Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Background
A post hoc analysis from a multiphase trial with open-label transition and maintenance phases, a double-blind relapse prevention phase, and an optional open-label extension examined the long-term tolerability with continuous once-monthly injectable paliperidone palmitate 39, 78, 117, or 156 mg (25, 50, 75, or 100 mg equivalents [mg eq] of paliperidone) in subjects with recently diagnosed (≤5 years; n = 216) versus chronic illness (>5 years; n = 429) schizophrenia.
Methods
Adverse events reported at a ≥2% margin between subgroups were identified. Relative risks (in the recently diagnosed compared with the chronically ill) and 95% confidence intervals (CI) were determined, and CI not including 1 were considered potentially significant.
Results
In both subgroups, the mean monthly dose was 109 mg (69.9 mg eq). Continuous mean exposures were 333.9 ± 271.9 and 308.7 ± 278.3 days in the recently diagnosed and chronic illness subgroups, respectively. Using the criteria outlined in the methods, nasopharyngitis was a potentially significant event reported in more chronically ill than recently diagnosed subjects at months 6, 9, 12, and endpoint (7.2% versus 2.8%; relative risk 0.384; 95% CI 0.163–0.907). Influenza (2.8% versus 0.7%; relative risk 3.9; 95% CI 1.003–15.730) and amenorrhea (3.2% versus 0.9%; relative risk 3.476; 95% CI 1.029–11.744) at endpoint were potentially significant events in more recently diagnosed than chronically ill subjects. Mean weight changes, sedation/somnolence, any extrapyramidal symptom-related or glucose-related events were generally similar between the groups. The mean prolactin level increased in both sexes in both subgroups (changes from baseline of +41.8 ng/mL and +26.5 ng/mL in recently diagnosed and chronic illness females and +12.3 ng/mL and +15.1 ng/mL in recently diagnosed and chronic illness males, respectively), and were higher in females with recently diagnosed illness than in females who were chronically ill (P = 0.0002 at endpoint). Prolactin-related events were reported by 7.9% of recently diagnosed subjects with schizophrenia and 3.5% of those who were chronically ill.
Conclusion
The long-term tolerability of paliperidone palmitate was generally similar in recently diagnosed schizophrenia subjects and those with more chronic illness, with the exception of some prolactin-related measures.
Introduction
In subjects with schizophrenia, the first 5–10 years of illness has been identified as a critical period for effective intervention to prevent biological as well as psychosocial deterioration,Citation1,Citation2–Citation7 optimize outcomes,Citation1,Citation8–Citation12 reduce the risk for symptom relapse,Citation13–Citation17 and possibly mitigate disease progression.Citation5,Citation18 However, during these early years, there are some barriers to effective intervention and treatment. These patients are often nonadherent with medications due to lack of insight into both their illness and the need for treatment, as well as forgetfulness, lack of social support, and personal choice.Citation19 Whereas recently diagnosed patients are often more responsive to antipsychotic medications than those with chronic schizophrenia,Citation14,Citation16,Citation20,Citation21 they may also be more sensitive to adverse drug effects.Citation5,Citation22–Citation28 In particular, reports suggest that various extrapyramidal symptoms (EPS), weight gain, prolactin-related effects, and sedation are more frequent and problematic for persons early in their illness.Citation8,Citation29–Citation36
Because early in the course of schizophrenia illness patients poorly adhere to daily therapy, it has been suggested that long-acting injectable antipsychotics may be a particularly appropriate treatment option for these patients. In addition to eliminating the daily need to remember to be adherent, these agents allow clinicians and caregivers to have immediate awareness of noncompliance. Guaranteed onboarding of antipsychotic medication allows clinicians to make more informed treatment decisions. Further, the long half-lives of these agents provide a wider window for missed doses (days or weeks rather than hours) before plasma levels drop below critical thresholds, where the risks for relapse, hospitalization, and suicide may be increased.Citation21,Citation37–Citation41
On the other hand, tolerability is often a key factor in medication choice for recently diagnosed patients and long-acting agents may pose such concerns for both clinicians and patients. Unfortunately, few studies have assessed the safety of injectable medications in patients with a recent onsetCitation42–Citation45 or first episode of psychosis,Citation46 and their use in recently diagnosed patients is generally limited. Despite well known adherence issues with oral medications and an associated high risk for subsequent relapse,Citation2,Citation37 long-acting agents are often reserved for more treatment-refractory patients.
