Abstract
Objective
The study aim was to assess the cumulative burden of polymorphisms located within four genetic loci previously associated with posttraumatic stress disorder (PTSD) among outpatients at risk for PTSD.
Methods
Diagnostic interviews were completed and DNA samples collected among 412 pain patients to determine if FKBP5 (rs9470080), COMT (rs4680), CHRNA5 (rs16969968), and CRHR1 (rs110402) single nucleotide polymorphisms were cumulatively associated with increased risk for PTSD.
Results
In bivariate analyses, it was found that a count of specific PTSD risk alleles located within FKBP5, COMT, CHRNA5, and CRHR1 genetic loci (allele range = 0–6, mean count = 2.92, standard deviation = 1.36) was associated with lifetime (t [409] = 3.430, P = 0.001) and early onset PTSD (t [409] = 4.239, P = 0.000028). In logistic regression, controlling for demographic factors, personality traits, and trauma exposures, this risk allele count remained associated with both lifetime (odds ratio = 1.49, P = 0.00158) and early onset PTSD (odds ratio = 2.36, P = 0.000093). Interaction effects were also detected, whereby individuals with higher risk allele counts and higher trauma exposures had an increased risk of lifetime PTSD (allele count × high trauma, P = 0.026) and early onset PTSD (allele count × high trauma, P = 0.016) in these logistic regressions. Those with no or few risk alleles appeared resilient to PTSD, regardless of exposure history.
Conclusion
A cumulative risk allele count involving four single nucleotide polymorphisms located within the FKBP5, COMT, CHRNA5, and CRHR1 genes are associated with PTSD. Level of trauma exposure interacts with risk allele count, such that PTSD is increased in those with higher risk allele counts and higher trauma exposures. Since the single nucleotide polymorphisms studied encompass stress circuitry and addiction biology, these findings may have implications for neuropsychiatric research and treatment.
Introduction
While studies suggest that most adults have experienced lifetime traumatic events, relatively few of them develop posttraumatic stress disorder (PTSD).Citation1–Citation3 Available twin and family studies indicate that PTSD is at least moderately heritable, with approximately 30% of variance accounted for by genetic factors.Citation4 To date several genetic components for PTSD have been identified that may explain this risk.Citation5–Citation7 These include biologic pathways involving the hypothalamic-pituitary-adrenal (HPA), locus coeruleus-noradrenergic, and the limbic systems, among others.Citation6,Citation8–Citation11 In the current study, genetic risk factors for PTSD were assessed among outpatients with chronic, nonmalignant pain, a condition often associated with PTSD.Citation12
For the current study, four genetic markers were assessed using a cumulative risk allele model to test for an association with PTSD among outpatients, similar to what has been undertaken to predict complex genetic associations in other clinical areas.Citation13 To assess PTSD, a validated questionnaire based on the Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition was used.Citation2,Citation14–Citation17 Extending previous research,Citation9 single nucleotide polymorphisms (SNPs) located within the FK506 binding protein-5 (FKBP5), catechol-O-methyltransferase (COMT), cholinergic receptor nicotinic alpha-5 (CHRNA5), and the corticotropin-releasing hormone receptor-1 (CRHR1) gene clusters were specifically genotyped and these markers were assessed for cumulative risk for PTSD.
The COMT gene is associated with anxiety disorders, psychosis, depression, and other conditions involving catecholamine pathway regulation and has recently been associated with PTSD.Citation9,Citation18,Citation19 The FKBP5 gene regulates glucocorticoid receptor sensitivity, is functionally involved in HPA axis activity, and is associated with PTSD.Citation5,Citation6,Citation9,Citation20 The CHRNA gene cluster, which encodes components of the nicotinic acetylcholine receptor, is associated with nicotine dependence and cigarette smoking,Citation21,Citation22 substance misuse,Citation23 and recently PTSD.Citation9 The CRHR1 gene is a polypeptide hormone and neurotransmitter involved in corticotropin-releasing hormone activity associated with the stress response. Studies suggest that this gene also regulates HPA axis function and is associated with the impact of traumatic stress exposure and PTSD.Citation20,Citation24
Methods
Subjects
Study subjects were adult outpatients (≥18 years old) who were prescribed pain medications for nonmalignant pain for ≥4 months.Citation14,Citation23 Chronic pain patients typically have a high prevalence of PTSD.Citation25,Citation26 The mean age of patients was 55 years old (standard deviation = 13.4) and the prevalence of lifetime PTSD was 15% (95% confidence interval [CI] = 11.7–18.1). The sample was randomly selected from among a population of chronic pain outpatients identified by query of the electronic health records of the Geisinger Clinic, an integrated health system that serves residents of 40 central and northeastern Pennsylvania counties.Citation14 Geisinger’s ambulatory clinics have used the Epic (Epic Systems Corporation, Verona, WI) outpatient electronic health record system since 2001. With patient consent, trained and supervised interviewers administered structured diagnostic telephone interviews from August 2007 through November 2008.
