biological reaction of the body when stimulated by various psychological, social, and environmental factors, which is now considered as one of the leading causes of depression.Citation6 Studies have found that long-term stress can lead to changes in the body’s neuroendocrine system and the immune system.Citation7 When the body is subjected to external stress, it firstly acts on the hippocampus area of the brain and stimulates the stress response of the body.Citation8
MicroRNAs (miRNAs) are highly conserved endogenous non-coding small molecule RNAs, which can inhibit gene expression by complementary binding to mRNA of the target genes.Citation9 Recent studies have found that miRNAs are not only involved in the progression of tumor diseases, but also related to a variety of psychiatric diseases, such as bipolar disorder, drug dependence, autism, schizophrenia, and stress-induced depression and resilience.Citation10–Citation13 MiR-144 is considered as a tumor suppressor and is poorly expressed in a variety of human tumors.Citation14,Citation15 Sun et alCitation16 found that miR-144 was poorly expressed in thyroid papillary carcinoma and could inhibit proliferation of thyroid papillary carcinoma cells by targeting E2F8, thus playing an anti-tumor role. Han et alCitation17 reported that miR-144 was down-regulated in ovarian cancer cells and inhibited SKOV3/OVCAR3 cell proliferation and migration by down-regulating the expression of RUNX1. However, Short et alCitation18 found that elevated paternal glucocorticoid exposure alters the small noncoding RNA profile including miR-144 in sperm and then modifies anxiety and depressive phenotypes in the offspring. Wang et alCitation19 reported that circulating microRNA-144-5p is associated with depressive disorders. These reports suggest that aberrant expression of miR-144 is closely related to emotion disease. However, up to now, whether and how miR-144 is involved in depression remains unknown.
Protein tyrosine phosphatase 1B (PTP1B) is a negative regulator of the leptin and insulin signaling pathways. It has been reported that PTP1B plays an important role in maintaining energy homeostasis.Citation20 Dysregulated cellular energy homeostasis and energy depletion have been known to be involved in stress and depression.Citation21 Moreover, PTP1B was reported to be the target of a variety of miRNAs to regulate the cancer development and energy control.Citation22 Whether it is also involved in the regulation of depression by interaction with miR-144 needs to be investigated. Tyrosine kinase receptor B (TrkB) and brain-derived neurotrophic factor (BDNF) are key proteins in the MAPK signaling pathway involved in the pathogenesis of depression and the antidepressant effect of drugs.Citation23 BDNF is an important neurotrophic protective factor synthesized in the brain, with the highest content in the hippocampus and cerebral cortex.Citation24 The combination of BDNF and TrkB can promote the regeneration and differentiation of neurons and up-regulate the plasticity of neurons.Citation25 Studies have shown that the dysregulated expression of TrkB and BDNF in the hippocampal tissue are involved in the development of depression.Citation26 Therefore, regulation of the TrkB/BDNF signaling pathway is considered as the common mechanism of action of various antidepressant treatments.Citation27
Chronic unpredicted mild stress (CUMS) induced depression in animal models is very similar to the core symptoms of human depression, so it is widely used in the studies of anti-depression drugs.Citation28 In this study, the depression model of CUMS rats was established to explore the role of mechanisms of miR-144 in the hippocampus, so as to provide a certain theoretical basis for the treatment of depression.
Materials and Methods
Animals
Male Sprague-Dawley rats, weighing 200–220 g and aged 5 weeks, were selected as the study subjects at the experimental animal center of Qingdao Mental Health Center. Rats were kept in a 12/12 hour light/dark cycle environment at 22±2°C, with free access to food and water. This study followed the guidelines of the National Institutes of Health and was approved by the animal care committee of Qingdao Mental Health Center. The rats were kept for a week before the experiments to be adapted. Rats were also adapted for 24 hours a day before behavioral testing.
Establishment of CUMS Model
Rats were randomly divided into two groups. There was no difference in body weight, baseline sucrose preference, or total fluid intake between the two groups at the beginning of CUMS treatment. The control group rats were not treated with CUMS, and the CUMS group rats underwent CUMS treatment continuously for 35 days. Briefly, eight different pressure sources were used in the experiment, including congestion and isolation of housing, fasting for food and water, a 45° inclined cage, exposure to a disgusting smell, night lights, turning off the lights for 12 hours, 4°C under breeding, and rotation on the vibrator. Rats in the CUMS group were randomly administered with two types of pressure sources for 3 hours a day, with an interval of 4 hours between the two types of pressure sources. Body weight and sucrose preference of the rats were measured on the day of CUMS treatment and every 7 days thereafter. The behavioral and biochemistry experiments were conducted at the end of CUMS treatment.