Paliperidone palmitate (Janssen Pharmaceuticals, Titusville, NJ), the palmitate ester of paliperidone, is a long-acting, once-monthly, injectable, atypical antipsychotic for the treatment of schizophrenia.Citation47 Doses of paliperidone palmitate may be expressed as milligrams (mg) or as milligram equivalents (mg eq) of the pharmacologically active fraction, paliperidone (39, 78, 117, 156, and 234 mg of paliperidone palmitate corresponding to 25, 50, 75, 100, and 150 mg eq of active paliperidone). Its use for both acute and maintenance treatment of schizophrenia has been demonstrated in short-termCitation48–Citation51 and longer-termCitation52–Citation54 studies. To date, there are no published prospective studies of paliperidone palmitate in patients experiencing their first episode or early in their schizophrenia illness. A recent post hoc analysis of a large, international, 13-week, placebo-controlled trial reported on tolerability and efficacy of paliperidone palmitate in subjects with recently diagnosed schizophrenia receiving the recommended initiation doses (234 mg [150 mg eq] day 1 and 156 mg [100 mg eq] day 8).Citation55 These data are informative but do not address longer-term tolerability concerns. A recently completed relapse prevention trialCitation52,Citation54 of paliperidone palmitate provides valuable long-term exposure data that can help to address this question. A post hoc safety and tolerability analysis of this trial focusing on the recently diagnosed subgroup as compared with the more chronically ill subpopulation is reported herein.
Materials and methods
Design
This was a post hoc analysis of a five-phase international trial conducted from March 2005 to February 2008 (NCT00111189). Key subject inclusion criteria included being aged 18–65 years and a body mass index ≥ 15.0 kg/ m2, a diagnosis of schizophrenia for at least one year before screening, and a total Positive and Negative Syndrome Scale score below 120 at screening and at baseline, with no lower score eligibility limit. The study was conducted in accordance with the Declaration of Helsinki and consistent with Good Clinical Practice. Additional design details have been previously reported.Citation52,Citation54
Study phases and treatments
The five study phases () were: a screening/washout phase up to 7 days; an open-label, 9-week, transition phase for switching to paliperidone palmitate at 78 mg (50 mg eq) on days 1 and 8, followed by flexible dosing of 39, 78, or 156 mg (25, 50, or 100 mg eq) at week 5; an open-label 24-week maintenance phase with flexible paliperidone palmitate dosing (39, 78, or 156 mg monthly [25, 50, or 100 mg eq]) for the first 12 weeks followed by the established maintenance dose for the second 12 weeks; a randomized (1:1 ratio), double-blind, placebo-controlled, relapse prevention phase of variable duration (up to 63 weeks) for subjects stabilized on a fixed paliperidone palmitate dose during the maintenance phase; and an optional open-label extension phase up to 52 weeks with flexible paliperidone palmitate dosing (39, 78, 117, or 156 mg [25, 50, 75, or 100 mg eq]). Subjects who experienced a relapse, completed the relapse prevention phase, or received at least one injection of paliperidone palmitate when enrollment in the study was stopped were eligible to enter the open-label extension phase.
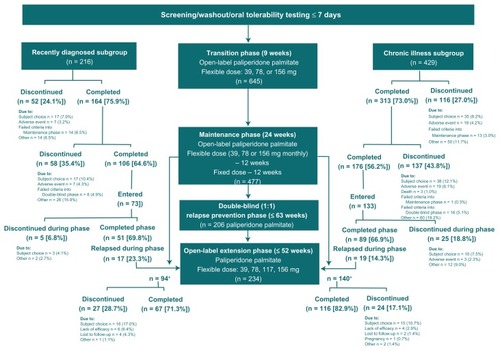
Figure 1 Study design and subject disposition in recently diagnosed and chronically ill subgroups treated continuously with paliperidone palmitate.
Post hoc population and tolerability assessments
The recently diagnosed subgroup was defined as those subjects within 5 years of their initial diagnosis of schizophrenia. The chronically ill subgroup included those subjects > 5 years out from their initial diagnosis of schizophrenia. This analysis was limited to subjects who received paliperidone palmitate continuously from entry into the open-label transition phase to study completion or discontinuation ().
Tolerability was evaluated by treatment-emergent adverse events reported from the open-label transition phase baseline through months 1, 3, 6, 9, and 12 and to the open-label extension endpoint. These included reports of adverse events (either reported by the subject voluntarily or collected by means of interviewing subjects in a nondirected manner), clinical laboratory tests, vital sign measurements, extrapyramidal symptom rating scales (Simpson Angus Scale, Barnes Akathisia Rating Scale, and Abnormal Involuntary Movement Scale) and findings on physical examination. Changes from baseline in physical assessments of weight and body mass index, and measurement of serum prolactin and glucose levels were recorded.
