Abstract
Migraine is a chronic, painful, and often disabling primary headache disorder, typically presenting with recurrent attacks that may be accompanied by a variety of neurological, gastrointestinal, and autonomic symptoms. Gastrointestinal symptoms in association with migraine including, nausea, vomiting, and gastroparesis, affect a large proportion of migraine sufferers. These symptoms may result in delays or inconsistencies in the absorption of oral treatments. Hence, the necessity for an innovative, non-invasive, parenteral delivery formulation for quick and effective treatment of migraine attacks is evident. Iontophoresis utilizes minimal amounts of electrical potential to support the fast transfer of ionized medication transdermally and into the general circulation. Two pharmacokinetic clinical trials have shown that iontophoretic delivery of sumatriptan through the skin produces quick and reproducible therapeutic plasma concentrations. A randomized, double-blind, multicenter, phase III study demonstrated superior efficacy versus placebo and excellent tolerability, with no triptan-related adverse events. The proportion of patients that were pain-free at 2 h post-treatment was 18% for the sumatriptan patch vs 9% for placebo (P = 0.0092; number needed to treat = 11.1). Upon approval from the Food and Drug Administration and other regulatory authorities, the iontophoretic transdermal delivery of sumatriptan will be a good choice for patients experiencing poor absorption of oral medication often associated with migraine and/or for those with intolerable triptan-related adverse events.
Introduction
Migraine is a primary headache disorder characterized by recurrent attacks of moderate to severe headache, often accompanied by nausea, vomiting, phonophobia, photophobia, and aggravation by exertion.Citation1–Citation3 The one-year prevalence is approximately 10% and varies little worldwide.Citation2 It affects both sexes in their most productive ages with a significant predominance in females.Citation2 The health and economic burden of migraine is significant.Citation2,Citation4 The financial cost of migraine has increased considerably during the past ten years, exceeding the level of 23 billion US dollars per year in 2002–2004.Citation5,Citation6 Today, the yearly estimated cost approaches $30 billion in the US.Citation7 The economic burden of migraine results from the disabling character of recurrent attacks, attributed to intense pain and autonomic symptoms, pre-event worry, comorbid depression, and anxiety. More than two out of three migraineurs experience nausea and one out of three experience vomiting during their migraine attacks.Citation2 Both symptoms are related to the intensity of pain,Citation8 although the exact link between pain and gastrointestinal symptoms in the migraine sufferer is not completely understood. Apart from discomfort that the gastrointestinal symptoms cause the patient, their presence may also result in postponing or even avoiding oral medications for the acute attack. In addition, migraine gastrointestinal symptoms have been demonstrated to decrease the migraineurs’ capability or even willingness to use oral medications for over one-third or more of migraine sufferers.Citation1 This is a significant issue, as clinical trials demonstrate that the efficacy of acute migraine treatment is much improved if medication is used early during an attack before central sensitization begins.Citation9,Citation10
Since oral medications may not be the optimal choice for those migraine sufferers that experience gastrointestinal symptoms, a non-oral formulation may be ideal in such migraineurs.Citation7 There are non-oral formulations available in the US for the treatment of migraine attacks, including three nasal sprays (sumatriptan, zolmitriptan, and dihydroergotamine) and three injectable formulations of sumatriptan. Nasal sprays aim at nasal mucosal absorption, but a significant portion of the drug is actually swallowed and absorbed in the small intestine, which is not a fast or effective a route of delivery for migraine patients with gastric stasis.Citation11,Citation12 Additionally, patients prefer tablets over nasal sprays, even though nasal formulations exert their action more rapidly.Citation7,Citation13 Sumatriptan is rapidly absorbed after subcutaneous injections, but many migraineurs consider an injection to be an invasive, more complicated, and a rather uncomfortable choice.Citation13 Additionally, injected sumatriptan results in a higher recurrence rate and more adverse events than either oral or nasal formulations.Citation14
An alternative to currently available oral or parenteral anti-migraine treatments would be welcomed by many migraineurs. A comparison of the strengths and weaknesses of different routes of administration of acute migraine medications is displayed in .
Table 1 Comparison of strengths and weaknesses of different routes of administration of acute migraine attack medications: oral, nasal, injectable, and transdermal
The novel iontophoretic transdermal sumatriptan patch
A recent development in drug delivery systems includes delivery of medication via a skin patch.Citation7,Citation13 Transdermal formulations are already in use for the treatment of various disorders,Citation13 with the most common being the nitrate patch for treatment of coronary artery disease and hypertension. More recently, a transdermal delivery system has been approved for treatment of Alzheimer’s disease.