Stereotactic Injection of LV-miR-144 into the Hippocampus of the Rat
Lentivirus with miR-144 overexpressing vector was purchased from GenePharma (Shanghai, China; LV3-pGLVH1/GFP+Puro vector, D06003). The promoter was human H1 promoter, and the cDNA sequence was as follows: TGGGGCCCTGGCTGGGATATCATCATATACTGTAAGTTTGCGATGAGACACTACAGTATAGATGATGTACTAGTCCGGGCACCCCC. SD rats were randomly divided into five groups (n=6): con + LV-scramble group, con+ LV-miR-144 group, CUMS + LV-scramble group, CUMS + LV-miR-144 group, and CUMS + Fluoxetine group. CUMS modeling took 35 days. The LV-scramble or LV-miR-144 (1 μL) was injected into the hippocampus of rats on the 28th day of CUMS modeling. The rats in the CUMS + Fluoxetine group were intraperitoneally injected with the antidepressant Fluoxetine for 7 consecutive days (10 mg/kg/day). The stereotactic injection coordinates of the bilateral hippocampus were: 3.5 mm ventral surface of the skull, 2.5 mm lateral medial suture, and 4.8 mm posterior anterior fontanel. Stereotactic injection was performed at a constant rate within 1 minute and the needle was slowly retracted 5 minutes after injection. After injection, the rats were injected intraperitoneally with penicillin sodium for 3 consecutive days, 200,000 units per day, to prevent infection. Seven days after stereotactic injection, behavioral tests were performed on the rats in each group.
Primary Hippocampus Neuronal Cultures
Primary hippocampus neuronal cultures were prepared as previously described.Citation29 Hippocampus were first carefully dissected from the rat embryo brain. The neuronal cells were then dissociated with trypsin digestion. The dissociated cells were re-suspended in a neurobasal medium (Gibco, B2719) containing 2 mM GlutaMAX. Cells were diluted to 100 cells/mL. A total of 3 mL of cells suspension was added to each 35-mm poly-D-lysine-coated plate and cultured for 2 hours in 5% CO2 at 37°C. The plating medium was then replaced with 3 mL of neurobasal/B27 medium, and the culture was contained in 5% CO2 and 21% O2. The primary cultured cells were allowed to grow for 2 weeks before treatment. After the cultured neurons were treated with LV-scramble or LV-miR-144 for 48 hours, PTP1B protein expression was analyzed by Western blotting.
Behavioral Experiment
Sucrose Preference Assay
Two bottles of 1% sucrose solution were provided to each group of rats within 24 hours, and then one bottle of sucrose solution was replaced with water. After the rats were fasted and deprived of water for 24 hours, 100 mL water and 100 mL 1% sucrose solution were provided for 3 hours. The consumptions of water and sucrose solution were recorded and the relative sucrose preference was calculated. The relative sucrose preference was calculated as the percentage of consumed sucrose solution volume in the total volume of consumed water and sucrose solution.
Elevated Maze
The elevated maze was 60 centimeters above the ground and consists of two relatively open arms and two relatively closed arms. The rats were placed in the central square to explore the maze freely for 5 minutes, and the SMART video tracking system was used to record the time the rats used in the open arm (non-anxious state).
Open Field Test
In addition to the sucrose preference test, the open field test and forced swimming test were also used to evaluate the depressions-like behavior changes in mice.Citation30 The rats were placed alone in the dim (25 Lux) open field room (100×100×45 cm) for 1 hour, and locomotion of the rats was recorded by the SMART video tracking system.
Morris Water Maze
Morris water maze was used to test spatial learning and memory, which has been described in previous publications. The maze apparatus consisted of a large circular pool (150 cm in diameter by 50 cm high) filled with water to a depth of 22 cm with water at 22±2°C. A transparent plexiglas escape platform (15 cm in diameter) was placed 2 cm below the opaque water surface and was kept in the target quadrant to hold the rats at the same location throughout the training period. The water is made opaque by addition of non-toxic and odorlessness black paint. Consistent visual cues were located in the test room outside of the maze. And the room was indirectly illuminated by several lamps, which delivered diffuse, dim, white lighting allowing the rats to see numerous distinct extra-maze visual cues. The lighting conditions were consistent throughout the task. A total of four trials per day with an inter-trial interval of approximately 10 minutes were performed across five consecutive days. Rats were allowed 60 seconds to find and mount the escape platform. Latency to escape onto the platform was recorded if it was less than 60 seconds. If the rat did not find the platform within 60 seconds, the latency time was recorded as 60 seconds and the experimenter guided the rat to the platform. Regardless of whether it found the platform, the rat was placed on the platform for 60 seconds. The time spent in the target quadrant was recorded. There were a very small number of animals floated from our research. Data from all trials were recorded using a video tracking system MT-200.
qRT-PCR
The rats in each group were euthanized after the behavioral test. Rat brain tissues were ground in liquid nitrogen and total RNAs were extracted using Trizol (Invitrogen) reagent. Total RNAs were reversely transcribed into cDNA using the Prime Script RT kit (Takara). PCR reactions were prepared using the SYBR Premix Ex TaqTM II (Takara). U6 was used as the internal control. Relative expression levels of each gene were calculated using 2−ΔΔCT method. The primer sequences used were listed in .