Analysis sets and statistical analysis
The safety population consisted of 216 recently diagnosed subjects and 429 chronically ill subjects who received paliperidone palmitate continuously from study entry through study completion or discontinuation. Demographic and baseline characteristics between groups were compared by t-test for continuous variables and Chi-square or Fisher’s exact test for categorical variables. Between-subgroup differences in continuous measures for post-baseline scores were assessed using analysis of covariance (model with fixed effects) for recently diagnosed versus chronically ill subgroups and country, and the baseline (transition phase) value as a covariate. Change from baseline within groups was assessed by paired t-test. Mean monthly doses of paliperidone palmitate (for days on drug only) and exposure in days were summarized.
Frequencies and percentages of adverse events were summarized from the time of first injection to months 1, 3, 6, 9, and 12 and endpoint. Adverse events reported during these time periods that occurred at a margin of ≥2% between the subgroups are reported and displayed by percentage rate in each treatment subgroup. The relative risk (RR) and 95% confidence interval [CI] in the recently diagnosed group compared with the chronically ill subgroup were determined. Unadjusted CI were utilized as a flagging device to identify potentially significant adverse events when the unadjusted CI did include 1. Any adjustment for multiplicity was considered to be counterproductive for identifying potential safety risks.
Any events related to extrapyramidal symptoms, glucose, or prolactin were also summarized. Logistic regression models examined the association between probability of these events with demographic and baseline characteristics that differed between subgroups. These included diagnosis status (recently diagnosed versus chronically ill), age, age at time of diagnosis of schizophrenia, race (black versus Caucasian or other versus Caucasian), weight, body mass index, and smoking status (yes or no).
Changes in weight and glucose and prolactin levels from transition phase baseline through open-label extension were summarized. In addition to analysis of covariance at endpoint, repeated-measures analysis of covariance assessed between-group differences using observed scores to compare mean response profiles over time. The P values for the group effect and group-by-visit interactions were provided. The group effect measured the deviation from the hypothesis of equality of mean changes between groups, averaged over the treatment duration. The group-by-visit interaction tested the hypothesis of parallel response profiles over time. Multiple linear regression models examined relationships between changes in weight, and glucose and prolactin levels, with demographic and baseline characteristics that were found to be different at baseline.
Results
Subject baseline demographics, disposition, and dosing
Subjects in the recently diagnosed subgroup were younger than the chronically ill subgroup at study entry, with an older age at the time of diagnosis of schizophrenia, and with a lower weight and body mass index (). There was a higher percentage of Caucasians and a lower percentage of smokers in the recently diagnosed subgroup than in the chronically ill subgroup. The mean years of illness was 2.9 ± 1.5 (range 1.0–5.0) in the recently diagnosed subgroup and 16.2 ± 8.1 (range 6.0–47.0) in the chronically ill subgroup. The subgroups were similar with respect to sex (male 60.2% and 58.0%, respectively), mean total Positive and Negative Syndrome Scale score (70.7 and 73.3, P = 0.0811, respectively), and Clinical Global Impressions Scale scores (not ill to moderate in 87.0% and 81.8%, respectively). Completion rates were generally similar between the subgroups during each study phase (). Among subjects receiving paliperidone palmitate who entered the double-blind relapse prevention phase, the time to relapse was not significantly different between the recently diagnosed and chronically ill subgroups (P = 0.0999, log-rank test).
Table 1 Transition baseline demographics and characteristics that differed between subgroups
The mean monthly dose was similar in each subgroup (approximately 109 mg [69.9 mg eq]), with a mean duration of exposure of 333.9 ± 271.9 days in the recently diagnosed subgroup and 308.7 ± 278.3 days in the chronically ill subgroup. During the study, 42.1% of those in the recently diagnosed subgroup received benzodiazepines and 10.2% received medications for extrapyramidal symptoms, respectively. In the chronically ill subgroup, rates were 46.4% for benzodiazepines and 17.0% for medications used to treat extrapyramidal symptoms.
Reported adverse events
During the month following the first injection, 31.5% (68 of 216) of recently diagnosed and 42.7% (183 of 429) of subjects in the chronically ill subgroup reported an adverse event (). In general, incidence rates, RR, and 95% CI suggested that adverse events were less likely in the recently diagnosed subgroup than in the chronically ill subgroup at each time interval.
Table 2 Subjects with at least one adverse event in the recently diagnosed and chronically ill subgroups (from time since first injection to specified time point)
In the month following the first injection, no adverse events were reported at a margin of ≥2% in more of the recently diagnosed subjects than in those with chronic illness (). Insomnia, worsening of schizophrenia, and agitation were reported at a margin of ≥2% in more of the chronically ill subjects than in recently diagnosed subjects. These differences in incidence were not considered potentially significant based upon the 95% CI. Nasopharyngitis was reported by more subjects with chronic illness than by those in the recently diagnosed subgroup of subjects at months 6 (4.9% versus 1.4%; RR 0.28; 95% CI 0.086–0.941 respectively), 9 (5.6% versus 1.9%; RR 0.33; 95% CI 0.116–0.942), and 12 (6.1% versus 2.3%; RR 0.38; 95% CI 0.149–0.981), and endpoint (7.2% versus 2.8%; RR 0.38; 95% CI 0.163–0.907); the 95% CI did not include 1 and were considered potentially significant. Influenza (2.8% versus 0.7%; RR 3.97; 95% CI 1.003–15.730) and amenorrhea (3.2% versus 0.9%; RR 3.48; 95% CI 1.029–11.744) were reported by more subjects in the recently diagnosed subgroup at endpoint. Given that the 95% CI did not include 1, these events were also considered potentially significantly different between subgroups.
