Abstract
Background
Major depressive disorder (MDD) is a heterogeneous mental disease that encompasses different subtypes and specifiers. Clinically targeted treatments have not been identified yet, although standardized strategies are recommended by several clinical guidelines. The main aim of this study is to respectively identify the precise treatment for three different subtypes of MDD (ie, melancholic, atypical, and anxious).
Methods
An 8-to-12-week, multicenter randomized controlled trial (RCT) with a parallel group design will be conducted to determine the most effective and appropriate treatment. A total of 750 adults diagnosed with MDD will be recruited, categorized into melancholic, atypical or anxious type based on the assessment of the Inventory of Depressive Symptomatology (IDS30) and the Hamilton Anxiety Scale (HAMA), and 1:1 randomly assigned to different intervention groups. Blood draw, EEG test, and MRI scan will be performed at baseline and endpoint. Clinical symptom and side-effects will be evaluated at critical decision points (CDP) including weeks two, four, six, eight, and 12 after treatment. The primary outcome is total score and reduction rate of the 17-Hamilton Depression Rating Scale (17-HDRS). The secondary outcomes include the scores of the Quick Inventory of Depressive Symptomatology-self-report (QIDS-SR), IDS30, HAMA and the Treatment Emergent Symptom Scale (TESS). All the data will be analyzed by SAS software.
Discussion
The study commenced recruitment in August 2017 and is currently ongoing.
Trial Registration
ClinicalTrials.gov Identifier: NCT03219008 (July 17, 2017).
Background
Major depressive disorder (MDD) is a highly prevalent, chronic, recurrent disorder with a range of adverse personal and societal consequences, affecting approximately 350 million people worldwide.Citation1 Despite the availability of effective modalities including pharmacological treatment, psychotherapy or a combination of both for MDD,Citation2,Citation3 there is still a large unmet need for treatment. For instance, only about 50% of MDD patients show a response and only about one in three attain remission within the initial-phase treatment.Citation4 Again, antidepressant medications are currently chosen using a trial-and-error approach because specific biomarkers which might be helpful for predicting antidepressant response or cueing one treatment over another have not been identified yet.Citation5
The dilemma we acknowledge is that MDD is a heterogeneous condition with a variety of pathophysiological mechanisms, symptomatic dimensions and treatment outcomes. Therefore, this disease is currently considered to encompass different subtypes and specifiers that have been embodied in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Actually, various subtypes with different clinical features have been proposed in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) report, which has demonstrated that of 3671 samples the overwhelming majority was the anxious subtype (44.6%) and the next was melancholic (19.7%) followed by atypical (17.1%), respectively.Citation6 And a substantial overlap of the three subtypes was found in analysis from the International Study to Predict Optimized Treatment in Depression (iSPOT-D) trial.Citation7
It seems to be reasonable that subtyping the different features might be beneficial for selecting from different treatment options. However, the researches on clinical efficacy of different treatment selection yield inconsistent conclusions when applied to these subtypes. More specifically, the STAR*D trial has demonstrated that patients with melancholic features responded more poorly compared to those with other subtypes in the initial phase with citalopram, but the differences were no longer significant once adjustment for baseline differences.Citation8 The study by Uher et alCitation9 has shown that the melancholic patients have a slightly worse response to antidepressant medications than do patients with nonmelancholic depression, while other studies failed to replicate this finding, eg, no differences in the Combining Medications to Enhance Depression Outcomes (CO-MED) trialCitation10 or remitting better among patients with melancholic depression in the Clinical Research Center for Depression (CRESCEND) study.Citation11 Interestingly, a similar phenomenon also occurs in several studies on the other two subtypes. For example, patients with anxious depression