Table 1 The Sequences of Primers
Measurement of Hormones and Inflammatory Factors
The hippocampus samples were homogenized using PBS, and the supernatants were collected after centrifuging at 2,500 rpm for 10 minutes, at 4°C. Protein content was determined by BCA protein assay kit. Serum and hippocampal concentrations of corticosterone (CORT), IL-1β, and TNFα were analyzed using commercial ELISA kits (Nuoyuan, Shanghai, China). Serum indexes were detected without any dilution, while hippocampus homogenate were diluted at 1:10 using PBS in duplicate wells. All experimental steps were performed according to the kit specifications. The plates were coated with rat antibodies. The OD values were recorded at 450 nm and the concentrations were calculated with a standard curve. The intra-assay coefficient of variation of CORT, IL-1β, and TNFα were all 5%. The sensitivities of the assays were 1 ng/mL, 1 pg/mL, and 1 pg/mL, respectively.
Western Blotting
Total proteins were extracted from brain tissues with RIPA lysate reagent. Protein samples were separated by SDS-PAGE and transferred to cellulose acetate membrane. After blocking using 5% skim milk powder solution for 2 hours, the primary antibody incubations (all at 1:1,000 dilutions) were performed overnight at 4°C with anti-PTP1B antibody (ab244207, Abcam), anti-phospho-TrkB (Tyr-706) (sc-135,645, Abcam), anti-TrkB (sc-11, Santa Cruz), anti-BDNF (ab108319, Abcam), and anti-GAPDH (ab8245, Abcam). Then, the membrane was incubated with the second antibody at room temperature for 1 hour. Quantity One software was used for quantitative analysis of protein bands, and GAPDH was used as the internal reference.
Dual-Luciferase Reporter Assay
HEK293T cells were purchased from Stem Cell Bank, Chinese Academy of Sciences. Bioinformatics analysis was performed to predict the binding sites of miR-144 in the 3ʹ-UTR of PTP1B. For the target prediction, three publically available databases including TargetScan, www.targetscan. Org/vert-71/PicTar, www.pictar.org, and miRanda, www.microrna.org, were used to predict the candidate targets of miR-144. A fragment of the mutant (MUT) or wild type (WT) 3-’UTR of PTP1B was cloned into the vector and then co-transfected with LV-scramble or LV-miR-144 into HEK293T cells for 48 hours. After transfection, cells were collected and dual-luciferase reporter assay (Promega) was performed.
Statistical Analysis
All data were reported as the mean±standard deviation (SD). The data were analyzed by one-way factorial analysis of variances (one-way ANVOA) and multiple comparisons were conducted. Significant effects of treatment were defined using Fisher’s method as a post hoc test, with P<0.05 considered as statistically significant.
Results
The Depressive Behaviors and Expression of miR-144 in the CUMS Rats
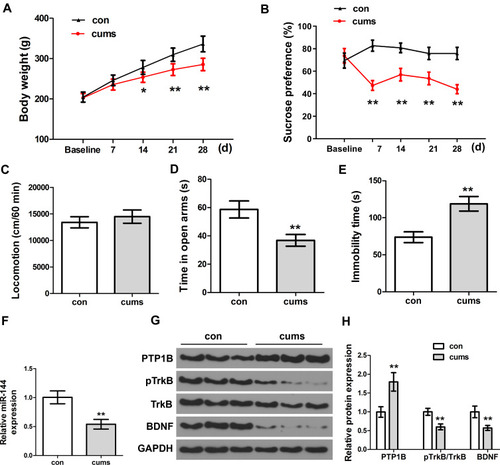
Firstly, the behavioral changes of depressed rats were observed and assessed by establishing the CUMS rat model. Compared with the control group, both weight and sucrose preference of CUMS rats were remarkably decreased with the extension of time ( and , P<0.01). In addition, the time in open arms of CUMS rats was significantly lower than that of the control group (, P<0.01), and the immobility time of CUMS rats was remarkably higher than that of the control group (, P<0.01). However, there was no significant difference between the locomotion of the CUMS group and the control group (, P>0.05). Therefore, the locomotion of the rat was not determined in the follow-up experiments. It was well-established that time in open armsCitation31 and immobility timeCitation32 could act as two important indicators for depression-like behaviors. The above results indicated that the CUMS-treated rats showed typical depression-like behaviors, and the CUMS rat model was successfully established. Next, we used qRT-PCR to determine the expression of miR-144 in the rat hippocampus. The results showed that the expression of miR-144 in the hippocampus of CUMS rats was significantly decreased compared with that in the control group (, P<0.01). This indicated that the expression of miR-144 might be suppressed in the hippocampus of CUMS rats. Moreover, the protein expression of PTP1B, pTrkB, and BDNF in the rat hippocampus was determined by Western Blotting. The results showed that, compared with the control group, the expression of PTP1B protein in the hippocampus of CUMS rats was significantly increased, while the expression of pTrkB and BDNF proteins was significantly decreased ( and , P<0.01). These results suggest that the depressive behavior of CUMS rats might be associated with the regulation of miR-144 and the PTP1B/TrkB/BDNF signaling pathways.