Phenotypic and confounding measures
Study interviewers administered diagnostic surveys using instruments used in past research.Citation2,Citation16,Citation17 To meet criteria for lifetime PTSD, patients had to meet the full diagnostic criteria for PTSD, known as the “A through F” criteria.Citation2,Citation15 These criteria include experiencing intense fear (criterion A), re-experiencing the event (criterion B), avoidance of stimuli associated with the event (criterion C), experiencing increased arousal (criterion D), experiencing symptoms for a month or more (criterion E), and experiencing psychological distress or impairment (criterion F). To be diagnosed with early onset PTSD, the patient had to meet the A–F diagnostic criteria for PTSD before the age of 35 years. Research typically suggests that early onset PTSD likely has greater impact on trauma victims.Citation27,Citation28 Over half (53%) of the PTSD cases in the current study met this early onset definition. Research instruments relevant to childhood adversity, lifetime trauma exposure, and self-esteem were also administered.Citation17,Citation29–Citation31 To assess neuroticism, also a risk factor for PTSD,Citation32 the NEO Five-Factor Inventory was used.Citation33
The childhood adversity scale used was the Adverse Childhood Experiences scale, a widely used measure of childhood adversity with good reported reliability and validity.Citation29,Citation30,Citation34 Cronbach’s alpha for the Adverse Childhood Experiences scale in the current study was 88. The trauma scale used was a measure that has been extensively used in past trauma research and has been shown to have excellent predictive validity.Citation16,Citation17,Citation35 In the current study, this measure consisted of a count of the number of traumatic events (eg, combat exposure, sexual assault, major disaster) that the person experienced in his/her lifetime (range 0–9, mean = 2.19, standard deviation = 2.0). Self-esteem was measured by the Rosenberg Self-Esteem Scale, a widely used construct to measure self-esteem.Citation31,Citation36 In the current study, Cronbach’s alpha was 80 for this scale. The NEO Five-Factor Inventory is a personality measure widely used in neuropsychiatric research.Citation33,Citation37,Citation38 This scale is reported to have good reliability and validity.Citation37,Citation38 Cronbach’s alpha for the scales used in the NEO are good, typically between 0.76–0.89.Citation39
Given that the current study population was drawn from a sample of pain patients, the study results were also adjusted for prescription opioid dependence, level of pain impairment, and the number of opioid prescriptions received as potential confounders. Opioid dependence was based on whether the subject met criteria for Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition drug dependence and was based on a diagnostic interview.Citation14 Pain assessment was based on the Brief Pain Inventory, a widely used and validated instrument.Citation40,Citation41 The number of opioid prescriptions received over the past 3 years was based on electronic health record data. Finally, to potentially account for population stratification, the study results were also adjusted for ancestry status, based on country of origin, coded as Northern European, Eastern European, Southern European, and other. Non-Caucasians were excluded from these analyses. This study was approved by the Geisinger Clinic’s Institutional Review Board.