had much lower remission rates in the STAR*D trial,Citation12 but recent findings from the large iSPOT-D trial showed no associations between multiple characterizations of anxious depression and treatment outcome.Citation13 However, some findings reveal that patients with the atypical subtype have lower remission rates than those without atypical features,Citation14 while other studies have shown inconsistent results.Citation9,Citation15 Furthermore, previous studies have failed to identify a certain antidepressant which might be more effective than another antidepressant for specific subtypes, either selective serotonin reuptake inhibitors (SSRIs) or tricyclics.Citation9,Citation16
From our point of view, it seems to be plausible that the combination with different treatments could be a better choice than any monotherapies for different depression subtypes. Therefore, we have made bold but sound assumptions in the present trial. A single antidepressant as basic treatment and combination with another class of antidepressant, atypical antipsychotics (AAP) or lithium, repetitive transcranial magnetic stimulation (rTMS) or modified electroconvulsive therapy (MECT), as well as computerized cognitive behavioral therapy (cCBT) are designed to specify the three subtypes respectively. In practical terms, the considerations in therapeutic medications are mainly as follows:
Fluoxetine/bupropion for melancholic subtype: the study by Lin et alCitation17 has suggested that melancholic depression showed better improvement in symptoms than nonmelancholic depression, which started at week two and persisted through weeks three, four, and six after fluoxetine treatment.Citation17 Bupropion, as an atypical antidepressant, shares a broad range of biological properties with psychostimulants and is thought highly of as a promising treatment for fatigue or decreased energy, commonly observed in melancholic depression.Citation18,Citation19
Fluvoxamine/AAP or lithium for atypical subtype: atypical depression is characterized by reversed diurnal mood variation, hypersomnia, significant weight gain, or increase in appetite which may be reasonable to speculate on rhythmic disturbances related to melatonin regulation in this subtype.Citation20 Fluvoxamine, different from other antidepressants, has been found to significantly promote the bioavailability of melatonin and markedly increase the melatonin blood concentrations via a pharmacokinetic inhibitory effect on cytochrome P450 enzymes.Citation21 The AAP or lithium is augmented, especially in light of the concerns about potential risk of manic/hypomanic switch and mood instability, since atypical features might increase suspicion of bipolarity.Citation22
Venlafaxine or duloxetine/mirtazapine for anxious subtype: serotonin and norepinephrine reuptake inhibitor (SNRI) including venlafaxine or duloxetine has been recommended for the treatment of MDD with significant anxious or somatic symptoms and various anxiety disorders. A combined treatment with SNRI (venlafaxine in particular) and mirtazapine is referred to as “California rocket fuel” in light of its higher than average potency and efficacy by pharmacological synergistic effect.Citation23 A double-blind randomized study by Blier et al has demonstrated that this combination seemed to perform very well and rapidly in that patients receiving this treatment achieved the highest remission rate and had no dropouts attributed to lack of efficacy.Citation24
The primary aim of the present study is to respectively identify the precise treatment for melancholic, atypical and anxious subtypes of MDD by comparing efficacy and safety between different intervention strategies including monotherapy, combination of various class of medication, physical treatment and psychotherapy. Furthermore, the collection of blood sample, brain electrophysiological and neuroimaging data will be included in this study in order to identify potential biomarkers useful for diagnostic subtyping and efficacy prediction.
Methods/Design
Organizational Structure
This 8 to 12-week, multicenter, parallel group, RCT study includes eight clinical sites (see Supplement). The principal investigator set up a coordination group that comprised of clinical trial coordinators (CTC) from each site. CTCs assist in the recruitment, evaluation, follow-up of participants at each site. Clinical data are collected by trained evaluators who have passed inter-rater training.