Figure 1 The depressive behaviors and expression of miR-144 in the CUMS rats. (A) Weight changes of rats in each group. (B) The relative sucrose preference of rats in each group was tested by Sucrose preference assay. (C) Locomotion of rats in each group was tested by open field test. (D) The time in open arms of rats in each group. (E) Immobility time of rats in each group. (F) Determination of the expression of miR-144 in the hippocampus by qRT-PCR. (G, H) Determination of the protein expression of PTP1B, pTrkB, and BDNF in the hippocampus by Western Blotting. Data are presented as mean±SD from at least three independent experiments.
Effect of miR-144 on Depressed Behaviors in CUMS Rats
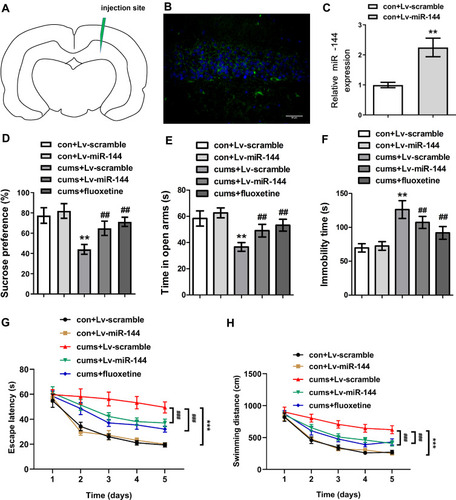
In order to explore the effect of miR-144 on the depressive behaviors in CUMS rats, LV-scramble or LV-miR-144 was stereotaxically injected into the bilateral hippocampus of rats ( and ), and the results showed that the expression of miR-144 was significantly increased after transfection. In addition, compared with rats in the con + LV-scramble group, the sucrose preference and the time in open arms of rats in the CUMS + LV-scramble group were significantly reduced, while the immobility time was significantly increased, indicating that rats in the CUMS + LV-scramble group had significant depressive behaviors (). In addition, the sucrose preference and the time in open arms of rats in the CUMS + LV-miR-144 group were remarkably higher than that in the CUMS + LV-scramble group ( and , P<0.01), while the immobility time was significantly lower than that in the CUMS + LV-scramble group (, P<0.05). Furthermore, the results showed that CUMS rats had longer escape latency than the control group rats, whereas miR-144 or fluoxetine treatment could reverse the learning and memory impairment induced by CUMS (, P<0.001). CUMS significantly increased swimming distance, whereas miR-144 or fluoxetine treatment could reverse the increased swimming distance induced by CUMS (, P<0.001). These results indicated that overexpression of miR-144 could significantly alleviate the depressive behaviors of CUMS rats, which was similar to the effect of depression treatment drug Fluoxetine.
Figure 2 Effect of miR-144 on depressive behaviors in CUMS rats. (A) The relative sucrose preference of rats in each group. (B) The time in open arms of rats in each group was tested by Elevated maze. (C) Immobility time of rats in each group. (D) The sucrose preference of rats in each group. (E) The time in open arms of rats in each group. (F) The immobility time of rats in each group. (G) The escape latency of rats in each group. (H) The swimming distance of rats in each group. Data were presented as mean±SD from at least three independent experiments.