DNA collection and genotyping
Following the study interview, buccal swab kits were mailed to consenting adults. Altogether, 412 returned the buccal swab kit with adequate DNA for the current analyses. Two subjects were identified as non-Caucasian and were not included in the current study analyses, as noted. The candidate genes studied (and corresponding SNPs) were: COMT (rs4680), FKBP5 (rs9470080), CHRNA5 (rs16969968), and CRHR1 (rs110402). The SNPs for the four target loci were originally selected using linkage disequilibrium tagging with consideration of prior evidence and functional annotation.Citation42 For each gene, the location of the main association signals related to PTSD were determined from the relevant literature. The linkage disequilibrium structure of the gene in the HapMap Caucasian sample (release 23a; http://www.hapmap.org) was then examined using the Gabriel algorithm.Citation43 The algorithm of de Bakker implemented in the software HaploView 4.0 (Daly Lab, Broad Institute, Cambridge, MA) was then used to select tagged SNPs for the target block in each gene.Citation44 The Gabriel algorithm delineates boundaries of genomic markers identified as haplotype blocks with little evidence for historical recombination, within which only a few common haplotypes are observed and that can be tagged for association analysis using unsaturated SNP subsets.Citation43 The de Bakker approach selects tags from empirical data based on linkage disequilibrium to maximize the cost-effectiveness of using a limited number of typed SNPs in a locus.Citation44
Genotyping was performed on an Applied BioSystems® 7500 Real-Time Polymerase Chain Reaction System using TaqMan® kits (Applied BioSystems, Foster City, CA), following the manufacturer’s protocols. Quality control measures included visual inspection of the allelic discrimination plots, monitoring concordance of cross-plated duplicate pairs, monitoring the overall call rate, and monitoring agreement with Hardy–Weinberg expectations.Citation45
Statistical analyses
Based on previous research,Citation9 a bivariate association with PTSD was tested for the four target SNPs in the current study and each of these was found to be significant (P < 0.05). A cumulative risk allele model that included these four SNPs was then developed, as has been done in other clinical areas.Citation13,Citation46 As suggested, each of these target SNPs had been associated with PTSD in previous reports. For each SNP studied, as previously discussed, an additive coding scheme (ie, subjects assigned zero, one, or two according to the number of copies of the minor allele) was tested followed by a dominant/recessive coding scheme (ie, carriers and homozygotes for the tested allele assigned a value of one, versus homozygotes for the alternate allele assigned a value of zero) if the additive model was not significant. Only one SNP tested was reduced to a dominant/recessive model ().Citation9 A count of alleles across these loci resulted in a risk score ranging from 0–6 (mean = 2.92, standard deviation = 1.36). For descriptive analyses, chi-squared tests were used to assess the associations between the main variables of interest and PTSD status (). In addition, independent t-tests were also used to assess the bivariate association between risk allele counts and key exposure variables, which included trauma exposure, adversity exposure, neuroticism level, self-esteem, as well as PTSD status (). Next, multivariate logistic regressions were used to test for an association of genetic risk allele count with PTSD status, controlling for potential confounding ().Citation42,Citation47 Gene × environmental exposure interaction effects were specifically tested for (), since these have been previously reported for some of these loci.Citation5,Citation20 For these interaction assessments, lifetime trauma and childhood adversity were classified as high versus not, based on the upper quintile or quartile range for lifetime trauma and childhood adversity, respectively. Specific gene × environment interaction effects were statistically evaluated by use of a cross-product term for risk allele-count × trauma exposure (or childhood adversity) in multivariate logistic regressions that included the main effects (ie, risk allele count and the respective environmental exposure) and predicted PTSD status. As suggested, given the current study population, prescription opioid dependence, pain impairment, and the number of opioid prescriptions received were also assessed as potential confounders as a final regression step. For data analyses, Stata® version 11.2 (StataCorp LP, College Station, TX) was used.
Table 1 Single nucleotide polymorphisms included in risk allele model
Table 2 Lifetime posttraumatic stress disorder and early onset posttraumatic stress disorder by study variables
Table 3 Mean risk allele counts by posttraumatic stress disorder status and key study variables (mean risk allele count = 2.92, standard deviation = 1.36)
Table 4 Multivariate logistic regressions predicting lifetime and early onset posttraumatic stress disorder from risk allele count, controlling for key risk factors and potential confounders
Table 5 Multivariate logistic regression interactions for lifetime and early onset posttraumatic stress disorder showing risk allele count × high trauma exposure effect
Results
The bivariate analysis confirmed that all four target SNPs were associated with lifetime PTSD (P < 0.05), including FKBP5 (rs9470080), COMT (rs4680), CHRNA5 (rs16969968), and CHRR1 (rs110402) SNPs. For each of these target SNPs, shows the chromosomal location, minor allele frequency, functional annotation, and Hardy–Weinberg equilibrium results. All four SNPs studied met expectations for Hardy–Weinberg equilibrium (P > 0.025). shows the association between lifetime and early onset PTSD and the main study variables. As can be seen, lifetime PTSD is associated with female sex, younger age, being unmarried, having lower household income, having higher trauma exposure, having higher childhood adversity, having higher neuroticism, and having lower self-esteem (all P values <0.05).