Participants
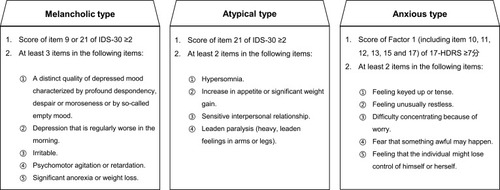
The study is ongoing with recruitment having begun in August 2017, and aims to recruit 750 participants diagnosed with MDD according to the criteria of the DSM-5. The inclusion criteria are as follows: (1) aged 18–65 years; (2) score of 17-HDRS >20; (3) meeting the criteria for melancholic, atypical or anxious subtype (see ); (4) first episode or recurrent episode with medication, rTMS, or MECT treatment ≤4 weeks prior to enrollment. The exclusion criteria are as follows: (1) a history of another DSM-IV Axis I disorder (eg, bipolar disorder, schizophrenia, schizoaffective disorder, and autism disorder); (2) obvious suicidal ideation, attempt or behavior (eg, score of item 3 of 17-HDRS ≥3); (3) hard to define as melancholic, atypical or anxious subtype; (4) a history of treatment resistant depression; (5) serious physical illness (eg, brain tumor or damage) or condition (eg, current pregnancy or breastfeeding) that may interfere with the study protocol.
Study Design and Procedure
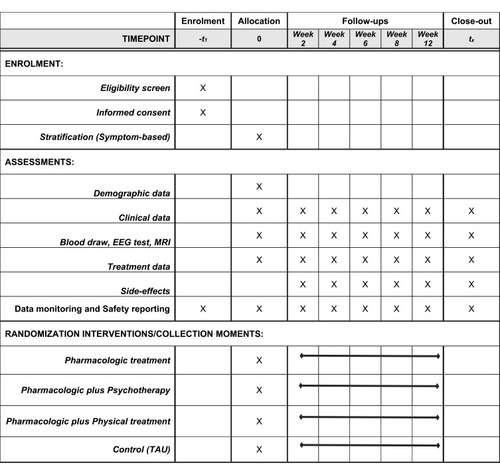
displays standard protocol items according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT).Citation25,Citation26
Randomization and Interventions
All participants are categorized into melancholic, atypical, or anxious subtypes, with 250 individuals in each group, based on our criteria shown in . Then the patients in each subtype group will be 1:1 randomly assigned to different intervention groups by random cipher. The detailed intervention strategies for the different subtypes are seen in .
Figure 3 Flow of participants through the study.
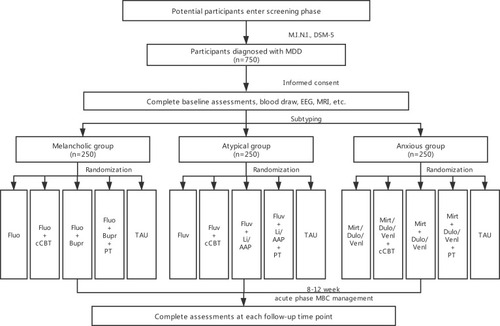
Doses for medications are adjusted by the clinicians according to routine clinical practice within the dose ranges in directions. The usage of AAP is decided by clinicians depending on the clinical guideline and experience. Physical treatment including rTMS and MECT is chosen by patient’s personal preferences to operate following routine clinical procedure. cCBT is adopted by the patient at home for six weeks according to manual. Treat as usual (TAU) is defined as the routine care that subjects receive once they are diagnosed with MDD. In practice, this means that TAU may include pharmacologic treatment, psychotherapy, physical treatment or a combination of them. We will not interfere with TAU, but we will assess carefully according to study protocol.
Clinical Visits and Assessments
At screening, the Mini-International Neuropsychiatric Interview (MINI) is used to confirm the criteria for MDD. Clinical symptoms and side-effects will be evaluated at critical decision points (CDP) including weeks two, four, six, eight, and 12 after treatment. Depressive symptom severity is assessed using 17-Hamilton Depression Rating Scale (17-HDRS), the Quick Inventory of Depressive Symptomatology-self-report (QIDS-SR) and the Inventory of Depressive Symptomatology (IDS30). The Hamilton Anxiety Scale (HAMA) is used to evaluate the anxiety symptoms. Adverse events are evaluated by the Treatment Emergent Symptom Scale (TESS).