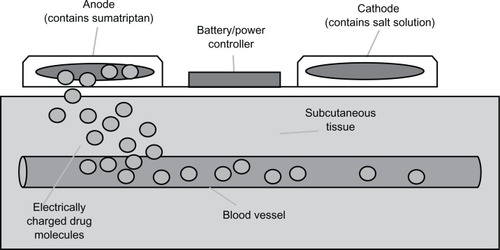
A recent technological advance in patch formulations is the use of electrical potential to promote a medication’s movement through the skin. This iontophoretic delivery system involves a novel sumatriptan patch developed for the treatment of migraine attacks (NP101-Zelrix®) from NuPathe Inc, (Conshohocken, PA) that has been awaiting Food and Drug Administration approval. This device consists of a thin patch containing two shallow wells filled with chemicals that act as electrodes, each with nonwoven pads placed on top. The cathode well contains negatively charged salt solution, while the anode well contains positively charged sumatriptan solution.Citation15 By applying a low-intensity electrical current between the two electrodes, movement of the ionized drug is triggered away from the anode and through the skin. The positively charged anode chamber repels the positively charged drug transdermally and into subcutaneous capillaries and the systemic circulation. It should be noted that this is a non-invasive process and no disruption of the skin takes place. The rate and amount of medication delivery is controlled by a microprocessor, which is activated by the user.Citation13 The principles of the iontophoretic drug delivery system are described in .
Pharmacokinetic profile
Two trials have been performed to evaluate the pharmacokinetic profile of transdermal iontophoretic delivery of sumatriptan.Citation16,Citation17 The first trial was conducted in eight healthy adults in order to assess the pharmacokinetic profile of four prototype sumatriptan patches in comparison to the subcutaneous sumatriptan injection (6 mg) and an oral sumatriptan tablet (50 mg).Citation16 All participants received all six formulations along with pharmacokinetic parameter assessment. This study demonstrated a linear relationship between the quantity of sumatriptan delivered and the amount of the electric potential used, supporting the opinion that iontophoresis is an effective method for delivering this drug. In addition, this trial indicated that the transdermal sumatriptan delivery has a good tolerability profile, with application site reactions being the most common adverse events, and virtually no triptan adverse events. A second pharmacokinetic study used an open label, randomized, crossover design and included 25 healthy adults (aged 21–57 years) that were treated with a single dose of two different sumatriptan iontophoretic patches and three marketed formulations of sumatriptan (100 mg tablet, 6 mg subcutaneous injection, and 20 mg nasal spray).Citation17 Patch number I contained 120 mg of sumatriptan succinate, while patch number II contained 104 mg of sumatriptan succinate. The study results demonstrated that the Cmax for both patches tested was more consistent among participants than either that for the 20 mg nasal spray or 100 mg tablets.Citation17 The Cmax for patch I was 24.8 ng/mL (coefficient of variation [CV] of 26.4%) compared with a Cmax of 12.5 ng/mL (CV of 43.8%) for the nasal spray, and a Cmax of 51.6 ng/mL (CV of 37.9%) for the tablet, showing greater consistency in the quantity of sumatriptan delivered by the patch in comparison to the oral tablet or nasal spray deliveries. Since there is evidence to suggest compromised gastric motility and reduced absorption among migraine sufferers during an acute attack, differences in consistency of plasma concentrations may play a serious role in response to medication. In this study, the subcutaneous sumatriptan injection’s Cmax (6 mg sumatriptan) was approximately 3.3 times that of patch I,Citation17 which may explain the absence of typical “triptan-related” adverse events observed with the use of patch in this study. Instead, subcutaneous sumatriptan treatment resulted in typical adverse events being reported. Hence, “triptan-related” adverse events may in fact be attributed to the high Cmax that happens with delivery via subcutaneous injection, and even the tablet. In this study, no serious adverse events were reported with the patch, while the most common adverse events included application-site pruritus and irritation. In summary, the results of the two pharmacokinetic studies indicate that administration of sumatriptan transdermally is fast, consistent, and well-tolerated without typical triptan-related adverse effects.Citation17 Patch number I, containing 120 mg of sumatriptan succinate, was selected for further development under the commercial name Zelrix™.
Clinical efficacy and safety
The results of a randomized, parallel group, double-blind, placebo-controlled, phase III trial of Zelrix for the treatment of migraine attacks have been reported, although not yet fully published.Citation18,Citation19 This trial included 530 adult patients, aged 18–65 years, with migraine (meeting International Classification of Headache Disorders II criteria) from 37 centers in the United States. Participants used Zelrix or placebo to treat a single moderate to severe intensity migraine attack.