shows the unadjusted risk allele count results by PTSD status and key study variables. The prevalence of lifetime PTSD in the study was 14.7% (95% CI = 11.7–18.1) and the prevalence of early onset PTSD was 7.6% (95% CI = 5.1–10.7) (). As can be seen, both lifetime (P = 0.001) and early onset (P = 0.000028) PTSD were associated with higher risk allele counts. Trauma exposure, childhood adversity, neuroticism, and self-esteem were not associated with risk allele counts (all P values >0.05).
presents the multivariate logistic regression results for lifetime and early onset PTSD. As can be seen, after controlling for demographic factors, trauma exposures and psychological traits (ie, neuroticism and self-esteem), risk allele count was significant for lifetime PTSD (odds ratio [OR] = 1.49, P = 0.00158). Noteworthy is that high trauma exposure (P = 0.049) and high neuroticism (P = 0.0003) were significant in this adjusted lifetime PTSD model. For early onset PTSD, the multivariate logistic regression results were highly significant (OR = 2.36, P = 0.000093). In contrast to lifetime PTSD, significant variables in this early onset model included high trauma exposure (P = 0.0011) and high childhood adversity (P = 0.0074). These logistic regression data suggest that those with six or more risk alleles have about nine times greater risk of lifetime PTSD (6 × 1.49 ≈ 9) and that those with six or more risk alleles have about 14 times greater risk of early onset PTSD (6 × 2.36 ≈ 14) than those with no risk alleles, respectively ().
Following assessment of main effect models, interaction effects were evaluated for allele count × high trauma and allele count × high adversity exposure for both lifetime and early onset PTSD (). These interaction effects were significant for risk allele count × high trauma exposure for both lifetime (OR = 2.05, P = 0.026) and early onset PTSD (OR = 3.47, P = 0.016) (). These interactions suggest that individuals with higher risk allele counts and higher trauma exposures had an increased risk of PTSD. However, the interaction effect for risk allele count × high childhood adversity was not significant in either the lifetime or early onset PTSD model (available upon request).
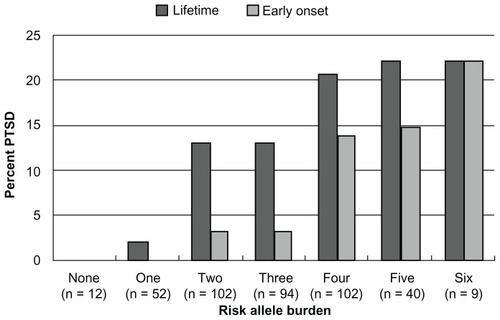
shows the prevalence of both lifetime and early onset PTSD by risk allele burden. As seen, those with less than two risk alleles have a low prevalence of PTSD. By comparison, those with four or more risk alleles have a prevalence of lifetime PTSD of more than 20%. These data suggest that those with no or few risk alleles may be resilient to PTSD, regardless of environmental exposures, since these exposures were not associated with allele burden () or with significant confounding of the association between allele count and PTSD (). This suggests that the main exposures known to cause PTSD – psychological trauma – did not vary by risk allele count. However, those with higher risk allele counts and high trauma exposure have an increased risk of PTSD (), indicating that a gene × environmental effect is present for risk allele count by level of trauma exposure.
Figure 1 Prevalence of lifetime posttraumatic stress disorder and early onset posttraumatic stress disorder by risk allele burden.
Abbreviation: PTSD, posttraumatic stress disorder.
As suggested, since the study population included Caucasian pain patients on pain medications, pain status, drug dependence status, the number of prescription orders for pain medicines were also controlled for, but these variables had little impact on the final regression results. Based on previous research, since a dominant model for SNP marker rs9470080 (FKBP5) – and not the other three markers – was used, a risk allele model that included all four SNPs was also assessed as additive risk allele markers (ie, all four SNPs coded zero, one, or two). This additive model produced similar results in the logistic regressions. Finally, ancestry status was also entered into the final logistic regression models and this adjustment also made little difference.
