Abstract
Suicide is a serious social problem in many countries, including Japan. The majority of people who commit suicide suffer from depression. Suicide attempt patients suffering from serious physical injuries are initially treated in hospital emergency departments. The present post hoc analysis examined data from patients admitted to an emergency hospital for treatment of physical injuries, resulting from a suicide attempt, and initial psychiatric treatment for depression and prevention of future suicide attempts. The effects on depressive symptoms were studied in two groups of patients using the 17-item Hamilton depression scale (HAMD). One group (n = 6) had received intravenous tricyclic antidepressants (TCA) (amitriptyline or clomipramine) while the other group (n = 7) had been treated orally with milnacipran, a serotonin and norepinephrine reuptake inhibitor antidepressant. Prior to treatment the four highest scoring items on the HAMD scale were the same in both groups namely, item 1 (depressed mood), item 3 (suicidality), item 7 (interest in work and activities), and item 10 (psychic anxiety). After 1 week of treatment, mean global HAMD scores were significantly reduced in both groups. Treatment resulted in a significant reduction of five HAMD items in the TCA group, whereas in the milnacipran group 12 HAMD items were significantly reduced. Suicidality (item 3) was significantly improved by 1 week treatment with milnacipran, but not by TCAs. Milnacipran rapidly improved a wide range of depressive symptoms, including suicidality within the first week. The improvement with milnacipran would appear to be, at least, equivalent to that achieved with TCAs, possibly affecting a wider range of symptoms. Since milnacipran has been shown in comparative studies to be better tolerated than TCAs, this antidepressant offers an interesting option for the treatment of suicidal patients in an emergency setting.
Introduction
The increase in suicides has become a serious social problem in recent years in many countries, including Japan. The number of suicides in Japan passed 30,000 per year in 2000 and shows no sign of decreasing.Citation1 The number of suicide attempts is probably at least ten times greater than the number of completed suicides.Citation1 It has been estimated that 90% of people who commit suicide suffer from psychiatric disordersCitation2 and 70% of them suffer from depression. It is widely accepted that the use of psychiatric treatment to prevent first-time and subsequent suicide attempts is essential in order to reduce the number of deaths due to suicide.Citation3–Citation5
After a suicide attempt patients suffering from serious physical injuries, including traumatic injury, are usually admitted to hospital emergency departments. In addition to treatment for their physical injuries, patients often receive acute psychiatric treatment for depression and to prevent future suicide attempts. There have been few studies of the efficacy of antidepressants in this difficult patient group. A recent study that reviewed long-term suicide prevention strategies in suicide attemptersCitation6 concluded that there is a need for further research to evaluate different strategies. A 6-month open trial in adolescents with a recent suicide attempt (Treatment of Adolescent Suicide Attempters [TASA] study) showed that intensive antidepressant and psychological therapy produced rates of improvement and remission of depression comparable to those observed in nonsuicidal adolescents with depression.Citation7 The authors underlined the importance of events occurring within the first weeks after a suicide attempt and emphasized the importance of increased therapeutic contact early in treatment. To the authors’ knowledge, there has been no study on the effects of antidepressant treatment in patients immediately following a suicide attempt.
The present study analyzed the records of patients admitted to Juntendo Shizuoka Hospital (Japan) for treatment of physical injuries resulting from a suicide attempt. They were also administered antidepressants to treat depressive symptoms and to prevent suicide. The therapeutic effects of the antidepressants were studied in two groups of patients using the 17-item Hamilton depression scale (HAMD). One group of patients had received the classical treatment (in Japan at the time of the treatment) of intravenous tricyclic antidepressants (TCAs). The other group had been treated with milnacipran, a serotonin and norepinephine reuptake inhibitor (SNRI) antidepressant. Milnacipran is one of the non-TCAs most frequently prescribed in this hospital due to its limited adverse effects, low risk of drug interactions, and absence of cardiac toxicity.Citation8
Subjects and methods
This ad hoc retrospective study analyzed data from suicidal patients who were admitted to the Juntendo Shizuoka Hospital (Japan) for treatment for physical injuries following a suicide attempt from January 2003 to March 2005. In order to be included in the analysis patients had to (1) have been diagnosed with major depressive disorder according to International Classification of Diseases-10 (ICD-10) criteria at hospital admission with an initial HAMD score of at least 26, (2) have been taking no psychotropic medication (including antidepressants) at the time of their suicide attempts, and (3) have full psychiatric records, including HAMD scores, for at least 1 week following initiation of antidepressant treatment.