Effect of miR-144 on PTP1B/TrkB/BDNF Signaling Pathway in Hippocampus of CUMS Rats
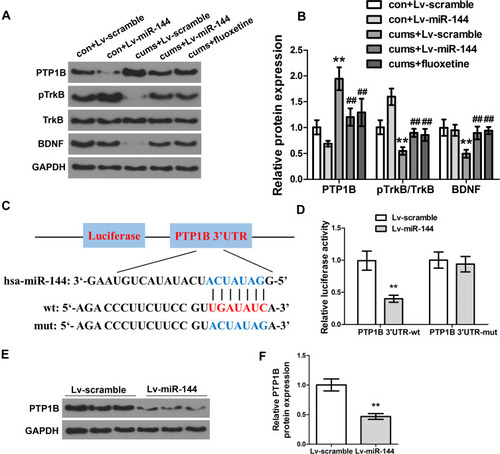
To investigate the effect of miR-144 on the PTP1B/TrkB/BDNF signaling pathway in the hippocampus of CUMS rats, expression of proteins PTP1B, pTrkB, and BDNF in the rat hippocampus were determined by Western Blot. The results showed that the expression of PTP1B in the hippocampus of the CUMS + LV-miR-144 group was significantly lower than that of the CUMS + LV-scramble group (P<0.01), while the expression levels of pTrkB and BDNF were significantly higher than that in the CUMS + LV-scramble group ( and , P<0.01). This indicated that LV-miR-144 treatment inhibited the expression of PTP1B and up-regulated the protein expression of pTrkB and BDNF in the hippocampus of the CUMS rat. Next, we used bioinformatics analysis to predict that PTP1B was a potential target of miR-144 (). Meanwhile, the mutant (MUT) or wild type (WT) PTP1B 3-’UTR vector was co-transfected with LV-scramble or LV-miR-144 into HEK293T cells and the luciferase activity was assessed. It showed that, after co-transfection with LV-miR-144, luciferase activity of the PTP1B 3-’UTR-WT group was significantly lower than that of the PTP1B 3ʹ-UTR-MUT group (, P<0.01). Furthermore, the expression of PTP1B in primary hippocampal neurons transfected by LV-scramble or LV-miR-144 was determined by Western Blot. The results showed that, compared with the LV-scramble group, the expression of PTP1B in the primary hippocampal neurons transfected with LV-miR-144 was significantly down-regulated ( and , P<0.01). These results suggested that PTP1B was a direct target of miR-144. Corticosterone (CORT), Interleukin-1β, and TNFα were reported to be key mediators in depression. We then investigated the level of CORT, IL-1β, and TNFα in the serum and hippocampus of CUMS rats by ELISA. Supplementary Figure S1A and B showed the CORT concentration changes in both Serum and hippocampus, in the groups of con + Lv-scramble, con + Lv-miR-144, CUMS + Lv-scramble, CUMS + Lv-miR-144, and CUMS + fluoxetine. In comparison with the con + Lv-scramble group, the CORT concentrations in the CUMS + Lv-scramble group was greatly elevated in both the serum and hippocampus. Compared with the con + Lv-miR-144, the CORT concentration in CUMS + Lv-miR-144 has a slight elevation. Similar results were observed in CUMS + fluoxetine. In order to examine the inflammatory levels, concentrations of TNF-α and IL-1β in the serum and hippocampus were detected. As shown in supplementary Figure 1C–F, the levels of TNF-α and IL-1β in the CUMS + Lv-scramble group were greatly elevated in both serum and hippocampus, compared with con + Lv-scramble. In contrast to the con + Lv-miR-144 group, the concentration of TNF-α and IL-1β in the CUMS + Lv-miR-144 groupwere slightly higher. Similar results were also observed in CUMS + fluoxetine.
Figure 3 Effect of miR-144 on the PTP1B/TrkB/BDNF signaling pathway. (A, B) Detection of the protein expression of PTP1B, pTrkB, and BDNF in the hippocampus by Western Blotting. (C) Schematic diagram of miR-144 binding sites in PTP1B 3ʹ-UTR. (D) Detection of the luciferase activity of PTP1B 3ʹ-UTR-WT and PTP1B 3ʹ-UTR-MUT genes in HEK293T cells. (E, F) Detection of the protein expression of PTP1B in primary hippocampus neurons transfected with LV-scramble or LV-miR-144 by Western Blotting. Data were presented as mean±SD from at least three independent experiments.
Discussion
Depression is a type of mental disorder mainly characterized by low mood, which seriously affects the quality-of-life of patients.Citation33 Long-term external stress was found to be a major contributor to depression.Citation34 The hippocampus is the central brain region that receives long-term external stress and plays an important role in the development of depression.Citation35 Increasing numbers of studies have proved that miRNAs are involved in the occurrence and development of mental diseases.Citation36,Citation37 MiR-144 is a tumor suppressor miRNA, which is down-regulated in a variety of human tumors, such as thyroid papillary cancer, ovarian cancer, non-small cell lung cancer, pancreatic cancer, etc.Citation14,Citation38,Citation39 However, the role of miR-144 in depression remains unclear. In the present study, the depression model of CUMS rats was established to explore the involvement of miR-144 in the hippocampus area of CUMS rats. Depression is characterized by inhibition of psychokinesis, hopelessness, anhedonia, and decreased interest.Citation40 In animal experiments, sucrose preference test, elevated maze test, open field test, and forced swimming test are often used to evaluate the degree of depression.Citation41 The results of this study showed that the body weight, sucrose preference, and time in open arms significantly decreased in CUMS model rats, while the immobility time significantly increased, indicating the successful establishment of the depression model in rats. Meanwhile, qRT-PCR results showed that miR-144 was down-regulated in the hippocampus of CUMS rat, suggesting that miR-144 might be associated with depressive behavior in rats.