Discussion
Examination of risk allele counts by PTSD status, as coded in the regression models, suggested that the “A” allele of rs16969968 (CHRNA5) and rs4680 (COMT), the “T” allele of rs9470080 (FKBP5), and the “G” allele of rs110402 (CRNR1) are more common among PTSD cases than non-PTSD cases. Thus, this study confirmed that SNP markers rs16969968(A), rs9470080(T), rs4680(A), and rs110402(G) were each individually associated with PTSD. When these SNP markers were combined into a cumulative risk allele model (), the bivariate t-test results were significant for both lifetime (P = 0.001) and early onset PTSD (P = 0.000028). In multivariate analyses controlling for potential confounders, both lifetime (OR = 1.49, P = 0.00158) and early onset PTSD (OR = 2.36, P = 0.000093) remained significant. Interactions were also detected for risk allele count × trauma exposure for both lifetime (P = 0.026) and early onset PTSD (P = 0.016). The final cumulative risk model suggests that the risk for lifetime PTSD was about nine times higher among those with six or more risk alleles (1.49 × 6 ≈ 9), compared to those with no risk alleles. For early onset PTSD, this risk was about 14 times higher among those with six or more risk alleles (2.36 × 6 ≈ 14).
FKBP5 polymorphisms are known to regulate the cortisol-binding affinity and nuclear translocation of the glucocorticoid receptor and polymorphisms at the FKBP5 locus have been reported to interact with exposure to child adversity in predicting PTSD.Citation5,Citation48 COMT polymorphisms have been found to affect fear extinction and are thought to play a role in the etiology of anxiety disorders.Citation9,Citation19,Citation49 The CHRNA5 gene has been associated with smoking and nicotine dependence.Citation21,Citation22 PTSD is known to be associated with cigarette smoking.Citation50 This locus has recently been associated with PTSD and is likely involved in mammalian fear circuitry.Citation9 Research suggests that the CRHR1 gene regulates HPA axis function in conjunction with exposure to early life trauma.Citation20 In addition, corticotropin-releasing hormone is thought to play a role in the pathophysiology of stress-related psychiatric disorders, such as major depressive disorder and PTSD.Citation24 It has been suggested that corticotropin-releasing hormone contributes to alterations in memory formation in PTSD cases and that this hormone influences hippocampal regulation of the HPA axis.Citation24 Note that the assignment of risk alleles for the SNP variants examined in the current study agree with prior findings for the FKBP5, CRHR1, and COMT genetic variants.Citation5,Citation51–Citation53 A 2011 paper, based on this same cohort,Citation9 was the first to associate a CHRNA5 genetic variant with PTSD, so the risk allele assignment in the current paper would be consistent with that earlier paper.
It is noteworthy that research suggests that PTSD is associated with an increased prevalence of chronic health conditions, including cardiovascular disease, rheumatoid arthritis, and other chronic diseases.Citation54–Citation60 Studies suggest that PTSD may result in inflammatory injuries through overactivation of the HPA and sympathetic-adrenal-medullary stress axes, subsequently followed by hypocortisolism related to molecular downregulation of these systems.Citation54,Citation61,Citation62 Epigenetic-related phenomena are also suspected.Citation63,Citation64 Consistent with these findings, current research suggests that low-grade systemic inflammatory activity is common in PTSD.Citation65–Citation68 This PTSD-disease link also could be related to adverse health behaviors, such as cigarette smoking and substance misuse related to self-regulation of aversive psychological states brought on by PTSD.Citation69,Citation70
Study limitations for this research include the fact that the interview data were based on self-report, the total sample size was limited, and the study participants were more often female and drawn from a pain population. Also, multiple comparisons were not adjusted for and population stratification using genetic methods were not taken into account, although final regression results were adjusted for reported ancestry and all non-Caucasians were eliminated from the analyses to control for stratification. These factors may have biased the results and could limit study generalization. Also, the total number of PTSD cases in the study was limited (n = 59). Thus, the findings will require further replication.
The current research suggests that FKBP5, COMT, CHRNA5, and CRHR1 genetic loci involving biologic pathways encompassing inflammatory mechanisms, nicotine dependence, substance misuse, sleep regulation, and fear circuitry, among others, are associated with PTSD and interact with levels of trauma exposure.Citation9 These genetic loci seem worthy of research related to the behavioral genetics of PTSD as well as related to chronic disease onset. For example, researchers have reported that CHRNA gene is associated with lung cancer.Citation71 This gene was also recently associated with cigarette smoking and nicotine dependence.Citation23 Thus, the causal pathway for lung cancer involving these loci appears to involve nicotine addiction associated with alterations in nervous system molecular biology. Without this compulsive addiction behavior, there would likely be insufficient exposure to cigarette smoke to result in lung cancer for most individuals.