In addition, blood draw, EEG test, and MRI scan will be performed at week 0 (baseline) and weeks 8 or 12 (endpoint). Five milliliters of venous blood will be collected from each subject in anticoagulant-free tubes between 07:00 and 09:00 am after an overnight fast. The blood will be centrifuged at 3000× g for 20 min at 4°C within two hours for serum separation and stored at −80°C until it is assayed. Repeated freeze–thaw cycles are avoided. Serum levels of target protein will be measured in duplicate by the ELISA method (or, ultimately, any other measure of protein detection). All samples are performed by operators that are blind to the subjects’ clinical status.
CTCs remain in contact with participants to enhance participation and minimize premature discontinuation. All participants are encouraged to continue the same treatment used in the 8 to 12-week period after completion of this study, if effective and acceptable.
Outcomes
The primary outcome is the reduction rate in 17-HDRS total score from baseline to the end of the study. Remission is defined as 17-HDRS total score of ≤7 and response is defined as ≥50% decrease from 17-HDRS total score at baseline. The secondary outcomes include the changes in the scores of QIDS-SR, IDS30 and HAMA from the baseline to the endpoint. The result of TESS will be used to represent the acceptability of intervention.
Sample Size Estimation
The sample size for this study was calculated with regard to the k-proportions comparison between treatment conditions. Considering a significance level of 0.05, an 80% statistical power, according to the following formulas to compute sample size and power, respectively (pA=0.5, pB=0.35, τ=3):
A total sample of 222 patients in each subtype group is required. Considering drate of 10%, 250 cases will be recruited. Cases will then be 1:1 randomly assigned to five different intervention groups of which N=50 per treatment arm.
Concomitant Medication
Except for the medications mentioned in , other psychotropic drugs are prohibited during the study. Meanwhile, sedative drugs are not recommended for insomnia during this study, with the exception of nonbenzodiazepines (eg, zopiclone, zolpidem, etc) or benzodiazepines but limited to lorazepam if need be. The use of medication for physical diseases is permitted but with fix-dose procedure as far as possible. All the concomitant medication will be recorded in detail.
Data Monitoring and Safety Reporting
Various aspects of the study including participant recruitment, protocol compliance and serious adverse events (SAEs) will be monitored and reported by CTCs every two months. All SAEs (see ) are recorded in the CRF and on the “Serious Adverse Events Report” form. The form is supposed to record the detailed information of SAEs, such as the severity, date of onset and duration, whether it is caused by exposure to study inventions, what actions have been taken, etc. Timely and accurate safety reports must be submitted to the principal investigator and the institutional review board. The principle investigator should ensure all measures necessary for resolution of the SAE are taken. All medications for treatment of the SAE are recorded in the CRF. The SAE follow-up will continue through the last day of the study.
Table 1 Serious Adverse Event
Discontinuation
The end of the study is defined as participants finishing the evaluation at the end of 12 weeks, or withdrawal from the study. Participants are free to withdraw from the study at any time for any reason. They can also be withdrawn by the investigator, if necessary, to protect their safety or integrity of the study data. The principal investigator can withdraw a participant from the study for any of the following reasons: (1) a protocol disobedience or noncompliance that might compromise the study data; (2) a participant’s request to discontinued the study; (3) occurrence of SAE or intolerance to the intervention; (4) occurrence of severe physical disease whether it is related to the study or not; (5) loss of follow-up.
Quality Control
Quality control mainly involves two issues: rater training and clinical data acquisition. (a) All clinical staff should finish standardized rater training and be certificated by each site CTC. Take the 17-HDRS for example, at least three training sessions (videotaped interviews) with depressed patients were organized, and raters need to reach the agreement of ratings with reference to expert standards. Moreover, CTCs at each site are trained and certified in protocol implementation and data collection methods for accreditation. To minimize cross-site differences, the central coordinator supervises the procedural quality control of clinical data procedures based on the guidelines of ICH-GCP, including Source Document Verification, Data Integrity Checks, etc. Entry of data into a portable android device (PAD) using a clinical trial data management system that performs simple range and missing data item checks. Data will be synchronized in real time and transferred to the central coordinating team, assuring capacity to assess the ongoing trial. (b) As quality control for EEG and fMRI, every platform used standardized instructions and recording. The data preprocessing step will carry a specific series of artifact correction and rejection procedures.