The patient records analyzed represented approximately 20% of all the patients admitted to Juntendo Shizuoka Hospital following suicide attempts during this period.
In addition to the treatment of their injuries, each patient was examined by a consultant psychiatrist and the best antidepressant treatment, in his opinion, was prescribed. In choosing the treatment, the physical injuries resulting from the suicide attempt and their treatment, as well as the psychiatric condition of the patient were considered. Thirteen patients, treated with a TCA (n = 6) (amitriptyline or clomipramine) or the SNRI, milnacipran (n = 7), were included in this analysis ( and ). TCAs were initially administered by intravenous infusion and later switched to oral administration in some cases. With the exception of a single patient on a combination of intravenous amitriptyline and fluvoxamine (), the antidepressants were administered as monotherapy. No other psychotropic agents were prescribed.
Table 1 Clinical details of patients administered tricyclic antidepressants
Table 2 Clinical details of patients administered milnacipran
HAMD was used to evaluate clinical symptoms of depression. The first assessment was made at the time of initiation of antidepressant therapy and subsequently after 1, 2, and 4 weeks of treatment where possible. Juntendo Shizuoka Hospital is an emergency hospital in a tourist region sadly famous for suicide attempts. Most of the patients were thus discharged or transferred to other hospitals, often in their hometown, within two weeks of admission. Therefore, it was only possible to observe a few patients for more than a week or two. For this reason HAMD ratings have only been analyzed at treatment initiation and after 1 week of treatment.
There is considerable evidence that mild depression often improves spontaneously even in the absence of antidepressant treatment, the so-called placebo effect.Citation9 In order to reduce any possible bias due to the inclusion of patients with mild depression, only patients with baseline HAMD scores of 26 or more were included.
Results
The clinical characteristics of the two patient groups and their treatment details are given in and . The mean initial HAMD value of the TCA group was higher than the milnacipran group (), although this difference was not quite significant (P = 0.0512). The four highest scoring items of the HAMD at baseline – item 1 (depressed mood), item 3 (suicidality), item 7 (interest in work and activities), and item 10 (psychic anxiety) – were the same in both groups ( and ).
Figure 1 Mean scores of individual HAMD items at baseline (dark gray columns) and after 1 week of tricyclic antidepressant treatment (light gray columns). The HAMD items are as follows: (1) depressed mood, (2) feeling of guilt, (3) suicide, (4) early insomnia, (5) middle insomnia, (6) late insomnia, (7) work and activities, (8) retardation, (9) agitation, (10) psychic anxiety, (11) somatic anxiety, (12) gastrointestinal somatic symptoms, (13) general somatic symptoms, (14) genital symptoms, (15) hypochondriasis, (16) loss of weight, and (17) insight.
Abbreviation: HAMD, 17-item Hamilton depression rating scale.
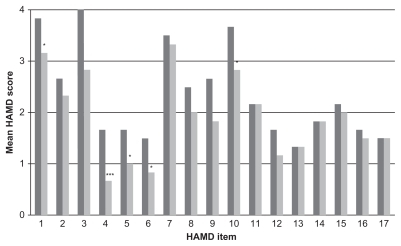
Figure 2 Mean scores of individual HAMD items at baseline (dark gray columns) and after 1 week of milnacipran treatment (light gray columns). The HAMD items are as follows: (1) depressed mood, (2) feeling of guilt, (3) suicide, (4) early insomnia, (5) middle insomnia, (6) late insomnia, (7) work and activities, (8) retardation, (9) agitation, (10) psychic anxiety, (11) somatic anxiety, (12) gastrointestinal somatic symptoms, (13) general somatic symptoms, (14) genital symptoms, (15) hypochondriasis, (16) loss of weight, and (17) insight.
Abbreviation: HAMD, 17-item Hamilton depression rating scale.
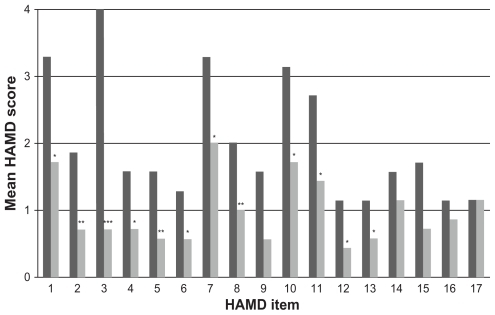
Table 3 Mean HAMD ratings in the two treatment groups before and after 1 week of treatment
In the TCA group, five of the 17 items (item 1 [depressed mood], item 4 [early insomnia], item 5 [middle insomnia], item 6 [late insomnia], and item 10 [psychic anxiety]) were significantly improved after 1 week of treatment (). In the milnacipran group, twelve of the 17 items (item 1 [depressed mood], item 2 [feeling of guilt], item 3 [suicidality], item 4 [early insomnia], item 5 [middle insomnia], item 6 [late insomnia], item 7 [interest in work and activities], item 8 [retardation], item 10 [psychic anxiety], item 11 [somatic anxiety], item 12 [gastrointestinal symptoms], and item 13 [general somatic symptoms]) were significantly improved after 1 week of treatment ().