In this trial, the primary endpoint was the percentage of patients experiencing headache freedom at 2 hour post patch application, which was reported to be 18% for active treatment vs 9% for placebo [P = 0.0092; Number Needed to Treat (NNT) = 11.1]. In addition, compared with placebo, significantly more patch patients achieved the secondary endpoints ( and ). These endpoints were photophobia-free at 2 hours (51% vs 36%, NNT = 6.66, P = 0.0028), phonophobia-free at 2 hours (55% vs 39%, NNT = 6.25, P = 0.00021), nausea-free at 2 hours (84% vs 63%, NNT = 4.8, P < 0.0001), no use of rescue medication (60% vs 40%, NNT = 5, P < 0.0001), 2 hours of headache relief (53% vs 29%; NNT = 4.2, P < 0.0001), relief from headache at 1 hour (29% vs 19%, NNT = 10, P = 0.0135), freedom from nausea at 1 hour (71% vs 58%, NNT = 7.7, P = 0.00251), and sustained headache pain relief for 2 to 24 hours post-patch activation (34% vs 21%, NNT = 7.7, P = 0.0015).
Figure 2 Results of a randomized, double-blind, placebo-controlled, phase III study of iontophoretic transdermal sumatriptan drug device for acute migraine.
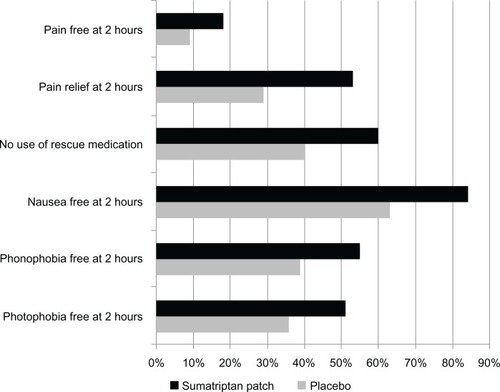
Table 2 Efficacy results from the phase III trial of the iontophoretic sumatriptan patchCitation18–Citation20
In this study, the sumatriptan transdermal delivery patch was well-tolerated. Treatment-emergent adverse events were reported by 51% of the active group and 45% of placebo participants, and were mostly transient mild or moderate intensity application site events. More specifically, application site adverse events were reported by 23% of patients on active treatment vs 15% on placebo. Application site paraesthesias were reported to be 12% and 19%, pruritus in the application site to be 8% and 7%, and reactions in the application site were 7% and 6%, respectively. There were virtually no triptan-related adverse events. The rate of withdrawal from trial due to adverse events was low and similar in both treatment groups (2%).Citation18–Citation20
The long-term safety and efficacy of the sumatriptan patch was examined in a subsequent open-label, extension study.Citation21 This study included 183 patients that applied a total of 2089 patches within a period of up to 12 months. In reference to adverse events, the most common were patch site application adverse events that occurred in 58.5% of patients. The incidence of probable allergic contact dermatitis and Cmax-related triptan adverse events was low (3.7% and 1.6%, respectively). The percentage of patients discontinuing the study because of adverse events was 13.6%, and the most common reasons were application site conditions (7.6%), allergic contact dermatitis (4.9%), nausea (0.5%), and dizziness (0.5%). With reference to efficacy, 2 h after patch activation for all patch treatments over the 12-month period, 23.8% of the initial migraine episodes were scored as headache-free, 58.2% as having headache pain relief, 78.9% as nausea-free, 60.1% as phonophobia-free, 53.4% as photophobia-free, and 20.7% as migraine-free.Citation21
Although the sumatriptan patch has not been approved in the USA, an idea of how it will be used can be drawn from protocol details in its clinical program.Citation21 The patch should be used at the beginning of a migraine attack, as is similar for all acute migraine treatments. The preferred application site of this thin, skin-colored patch will likely be the upper arm. The patch is set by a microprocessor to deliver the correct dose of sumatriptan for 4 hours, no matter how long it is left on the skin. It should be removed 4 hours after its application.
Conclusion
A parenteral route of drug delivery may be ideal for migraine sufferers who experience gastrointestinal symptoms and/or dysfunction, as the presence of gastroparesis may lead to poor oral absorption. Although injectable and nasal delivery systems that bypass the gastrointestinal tract are available, these approaches may not be acceptable to some patients and may be associated with lack of efficacy and/or an adverse events profile that limits their use. Many migraineurs continue to prefer oral administration of medications despite the lack of efficacy. A recent development in medication delivery formulations for migraine is the iontophoretic sumatriptan patch. Based on data from two pharmacokinetic studies and one phase III study, it appears that migraineurs with certain patient profiles may particularly benefit from this approach.Citation13 First, this patch should be helpful for patients that experience gastroparesis and small bowel absorption issues, resulting in delayed, partial, or inconsistent response to oral treatments; however, it should also be helpful for patients experiencing vomiting or intense nausea, since such migraineurs often postpone or even avoid oral therapy. Second, it should benefit migraineurs concerned about the possibility of vomiting after drinking water to ingest oral medications for treatment of acute migraine, those who delay using any type of medication for any reason, and those with apprehension about taking a sumatriptan injection due to triptan-related side effects.