Based on the successful establishment of the CUMS rat model, LV-scramble or LV-miR-144 was stereotaxically injected into the bilateral hippocampus of rats to evaluate the depressive behavior of rats in this study. Fluoxetine is a drug that can improve the depressive behavior of depression model animals, which is a commonly used positive control drug for depression researches.Citation42 Fluoxetine was also selected as a positive control drug for LV-miR-144 in this study. The results showed that the sucrose preference and time in open arms of the CUMS + LV-miR-144 group were significantly increased, while the immobility time was significantly decreased. Zhao et al found that the expression of miR-101 was down-regulated in the VLOs of the CUMS rat brain, and injection of miR-101 mimic could significantly alleviate the depressive behaviors of CUMS rats.Citation43 Higuchi et alCitation44 reported that the expression level of miRNA-124 was significantly decreased in the hippocampus tissue of CUMS rats, while overexpression of miR-124 in the hippocampus area could significantly reverse the depression-like behaviors of CUMS rats. The results of this study suggested that overexpression of miR-144 could significantly alleviate the depressive behavior of CUMS rats, and its antidepressant effect was similar to that of Fluoxetine.
PTP1B plays a role in maintaining the phosphorylation balance of tyrosine protein and is involved in various physiological processes such as cell growth, cell differentiation, gene transcription, immune response, and signal transduction.Citation45 It has been reported that PTP1B was a negative regulator of tyrosine phosphorylation of the tyrosine kinase TrKB, the receptor for brain-derived neurotrophic factor (BDNF).Citation46 The TrkB/BDNF signaling pathway is involved in the occurrence and development of depression.Citation47 Our results showed that the expression of PTP1B protein in the hippocampus of CUMS rats was significantly up-regulated, while the expression of pTrkB and BDNF were significantly down-regulated, suggesting that the depressive behaviors of CUMS rats might be related to the PTP1B/TrkB/BDNF signaling pathway. In order to further explore the effect of miR-144 on the PTP1B/TrkB/BDNF signaling pathway in the hippocampus of CUMS rats, LV-scramble or LV-miR-144 was stereo-injected into the bilateral hippocampus of rats. The results showed that LV-miR-144 treatment inhibited the expression of PTP1B in the CUMS rat hippocampus and up-regulated the expression of downstream pTrkB and BDNF. Serra et alCitation48 found that down-regulated expression of BDNF and TrkB in the hippocampal tissue of rats could aggravate the depression degree of depressed rats. Wei et alCitation49 reported that H2S exerted an antidepressant effect on CUMS rats by activating the BDNF/TrkB pathway. Therefore, the results of this study suggested that miR-144 might be related to PTP1B/TrkB/BDNF signal transduction in the hippocampus of CUMS rats. MiRNAs can inhibit gene expression by complementary binding to the target gene mRNA. It was reported that PTP1B can promote neuro-inflammation.Citation50 We confirmed that PTP1B was a direct target of miR-144. In addition, the expression of PTP1B in the hippocampus and primary cultured hippocampal neurons transfected with LV-miR-14 was significantly down-regulated, indicating that the expression of PTP1B was directly regulated by miR-144.
Conclusion
In conclusion, the present study assessed the mechanisms of miR-144 in the hippocampus area of CUMS rats. These results indicated that miR-144 could activate the downstream TrkB/BDNF signaling pathway by inhibiting the expression of PTP1B in the hippocampus of CUMS rats, and eventually play an anti-depressive role in the stress dysfunction hippocampus.
Disclosure
The authors report no conflicts of interest for this work.
References
- Chukhraev N, Vladimirov A, Zukow W, Chukhraiyeva O, Levkovskaya V. Combined physiotherapy of anxiety and depression disorders in dorsopathy patients. J Phys Educ Sport. 2017;17(1):414.