Similarly, the genetic components involved in PTSD, including the FKBP5, COMT, CHRNA5, and CRHR1 genes, may be associated with the pathophysiology of specific diseases following PTSD onset. Recently it was reported that CHRNA was not only associated with lung cancer but also with peripheral arterial disease.Citation22 PTSD has also been associated with metabolic syndrome.Citation72 Since the risk alleles studied appear to encompass multiple, interrelated disease pathways, these genetic markers may have research implications for neuropsychiatric research and treatment. The absence of PTSD among those with no or few risk alleles is intriguing and also worthy of investigation. The authors suspect that genetic resilience, in this case the absence of PTSD risk alleles, may also be associated with chronic disease resilience, but further research is required to confirm this hypothesis.
Previous presentation
Preliminary results from this study were presented at: The 31st Annual Anxiety Disorders Association of America Conference, New Orleans, LA, March 25, 2011.
Acknowledgments
Support for this study was provided in part by the Geisinger Clinic Research Fund (Grant No TRA-015) and the National Institute of Mental Health (Grant No R21-MH-086317) to Dr Boscarino.
Disclosure
The authors report no conflicts of interest in this work.
References
- KesslerRCSonnegaABrometEHughesMNelsonCBPosttraumatic stress disorder in the National Comorbidity SurveyArch Gen Psychiatry19955212104810607492257
- BoscarinoJAAdamsREPTSD onset and course following the World Trade Center disaster: findings and implications for future researchSoc Psychiatry Psychiatr Epidemiol2009441088789819277439
- BreslauNKesslerRCChilcoatHDSchultzLRDavisGCAndreskiPTrauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of TraumaArch Gen Psychiatry19985576266329672053
- SteinMBJangKLTaylorSVernonPALivesleyWJGenetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin studyAm J Psychiatry2002159101675168112359672
- BinderEBBradleyRGLiuWAssociation of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adultsJAMA2008299111291130518349090
- KoenenKCGenetics of posttraumatic stress disorder: review and recommendations for future studiesJ Trauma Stress200720573775017955543
- NemeroffCBBremnerJDFoaEBMaybergHSNorthCSSteinMBPosttraumatic stress disorder: a state-of-the-science reviewJ Psychiatr Res200640112116242154
- BroekmanBFOlffMBoerFThe genetic background to PTSDNeurosci Biobehav Rev200731334836217126903
- BoscarinoJAErlichPMHoffmanSNRukstalisMStewartWFAssociation of FKBP5, COMT and CHRNA5 polymorphisms with PTSD among outpatients at risk for PTSDPsychiatry Res2011188117317421440309
- SkeltonKResslerKJNorrholmSDJovanovicTBradley-DavinoBPTSD and gene variants: new pathways and new thinkingNeuropharmacology201262262863721356219
- RauchSLDrevetsWCNeuroimaging and neuroanatomy of stress-induced and fear circuitry disordersAndrewsGCharneyDSSirovatkaPJRegierDAStress-Induced and Fear Circuitry Disorders: Refining the Research Agenda for DSM-VArlington, VAAmerican Psychiatric Association2009215254
- McFarlaneACThe long-term costs of traumatic stress: intertwined physical and psychological consequencesWorld Psychiatry20109131020148146
- StillCDWoodGCChuXHigh allelic burden of four obesity SNPs is associated with poorer weight loss outcomes following gastric bypass surgeryObesity20111981676168321311511
- BoscarinoJARukstalisMHoffmanSNRisk factors for drug dependence among out-patients on opioid therapy in a large US healthcare systemAddiction2010105101776178220712819
- BoscarinoJAAdamsREFigleyCRMental health service use after the World Trade Center disaster: utilization trends and comparative effectivenessJ Nerv Ment Dis20111992919921278537
- GaleaSAhernJResnickHPsychological sequelae of the September 11 terrorist attacks in New York CityN Engl J Med20023461398298711919308
- ResnickHSKilpatrickDGDanskyBSSaundersBEBestCLPrevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of womenJ Consult Clin Psychol19936169849918113499
- CraddockNOwenMJO’DonovanMCThe catechol-O-methyl transferase (COMT) gene as a candidate for psychiatric phenotypes: evidence and lessonsMol Psychiatry200611544645816505837