Standardized EEG and MRI Data Collection and Preprocessing
The functional magnetic resonance imaging (fMRI) data is measured while participants are performing an auditory attentional task (standard P300). To determine which brain regions are correlated with each other, and to what degree, we employ a SAS macro to calculate the maximum activation in each brain region and to generate a summary graphic of the correlations among brain regions. It computes correlation matrix of all possible brain regions pairwise and output with different line widths reflecting magnitude of the Pearson or Spearman correlation coefficient (all lines representing correlations with a P<0.05).Citation27
At all experimental sites, resting EEG (eyes-open and eyes-closed conditions) is recorded and LDAEP measured using binaural tones (1000 Hz, 40 ms) at five intensities (60–100 dB SPL). EEG data will be interpolated to a common 72-channel montage (CU) and sample rate (256 Hz) to minimize cross-site differences, and a preprocessing pipeline for continuous EEG to extract epochs for source localization analyses (ie rACC theta activity).Citation28
Statistical Analysis
All the data will be managed by CTCs in each site and converge into the principal investigator. Primary analyses will be conducted based on an intent-to-treat principle, including all participants as randomized, regardless of treatment received or withdrawal from the study. Per protocol analysis including only participants who have completed the primary outcome will be analyzed. Missing data will be handled by multiple imputations with 100 iterative estimations per value, which is currently viewed as the gold standard with regard to missing data.Citation29 We will use hierarchical linear modeling, with mixed-effects repeated-measures models implemented in SAS software, version 9.4 PROC MIXED (SAS Institute Inc.), with the cutoff P-value set at 0.05 (two-sided). Random effects are fitted to the model accounting for additional sources of variation. Multiple logistic regression models will be used to give maximum sensitivity and specificity as well as further analysis.Citation30
Discussion
This is an 8 to 12-week, multicenter RCT study with a parallel group design which aims to determine the best and acceptable treatment for melancholic, atypical, and anxious subtypes of MDD in adults. In addition, clinically useful biomarker tools integrating peripheral blood test, electrophysiological and neuroimaging data that can be applied to for objectively diagnosing and subtyping as well as precisely predicting treatment response are expected to develop in this study. Participants are being recruited from eight clinical sites in China to assemble a broadly inclusive and representative population. Thus, study results should be widely generalizable.
Trial Status
Protocol version 3.0 (July 2017). The study commenced recruitment in August 2017 and is currently ongoing. Estimated study completion date is December 2020.
Abbreviations
MDD, major depressive disorder; DSM-5, Diagnostic and Statistical Manual of Mental Disorders; STAR*D, Sequenced Treatment Alternatives to Relieve Depression; iSPOT-D, International Study to Predict Optimized Treatment in Depression; CO-MED, Combining Medications to Enhance Depression Outcomes; CRESCEND, Clinical Research Center for Depression; SSRIs: selective serotonin reuptake inhibitors; AAP, antidepressant, atypical antipsychotics; rTMS, repetitive transcranial magnetic stimulation; MECT, modified electroconvulsive therapy; cCBT, computerized cognitive behavioral therapy; SNRI, serotonin and norepinephrine reuptake inhibitor; CTC, clinical trial coordinators; TAU, treat as usual; MINI, Mini-International Neuropsychiatric Interview; CDP, critical decision points; 17-HDRS, 17-Hamilton Depression Rating Scale; QIDS-SR, Quick Inventory of Depressive Symptomatology-self-report; IDS30, Inventory of Depressive Symptomatology; HAMA, Hamilton Anxiety Scale; TESS, Treatment Emergent Symptom Scale; SAEs, serious adverse events; ICF, informed consent form; ICH-GCP, International Conference on Harmonization Good Clinical Practice; CONSORT, Consolidated Standards of Reporting Trials.