- Wang J, Zhou QX, Lv LB, Xu L, Yang YX. A depression model of social defeat etiology using tree shrews. Dongwuxue Yanjiu. 2012;33(1):92–98.22345016
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22. doi:10.1038/nri.2015.526711676
- Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28. doi:10.1038/nm.424627918562
- Shemyakov S, Nikolenko V, Sarkisyan K. Age-related changes in morphometric parameters of hippocampal neurons in humans. Neurosci Behav Physiol. 2017;47(6):613–616. doi:10.1007/s11055-017-0442-y
- Strain JJ. The psychobiology of stress, depression, adjustment disorders and resilience. World J Biol Psychiatry. 2018;19(sup1):S14–S20. doi:10.1080/15622975.2018.145904930204561
- Moya-Perez A, Perez-Villalba A, Benitez-Paez A, Campillo I, Sanz Y. Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav Immun. 2017;65:43–56. doi:10.1016/j.bbi.2017.05.01128512033
- Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol. 2015;7(9):a018812. doi:10.1101/cshperspect.a01881226330519
- Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15(6):321. doi:10.1038/nrc393225998712
- Agarwal V, Bell GW, Nam J-W, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005. doi:10.7554/eLife.05005
- Reddy KB. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015;15(1):38.25960691
- Issler O, Chen A. Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci. 2015;16(4):201. doi:10.1038/nrn387925790865
- Sun XY, Song ZH, Si YW, Wang JH. microRNA and mRNA profiles in ventral tegmental area relevant to stress-induced depression and resilience. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:150–165. doi:10.1016/j.pnpbp.2018.05.02329864451
- Sheng S, Xie L, Wu Y, Ding M, Zhang T, Wang X. MiR-144 inhibits growth and metastasis in colon cancer by down-regulating SMAD4. Biosci Rep. 2019;39(3):BSR20181895. doi:10.1042/BSR2018189530745456
- Qu D, Yan B, Xin R, Ma T. A novel circular RNA hsa_circ_0020123 exerts oncogenic properties through suppression of miR-144 in non-small cell lung cancer. Am J Cancer Res. 2018;8(8):1387.30210911
- Sun J, Shi R, Zhao S, et al. E2F8, a direct target of miR-144, promotes papillary thyroid cancer progression via regulating cell cycle. J Exp Clin Cancer Res. 2017;36(1):40. doi:10.1186/s13046-017-0504-628270228
- Han S, Zhu J, Zhang Y. miR-144 potentially suppresses proliferation and migration of ovarian cancer cells by targeting RUNX1. Med Sci Monit Basic Res. 2018;24:40. doi:10.12659/MSMBR.90733329445078
- Short AK, Fennell KA, Perreau VM, et al. Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl Psychiatry. 2016;6(6):e837. doi:10.1038/tp.2016.10927300263
- Wang X, Sundquist K, Hedelius A, Palmer K, Memon AA, Sundquist J. Circulating microRNA-144-5p is associated with depressive disorders. Clin Epigenetics. 2015;7. doi:10.1186/s13148-015-0099-8
- Cho H. Protein tyrosine phosphatase 1B (PTP1B) and obesity. Vitam Horm. 2013;91:405–424.23374726
- Ostergaard L, Jorgensen MB, Knudsen GM. Low on energy? An energy supply-demand perspective on stress and depression. Neurosci Biobehav Rev. 2018;94:248–270. doi:10.1016/j.neubiorev.2018.08.00730145282
- Zhang J, Pan J, Yang M, et al. Up-regulating microRNA-203 alleviates myocardial remodeling and cell apoptosis through down-regulating PTP1B in rats with myocardial infarction. J Cardiovasc Pharmacol. 2019;74(5):474–481. doi:10.1097/FJC.000000000000073331725080
- Kasemeier-Kulesa JC, Morrison JA, Lefcort F, Kulesa PM. TrkB/BDNF signalling patterns the sympathetic nervous system. Nat Commun. 2015;6(1):8281. doi:10.1038/ncomms928126404565
- Miao H, Li R, Han C, Lu X, Zhang H. Minocycline promotes posthemorrhagic neurogenesis via M2 microglia polarization via upregulation of the TrkB/BDNF pathway in rats. J Neurophysiol. 2018;120(3):1307–1317. doi:10.1152/jn.00234.201829790836
- Fred SM, Laukkanen L, Vesa L, et al. Pharmacologically diverse antidepressant drugs disrupt the interaction of BDNF receptor TRKB and the endocytic adaptor AP-2. BioRxiv. 2019;591909.
- Colle R, Deflesselle E, Martin S, et al. BDNF/TRKB/P75NTR polymorphisms and their consequences on antidepressant efficacy in depressed patients. Pharmacogenomics. 2015;16(9):997–1013. doi:10.2217/pgs.15.5626122862
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(3):238. doi:10.1038/nm.405026937618
- Li R, Wang X, Qin T, Qu R, Ma S. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain. Behav Brain Res. 2016;296:318–325. doi:10.1016/j.bbr.2015.09.03126416673
- Volpicelli F, Speranza L, Pulcrano S, et al. The microRNA-29a modulates serotonin 5-HT7 receptor expression and its effects on hippocampal neuronal morphology. Mol Neurobiol. 2019;56(12):8617–8627. doi:10.1007/s12035-019-01690-x31292861
- Sáenz JCB, Villagra OR, Trías JF. Factor analysis of forced swimming test, sucrose preference test and open field test on enriched, social and isolated reared rats. Behav Brain Res. 2006;169(1):57–65. doi:10.1016/j.bbr.2005.12.00116414129
- Andreatini R, Bacellar L. The relationship between anxiety and depression in animal models: a study using the forced swimming test and elevated plus-maze. Braz J Med Biol Res. 1999;32(9):1121–1126. doi:10.1590/S0100-879X199900090001110464389
- Belovicova K, Bogi E, Csatlosova K, Dubovicky M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip Toxicol. 2017;10(1):40–43. doi:10.1515/intox-2017-000630123035
- Freeman MP. Perinatal depression: recommendations for prevention and the challenges of implementation. JAMA. 2019;321(6):550–552. doi:10.1001/jama.2018.2124730747953
- Hosseini A, Jalali M. The possible biological effects of long-term stress on depression. Medbiotech J. 2018;2(04):252–255.