- MontagCBuckholtzJWHartmannPCOMT genetic variation affects fear processing: psychophysiological evidenceBehav Neurosci2008122490190918729643
- GillespieCFPhiferJBradleyBResslerKJRisk and resilience: genetic and environmental influences on development of the stress responseDepress Anxiety2009261198499219750552
- SpitzMRAmosCIDongQLinJWuXThe CHRNA5-A3 region on chromosome 15q24-25.1 is a risk factor both for nicotine dependence and for lung cancerJ Natl Cancer Inst2008100211552155618957677
- ThorgeirssonTEGellerFSulemPA variant associated with nicotine dependence, lung cancer and peripheral arterial diseaseNature2008452718763864218385739
- ErlichPMHoffmanSNRukstalisMNicotinic acetylcholine receptor genes on chromosome 15q25.1 are associated with nicotine and opioid dependence severityHum Genet2010128549149920725741
- von WolffGAvrabosCStepanJVoltage-sensitive dye imaging demonstrates an enhancing effect of corticotropin-releasing hormone on neuronal activity propagation through the hippocampal formationJ Psychiatr Res201145225626120619419
- BeckJGClappJDA different kind of co-morbidity: understanding posttraumatic stress disorder and chronic painPsychol Trauma20113210110821765966
- PhiferJSkeltonKWeissTPain symptomatology and pain medication use in civilian PTSDPain2011152102233224021665366
- KesslerRCWangPSThe descriptive epidemiology of commonly occurring mental disorders in the United StatesAnnu Rev Public Health20082911512918348707
- SpinelliSCheferSSuomiSJHigleyJDBarrCSSteinEEarly-life stress induces long-term morphologic changes in primate brainArch Gen Psychiatry200966665866519487631
- DongMGilesWHFelittiVJInsights into causal pathways for ischemic heart disease: adverse childhood experiences studyCirculation2004110131761176615381652
- FelittiVJAndaRFNordenbergDRelationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) StudyAm J Prev Med19981442452589635069
- RosenbergMConceiving the SelfNew York, NYBasic Books1979
- CoxBJMacPhersonPSEnnsMWMcWilliamsLANeuroticism and self-criticism associated with posttraumatic stress disorder in a nationally representative sampleBehav Res Ther200442110511414744527
- CostaPTJrWidigerTAPersonality Disorders and the Five-Factor Model of Personality2nd edArlington, VAAmerican Psychological Association2002
- ChapmanDPWhitfieldCLFelittiVJDubeSREdwardsVJAndaRFAdverse childhood experiences and the risk of depressive disorders in adulthoodJ Affect Disord200482221722515488250
- BoscarinoJAAdamsREFigleyCRMental health service use 1-year after the World Trade Center disaster: implications for mental health careGen Hosp Psychiatry200426534635815474634
- BlascovichJTomakaJMeasures of self-esteemRobinsonJPShaverPRWrightsmanLSMeasures of Personality and Social Psychological Attitudes1San Diego, CAAcademic Press1991115160
- GoodwinRDFriedmanHSHealth status and the five-factor personality traits in a nationally representative sampleJ Health Psychol200611564365416908463
- TaylorMDWhitemanMCFowkesGRLeeAJAllerhandMDearyIJFive Factor Model personality traits and all-cause mortality in the Edinburgh Artery Study cohortPsychosom Med200971663164119483118
- CostaPTJrMcCraeRRRevised NEO Personality Inventory (NEO PI-R) and NEO Five Factor Inventory (NEO-FFI): Professional ManualLutz, FLPsychological Assessment Resources Inc1992
- CleelandCSRyanKMPain assessment: global use of the Brief Pain InventoryAnn Acad Med Singapore19942321291388080219
- TanGJensenMPThornbyJIShantiBFValidation of the Brief Pain Inventory for chronic nonmalignant painJ Pain20045213313715042521
- ZieglerAKonigIRA Statistical Approach to Genetic Epidemiology2nd edWeinheimWiley-VCH2010
- GabrielSBSchaffnerSFNguyenHThe structure of haplotype blocks in the human genomeScience200229655762225222912029063
- de BakkerPIYelenskyRPe’erIGabrielSBDalyMJAltshulerDEfficiency and power in genetic association studiesNat Genet200537111217122316244653
- StrachanTReadAPHuman Molecular Genetics4th edNew York, NYGarland Science2011
- ZhengSLSunJWiklundFCumulative association of five genetic variants with prostate cancerN Engl J Med2008358991091918199855