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The Ethical Review Board of Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine approved the study protocol (2017-07R). Any significant modifications to the protocol will be forwarded to the committee for approval. During the informed consent process, the nature, purpose, potential benefits and risks, and requirements of the study will be explained to all candidates by researchers, with sufficient time for them to resolve concerns about the study. Those who are willing to participate will be asked to sign and date the informed consent form (ICF). A copy of the signed ICF will be given to every participant. All participants will be asked for their permission to share their anonymized data across all collaborating study sites and to publish the results which will not contain identifiable information. This study is conducted in accordance with the Declaration of Helsinki of the World Medical Association.Citation31 All researchers will follow the guidelines for the International Conference on Harmonization Good Clinical Practice (ICH-GCP)Citation32 and the trial outcomes will be reported in line with the Consolidated Standards of Reporting Trials (CONSORT) guidelines.Citation33
Author Contributions
XHL, YW and HFZ are co-first authors and contributed equally to this work. The other authors are team members of this study and have made a lot of contribution in design of trial. YRF as the principal investigator of the study provided constructive advice and thorough revision for the manuscript. All authors have commented on and approved the final version of the paper. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
Sincere thanks to all the nursing staff at the eight hospitals involved for facilitating data collection at admission and discharge over a number of years. And we thank the patients who generously gave their time to participate in this study.
Disclosure
The authors declare that they have no conflict of interests in this work.
Additional information
Funding
References
- Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017;317(15):1517. doi:10.1001/jama.2017.3826
- Cuijpers P, Sijbrandij M, Koole SL, Andersson G, Beekman AT, Reynolds CF. The efficacy of psychotherapy and pharmacotherapy in treating depressive and anxiety disorders: a meta-analysis of direct comparisons. World Psychiatry. 2013;12(2):137–148. doi:10.1002/wps.2003823737423
- Cuijpers P, Sijbrandij M, Koole SL, Andersson G, Beekman AT, Reynolds CF. Adding psychotherapy to antidepressant medication in depression and anxiety disorders: a meta-analysis. World Psychiatry. 2014;13(1):56–67. doi:10.1002/wps.2008924497254
- Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi:10.1176/appi.ajp.163.1.2816390886
- Rush AJ, Fava M, Wisniewski SR, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25(1):119–142. doi:10.1016/S0197-2456(03)00112-015061154
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi:10.1176/ajp.2006.163.11.190517074942
- Arnow BA, Blasey C, Williams LM, et al. Depression subtypes in predicting antidepressant response: a report from the iSPOT-D trial. Am J Psychiatry. 2015;172(8):743–750. doi:10.1176/appi.ajp.2015.1402018125815419
- McGrath PJ, Khan AY, Trivedi MH, et al. Response to a selective serotonin reuptake inhibitor (citalopram) in major depressive disorder with melancholic features: a STAR*D report. J Clin Psychiatry. 2008;69(12):1847–1855. doi:10.4088/jcp.v69n120119026268
- Uher R, Dernovsek MZ, Mors O, et al. Melancholic, atypical and anxious depression subtypes and outcome of treatment with escitalopram and nortriptyline. J Affect Disord. 2011;132(1–2):112–120. doi:10.1016/j.jad.2011.02.01421411156
- Bobo WV, Chen H, Trivedi MH, et al. Randomized comparison of selective serotonin reuptake inhibitor (escitalopram) monotherapy and antidepressant combination pharmacotherapy for major depressive disorder with melancholic features: a CO-MED report. J Affect Disord. 2011;133(3):467–476. doi:10.1016/j.jad.2011.04.03221601287
- Xu K, Jiang W, Ren L, et al. Impaired interhemispheric connectivity in medication-naive patients with major depressive disorder. J Psychiatry Neurosci. 2013;38(1):43–48. doi:10.1503/jpn.11013222498077
- Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342–351. doi:10.1176/appi.ajp.2007.0611186818172020
- Braund TA, Palmer DM, Williams LM, Harris AW. Characterising anxiety in major depressive disorder and its use in predicting antidepressant treatment outcome: an iSPOT-D report. Aust N Z J Psychiatry. 2019;53(8):782–793. doi:10.1177/000486741983593330880405
- Gili M, Roca M, Armengol S, Asensio D, Garcia-Campayo J, Parker G. Clinical patterns and treatment outcome in patients with melancholic, atypical and non-melancholic depressions. PLoS One. 2012;7(10):e48200. doi:10.1371/journal.pone.004820023110213
- Stewart JW, McGrath PJ, Fava M, et al. Do atypical features affect outcome in depressed outpatients treated with citalopram? Int J Neuropsychopharmacol. 2010;13(1):15–30. doi:10.1017/S146114570900018219341509
- Joyce PR, Mulder RT, Luty SE, McKenzie JM, Rae AM. A differential response to nortriptyline and fluoxetine in melancholic depression: the importance of age and gender. Acta Psychiatr Scand. 2003;108(1):20–23. doi:10.1034/j.1600-0447.2003.00120.x12807373
- Lin CH, Huang CJ, Liu SK. Melancholic features in inpatients with major depressive disorder associate with differential clinical characteristics and treatment outcomes. Psychiatry Res. 2016;238:368–373. doi:10.1016/j.psychres.2015.11.00926899817
- Jefferson JW, Rush AJ, Nelson JC, et al. Extended-release bupropion for patients with major depressive disorder presenting with symptoms of reduced energy, pleasure, and interest: findings from a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2006;67(6):865–873. doi:10.4088/jcp.v67n060216848645
- Pae CU, Lim HK, Han C, et al. Fatigue as a core symptom in major depressive disorder: overview and the role of bupropion. Expert Rev Neurother. 2007;7(10):1251–1263. doi:10.1586/14737175.7.10.125117939764
- Herane-Vives A, de Angel V, Papadopoulos A, et al. Short-term and long-term measures of cortisol in saliva and hair in atypical and non-atypical depression. Acta Psychiatr Scand. 2018;137(3):216–230. doi:10.1111/acps.1285229397570
- Hartter S, Wang X, Weigmann H, et al. Differential effects of fluvoxamine and other antidepressants on the biotransformation of melatonin. J Clin Psychopharmacol. 2001;21(2):167–174. doi:10.1097/00004714-200104000-0000811270913
- Yatham LN, Kennedy SH, Parikh SV, et al. Canadian network for mood and anxiety treatments (CANMAT) and international society for bipolar disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170. doi:10.1111/bdi.1260929536616
- Thase ME. Antidepressant combinations: cutting edge psychopharmacology or passing fad? Curr Psychiatry Rep. 2013;15(10):403. doi:10.1007/s11920-013-0403-224052267
- Blier P, Ward HE, Tremblay P, Laberge L, Hebert C, Bergeron R. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281–288. doi:10.1176/appi.ajp.2009.0902018620008946
- Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan AW, King MT. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA. 2018;319(5):483–494. doi:10.1001/jama.2017.2190329411037
- Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi:10.1136/bmj.e758623303884
- Brensinger C, Herlim M, Bilker WB, Gur RC. A SAS® macro for visually displaying correlations between brain regions using fMRI data, SUGI. 2005 Available from: http://www2.sas.com/proceedings/sugi30/151-30.pdf. Accessed 93, 2020.
- Tenke CE, Kayser J, Pechtel P, et al. Demonstrating test-retest reliability of electrophysiological measures for healthy adults in a multisite study of biomarkers of antidepressant treatment response. Psychophysiology. 2017;54(1):34–50. doi:10.1111/psyp.1275828000259
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147–177. doi:10.1037/1082-989X.7.2.14712090408
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. SAS Institute, Incorporated; 1996.
- World medical A. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.28105324141714
- Dixon JR. The international conference on harmonization good clinical practice guideline. Qual Assur. 1998;6(2):65–74. doi:10.1080/10529419927786010386329
- Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi:10.1136/bmj.c33220332509