The iontophoretic sumatriptan patch, which is awaiting Food and Drug Administration approval, hopefully in January 2013, may prove to offer enhanced clinical benefits and become one of the first choices for patients experiencing symptoms consistent with gastrointestinal dysfunction associated with migraine.
Disclosure
M Vikelis has consulted for, advised, or spoken for AstraZeneca Hellas, Janssen-Cilag Hellas, and Novis Pharmaceuticals Hellas and is currently an employee of Novartis Hellas SACI.
DD Mitsikostas is on the advisory board of Allergan, Astellas Pharma, Bayer-Shering Hellas, Genesis Pharma, Merck Hellas, and Novartis Hellas; he has received honoraria from Menarini, has received payment for development of educational presentations including service on speakers’ bureaus for Eli Lilly, and has received travel/accommodation expenses covered or reimbursed from Janssen-Cilag, MSD, Novis, Pfizer, and UCB.
AM Rapoport has consulted or spoken for Allergan, MAP, Nautilus Neurosciences, NuPathe, Winston, and Zogenix.
No writing assistance or other support was utilized in the production of this manuscript.
References
- SilbersteinSDMigraine symptoms: results of a survey of self-reported migraineursHeadache19953573873967672955
- StovnerLjHagenKJensenRThe global burden of headache: a documentation of headache prevalence and disability worldwideCephalalgia200727319321017381554
- Headache Classification Subcommittee of the International Headache SocietyThe international classification of headache disorders: 2nd editionCephalalgia200424Suppl 1916014979299
- MenkenMMunsatTLTooleJFThe global burden of disease study: implications for neurologyArch Neurol200057341842010714674
- HawkinsKWangSRupnowMDirect cost burden among insured US employees with migraineHeadache200848455356318070057
- HawkinsKWangSRupnowMFIndirect cost burden of migraine in the United StatesJ Occup Environ Med200749436837417426520
- VikelisMRapoportAMNew drug delivery options for migraineExpert Rev Neurother201111677177321651323
- KelmanLTanisDThe relationship between migraine pain and other associated symptomsCephalalgia200626554855316674763
- BursteinRCollinsBJakubowskiMDefeating migraine pain with triptans: a race against the development of cutaneous allodyniaAnn Neurol2004551192614705108
- BursteinRLevyDJakubowskiMEffects of sensitization of trigemi-novascular neurons to triptan therapy during migraineRev Neurol (Paris)20051616–765866016141951
- FuseauEPetricoulOMooreKHPBarrowAIbbotsonTClinical pharmacokinetics of intranasal sumatriptanClin Pharmacokinet2002411180181112190330
- UemuraNOnishiTMitaniyamaABioequivalence and rapid absorption of zolmitriptan nasal spray compared with oral tablets in healthy Japanese subjectsClin Drug Investig2005253199208
- RapoportAMFreitagFPearlmanSHInnovative delivery systems for migraine: the clinical utility of a transdermal patch for the acute treatment of migraineCNS Drugs2010241192994020932065
- TepperSJMillsonDSafety profile of the triptansExpert Opin Drug Saf20032212313212904112
- PierceMWHeadache reliefDrug Discovery and Development [internet]. March 1, 2008. Available from: http://www.dddmag.com/Article-Anti-migraine-Drugs-Feature-New-Delivery-Methods.aspx. Accessed May 11, 2012.
- SiegelSJO’NeillCDubéLMA unique iontophoretic patch for optimal transdermal delivery of sumatriptan succinatePharm Res200724101919192617577644
- PierceMMarburyTO’NeillCSiegelSDuWSebreeTZelrix: a novel transdermal formulation of sumatriptanHeadache200949681782519438727
- GoldsteinJPugachNSmithTAcute anti-migraine efficacy and tolerability of Zelrix, a novel iontophoretic transdermal patch of sumatriptanResearch abstract presented at 14th International Headache CongressSeptember 10–13, 2009Philadelphia (PA)Headache201050350951520456145
- GoldsteinJPugachNSmithTRAcute anti-migraine efficacy and tolerability of Zelrix, a novel iontophoretic transdermal patch of sumatriptanCephalalgia200929Suppl 120
- GoldsteinJSmithTRPugachNA Sumatriptan iontophoretic transdermal system for the acute treatment of migraineHeadache [epub ahead of print].
- SmithTPierceMWGriesserJLong-term safety and efficacy of NP101, a sumatriptan iontophoretic patch for the treatment of acute migraineHeadache20125261262422352764