As explained in the methods section, most patients were transferred away from the Juntendo Shizuoka Hospital after a week or two and only incomplete data were available beyond 1 week’s treatment duration. In the TCA group, HAMD data were available after 2 weeks treatment for four patients. In all cases, HAMD values continued to decrease significantly (mean 35.5 ± 4.9 at week 1 to 19.3 ± 2.5 at week 2, P = 0.007). The two patients for whom data were available after 4 weeks of treatment had HAMD values of 7 and 13.
In the milnacipran group, HAMD data were available after 2 weeks treatment for two patients. For both of these, HAMD values continued to decrease (27 to 20 and 20 to 13 from week 1 to week 2, respectively). The single patient for whom data were available after 4 weeks treatment was in remission with a HAMD value of 3.
In the TCA group, treatment was initiated with an intravenous dose of 15–50 mg/day increasing within the first week to 30–50 mg/day (). In the milnacipran group, patients were rapidly titrated from the initial dose of 30 mg/day or 50 mg/day to 50 mg/day or 100 mg/day within a few days (). In spite of the rapid titration, both treatments were relatively well tolerated. In the TCA group, four of the six patients reported side effects. There was one case of dry mouth (sufficiently severe to cause problems of speech articulation), a manic switch in a bipolar patient, moderate drowsiness with constipation, and severe drowsiness with delirium. In the group of patients taking milnacipran, two of the seven patients reported side effects (mild drowsiness and palpitations). No aggravation of psychiatric symptoms was observed in either group. The suicidality item scored maximally at baseline in all patients so that no aggravation of suicidal tendencies could be observed on the HAMD score. No suicide attempts occurred during the duration of observation period covered by this analysis.
Discussion
All of the patients in the present analysis were initially treated for physical injuries resulting from their suicide attempts. They were all suffering from moderate to severe depression with strong suicidal ideation. Both antidepressant treatments resulted in rapid significant overall improvement in depressive symptoms as determined by the mean HAMD scores at the end of the first week (). Treatment with TCAs resulted in significant reduction in the severity of depressed mood, the insomnia items, and psychic anxiety. The rapid improvement in the insomnia items is possibly related to the well-known sedative effect of amitriptyline. Treatment with milnacipran had a significant effect on a wide range of symptoms. In addition to the symptoms improved by the TCAs, milnacipran also significantly improved feelings of guilt, suicidality, anhedonia (interest in work and activities), retardation, and somatic symptoms.
Of particular interest is the suicidality item. Since the patients had all made a very recent suicide attempt, this item was scored maximally (a score of four) in all patients at the pretreatment evaluation. After 1 week of TCA therapy, four out of six patients still had a score of three or four on this item, indicating the continued presence of strong suicidal tendencies. In the milnacipran group, after 1 week of treatment, six of the seven patients scored zero or one, suggesting a virtual absence of suicidal tendencies in most patients.
Milnacipran is not sedativeCitation8 and the significant reduction of the insomnia items is more likely to be part of a global antidepressant effect than a specific sedative effect. Such a global effect is also suggested by the significant improvement in all of the somatic symptoms.
The little data that were available at time-points beyond 1 week suggest a continuation of the improvement seen during the first week.
At the time of this study, TCAs were the standard treatment of depression, usually initiated by the intravenous route. Because of the problems of adverse effects of the TCAs, emergency psychiatrists have been particularly open to better tolerated alternative antidepressants such as selective serotonin reuptake inhibitors (SSRIs) and SNRIs. Some concerns have been raised that the use of SSRIs may increase the risk of suicide.Citation10,Citation11 Whether these concerns are founded or not, they have had the effect of dissuading psychiatrists in Japan from using SSRIs in overtly suicidal patients. Milnacipran has been shown, in comparative studies, to be equally effective to TCAs in treating depressive symptoms and better tolerated. Citation12,Citation13 In addition, a review of early clinical trials found that the risk of suicide and attempted suicide in clinical trials was considerably greater in patients treated with SSRIs than either milnacipran or TCAs.Citation8 A recent studyCitation14 has shown that during treatment of depressed patients with milnacipran, suicidal thoughts regressed progressively in parallel with other depressive symptoms. At no time was there any increase in suicidal thoughts even in patients who reported anxiety as an adverse event.Citation14 These reassuring data have encouraged emergency psychiatrists to use milnacipran, the first SNRI available in Japan, in suicidal patients. The data from the present study thus have both a theoretical and practical importance.