- ThomasDCStatistical Methods in Genetic EpidemiologyNew York, NYOxford University Press2004
- XiePKranzlerHRPolingJInteraction of FKBP5 with childhood adversity on risk for post-traumatic stress disorderNeuropsychopharmacology20103581684169220393453
- AmstadterABNugentNRKoenenKCAssociation between COMT, PTSD, and increased smoking following hurricane exposure in an epidemiologic samplePsychiatry200972436036920070134
- FuSSMcFallMSaxonAJPost-traumatic stress disorder and smoking: a systematic reviewNicotine Tob Res20079111071108417978982
- ValenteNLValladaHCordeiroQCatechol-O-methyltransferase (COMT) val158 met polymorphism as a risk factor for PTSD after urban violenceJ Mol Neurosci201143351652321080103
- TyrkaARPriceLHGelernterJSchepkerCAndersonGMCarpenterLLInteraction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivityBiol Psychiatry200966768168519596121
- DeYoungCGCicchettiDRogoschFAModeration of the association between childhood maltreatment and neuroticism by the corticotropin-releasing hormone receptor 1 geneJ Child Psychol Psychiatry201152889890621438878
- BoscarinoJAForsbergCWGoldbergJA twin study of the association between PTSD symptoms and rheumatoid arthritisPsychosom Med201072548148620410244
- BoscarinoJAA prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and preventionPsychosom Med200870666867618596248
- KubzanskyLDKoenenKCIs posttraumatic stress disorder related to development of heart disease? An updateCleve Clin J Med.200976Suppl 2S606519376986
- GlaesmerHBrahlerEGundelHRiedel-HellerSGThe association of traumatic experiences and posttraumatic stress disorder with physical morbidity in old age: a German population-based studyPsychosom Med201173540140621636658
- BenyaminiYSolomonZCombat stress reactions, posttraumatic stress disorder, cumulative life stress, and physical health among Israeli veterans twenty years after exposure to combatSoc Sci Med20056161267127715970236
- O’TooleBICattsSVTrauma, PTSD, and physical health: an epidemiological study of Australian Vietnam veteransJ Psychosom Res2008641334018157997
- AhmadiNHajsadeghiFMirshkarloHBBudoffMYehudaREbrahimiRPost-traumatic stress disorder, coronary atherosclerosis, and mortalityAm J Cardiol20111081293321530936
- BoscarinoJAPosttraumatic stress disorder and physical illness: results from clinical and epidemiologic studiesAnn N Y Acad Sci2004103214115315677401
- HeimCEhlertUHellhammerDHThe potential role of hypocortisolism in the pathophysiology of stress-related bodily disordersPsychoneuroendocrinology200025113510633533
- SmithAKConneelyKNKilaruVDifferential immune system DNA methylation and cytokine regulation in post-traumatic stress disorderAm J Med Genet B Neuropsychiatr Genet.2011156B670070821714072
- UddinMAielloAEWildmanDEEpigenetic and immune function profiles associated with posttraumatic stress disorderProc Natl Acad Sci U S A2010107209470947520439746
- GanderMLvon KanelRMyocardial infarction and post-traumatic stress disorder: frequency, outcome, and atherosclerotic mechanismsEur J Cardiovasc Prev Rehabil200613216517216575268
- SpitzerCBarnowSVolzkeHAssociation of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general populationJ Psychiatr Res2010441152119628221
- von KanelRHeppUKraemerBEvidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorderJ Psychiatr Res200741974475216901505
- BoscarinoJAVietnam veterans, postwar experiences and health outcomesFinkGEncyclopedia of Stress32nd edSan Diego, CAAcademic Press2007830838
- BoscarinoJAKirchnerHLHoffmanSNSartoriusJAdamsREPTSD and alcohol use after the World Trade Center attacks: a longitudinal studyJ Trauma Stress201124551552521882246
- VlahovDGaleaSResnickHIncreased use of cigarettes, alcohol, and marijuana among Manhattan, New York, residents after the September 11th terrorist attacksAm J Epidemiol20021551198899612034577
- HungRJMcKayJDGaborieauVA susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25Nature2008452718763363718385738
- WeissTSkeltonKPhiferJPosttraumatic stress disorder is a risk factor for metabolic syndrome in an impoverished urban populationGen Hosp Psychiatry201133213514221596206