- Roddy DW, Farrell C, Doolin K, et al. The hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biol Psychiatry. 2019;85(6):487–497. doi:10.1016/j.biopsych.2018.08.02130528746
- Wang Y, Wang J, Guo T, et al. Screening of schizophrenia associated miRNAs and the regulation of miR-320a-3p on integrin β1. Medicine. 2019;98(8).
- Saeedi S, Israel S, Nagy C, Turecki G. The emerging role of exosomes in mental disorders. Transl Psychiatry. 2019;9(1):122. doi:10.1038/s41398-019-0459-930923321
- Yamada Y, Arai T, Kojima S, et al. Regulation of antitumor miR‐144‐5p targets oncogenes: direct regulation of syndecan‐3 and its clinical significance. Cancer Sci. 2018;109(9):2919–2936. doi:10.1111/cas.1372229968393
- Yin Y, Cai J, Meng F, Sui C, Jiang Y. MiR-144 suppresses proliferation, invasion, and migration of breast cancer cells through inhibiting CEP55. Cancer Biol Ther. 2018;19(4):306–315. doi:10.1080/15384047.2017.141693429561704
- Brys ADH, Lenaert B, Van Heugten CM, Gambaro G, Bossola M. Exploring the diurnal course of fatigue in patients on hemodialysis treatment and its relation with depressive symptoms and classical conditioning. J Pain Symptom Manage. 2019;57(5):890–898.e4. doi:10.1016/j.jpainsymman.2019.02.01030776536
- He Z-X, Song H-F, Liu T-Y, et al. HuR in the medial prefrontal cortex is critical for stress-induced synaptic dysfunction and depressive-like symptoms in mice. Cereb Cortex. 2019;29(6):2737–2747. doi:10.1093/cercor/bhz03630843060
- Gobinath AR, Richardson RJ, Chow C, et al. Voluntary running influences the efficacy of fluoxetine in a model of postpartum depression. Neuropharmacology. 2018;128:106–118. doi:10.1016/j.neuropharm.2017.09.01728964735
- Zhao Y, Wang S, Chu Z, Dang Y, Zhu J, Su X. MicroRNA-101 in the ventrolateral orbital cortex (VLO) modulates depressive-like behaviors in rats and targets dual-specificity phosphatase 1 (DUSP1). Brain Res. 2017;1669:55–62. doi:10.1016/j.brainres.2017.05.02028549965
- Higuchi F, Uchida S, Yamagata H, et al. Hippocampal microRNA-124 enhances chronic stress resilience in mice. J Neurosci. 2016;36(27):7253–7267. doi:10.1523/JNEUROSCI.0319-16.201627383599
- Li ZP, Lee -H-H, Uddin Z, Song YH, Park KH. Caged xanthones displaying protein tyrosine phosphatase 1B (PTP1B) inhibition from cratoxylum cochinchinense. Bioorg Chem. 2018;78:39–45. doi:10.1016/j.bioorg.2018.02.02629533213
- Krishnan N, Krishnan K, Connors CR, et al. PTP1B inhibition suggests a therapeutic strategy for rett syndrome. J Clin Invest. 2015;125(8):3163–3177. doi:10.1172/JCI8032326214522
- Zhang J-C, Yao W, Hashimoto K. Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr Neuropharmacol. 2016;14(7):721–731. doi:10.2174/1570159X1466616011909464626786147
- Serra MP, Poddighe L, Boi M, et al. Expression of BDNF and trkB in the hippocampus of a rat genetic model of vulnerability (Roman low-avoidance) and resistance (Roman high-avoidance) to stress-induced depression. Brain Behav. 2017;7(10):e00861. doi:10.1002/brb3.86129075579
- Wei L, Kan L-Y, Zeng H-Y, et al. BDNF/TrkB pathway mediates the antidepressant-like role of H2S in CUMS-exposed rats by inhibition of hippocampal ER stress. Neuromolecular Med. 2018;20(2):252–261. doi:10.1007/s12017-018-8489-729704115
- Song GJ, Jung M, Kim J-H, et al. A novel role for protein tyrosine phosphatase 1B as a positive regulator of neuroinflammation. J Neuroinflammation. 2016;13(1):86. doi:10.1186/s12974-016-0545-327095436