This study has a number of weaknesses, most of them linked to the type of patients studied. The number of patients is small and the study duration is short. In spite of these shortcomings, robust statistically significant effects have been observed. The study is a post hoc analysis of medical records. The limitations that this introduces, including the impossibility to make intergroup comparisons, are compensated by the “real-life” situation observed. Since the treatment prescribed was that which was considered to be “the most appropriate for the patient,” it is quite possible that the patients treated with TCAs and milnacipran differed significantly in a number of ways. The TCA-treated patients had more severe symptoms as indicated by a higher mean HAMD score at baseline, although this was not significant with the limited number of patients studied.
Finally, the absence of a placebo group leaves open the possibility of a “placebo effect” or spontaneous improvement. If this were so, however, one would have expected the results to be similar for the two groups, which is not the case. In addition the nature of records analyzed precluded the exploration of potential confounding factors which could have influenced the improvement of depressive symptoms including suicidality.
In view of these shortcomings only the most conservative conclusions should be drawn from this analysis. Milnacipran appears to rapidly improve a wide range of depressive symptoms, including suicidality within the first week. The improvement would seem to be at least equivalent to that achieved with the classical treatment, TCAs, in similar patients and possibly affecting a wider range of symptoms. Since milnacipran has been shown in comparative trials to be better tolerated than TCAs,Citation8 it offers an interesting option for the treatment of suicidal patients in an emergency setting. Clearly, a replication of this analysis in a larger number of patients is warranted.
Acknowledgments
We thank Dr Daisuke Mochizuki and Dr Mike Briley for their helpful comments and suggestions in the preparation of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- Ministry of Health, Labor, and Welfare JapanStudy on Improvement of Health Statistics Documents concerning SuicideOutline of Suicide Prevention Measures2002
- BarracloughBBunchJNelsonBSainsburyPA hundred cases of suicide: clinical aspectsBr J Psychiatry19741253553734425774
- SakinofskyITreating suicidality in depressive illness. Part I: current controversiesCan J Psychiatry2007526 Suppl 171S84S17824354
- SakinofskyITreating suicidality in depressive illness. Part 2: does treatment cure or cause suicidality?Can J Psychiatry2007526 Suppl 185S101S17824355
- YoshimuraRTreatment of depression from the point of view of suicide preventionSeishin Shinkeigaku Zasshi20071099822833 Japanese18064872
- DaigleMSPouliotLChagnonFGreenfieldBMisharaBSuicide attempts: prevention of repetitionCan J Psychiatry2011561062162922014695
- VitielloBBrentDAGreenhillLLDepressive symptoms and clinical status during the Treatment of Adolescent Suicide Attempters (TASA) StudyJ Am Acad Child Adolesc Psychiatry20094810997100420854770
- MontgomerySAProstJFSollesABrileyMEfficacy and tolerability of milnacipran: an overviewInt Clin Psychopharmacol199611 Suppl 447518923127
- BaumeisterHInappropriate prescriptions of antidepressant drugs in patients with subthreshold to mild depression: time for the evidence to become practiceJ Affect Disord2011 [Epub ahead of print.]
- HealyDWhitakerCAntidepressants and suicide: risk-benefit conundrumsJ Psychiatry Neurosci200328533133714517576
- FergussonDDoucetteSGlassKCAssociation between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trialsBMJ2005330748839640315718539
- YamashitaIMatsubaraROnoderaIItoKOkadaFAsanoYClinical evaluation of milnacipran hydrochloride (TN-912) on depression and depressive states – phase ii clinical trial with imipramine hydrochloride as a control drugJ Clin Therap Med199511819842
- KasperSPletanYSollesATournouxAComparative studies with milnacipran and tricyclic antidepressants in the treatment of patients with major depression: a summary of clinical trial resultsInt Clin Psychopharmacol199611 Suppl 435398923125
- AvedisovaABorodinVZakharovaKAldushinAEffect of milnacipran on suicidality in patients with mild to moderate depressive disorderNeuropsychiatr Dis Treat2009541542019721721