Figure 2 Percentage, relative risk (recently diagnosed versus chronic illness), and 95% confidence intervals of adverse events reported by a margin of ≥2% in recently diagnosed or chronically ill subgroups.
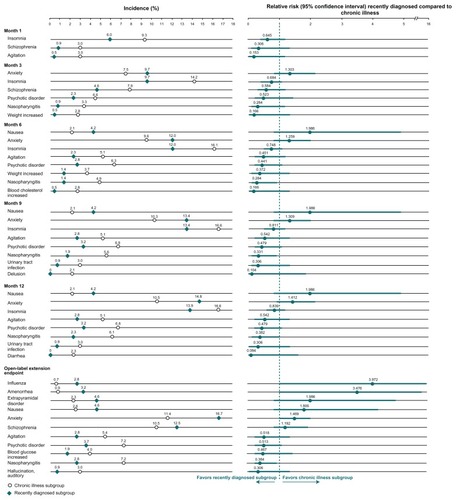
In the recently diagnosed subgroup, sedation was reported by none, one, or two subjects at each time point (0.0%–0.93%), with somnolence reported by one or two subjects at each time point (0.46%–0.93%), and five subjects at endpoint (2.31%). In the chronically ill subgroup, sedation was reported by two subjects at each time point (0.47%), with somnolence reported by 1–5 subjects at each time point (0.23%–1.17%).
Events related to extrapyramidal symptoms
Extrapyramidal symptom-related adverse event rates included parkinsonism, hyperkinesia, dystonia, tremor, and dyskinesia. Rates of any extrapyramidal symptom-related events were numerically lower in the recently diagnosed subgroup at each period assessed (through months 1, 3, 6, 9, and 12 and open-label extension). Rates ranged from 2.3% to 9.3% in the recently diagnosed subgroup and from 5.8% to 12.6% in the chronically ill subgroup (). Rates of individual extrapyramidal symptom-related events and their specific preferred terms are summarized in . Among the specific preferred terms, nonspecific extrapyramidal disorder was reported by more recently diagnosed subjects (4.6%) than by chronically ill subjects (2.3%), and akathisia was reported by more chronically ill subjects (3.3%) than recently diagnosed subjects (1.9%).
Figure 3 Percentage of Subjects with Any EPS-Related Event* from First Injection to Specified Timepoint, in Recently Diagnosed and Chronic Illness Subjects.
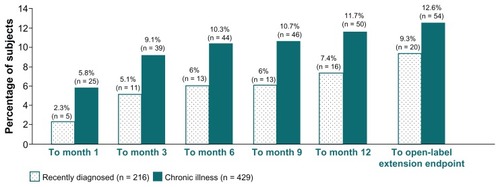
Table 3 Extrapyramidal symptom-related adverse events from first injection through open-label extension phase, in recently diagnosed and chronically ill subgroups
Logistic regression models showed that none of the baseline characteristics that differed between the subgroups was associated with risk of extrapyramidal symptom-related events (ie, age, P = 0.7057; weight, P = 0.8921; body mass index, P = 0.9823; age at diagnosis of schizophrenia, P = 0.7727; current smoking status, P = 0.3507; and race [black, P = 0.0950; other, P = 0.1602]).
Weight changes
Weight changes (least squares mean ± standard error) at endpoint were 2.6 ± 0.9 kg in the recently diagnosed subgroup and 3.4 ± 0.7 kg in the chronically ill subgroup (P = 0.42; least squares mean difference 0.8 ± 0.99, 95% CI: −1.15–2.75). Observed scores at each time point were evaluated using repeated-measures analysis of covariance. Average changes from baseline to endpoint between groups were similar (between-group comparison, P = 0.4342); this finding did not differ throughout the trial (group-by-visit interaction, P = 0.9520). Linear regression models assessing the impact of baseline differences did not reveal any potential associations (ie, age, P = 0.5117; weight, P = 0.1504; body mass index, P = 0.6557; age at schizophrenia diagnosis, P = 0.9309; current smoking status, P = 0.1172; and race, [black, P = 0.5444; other, P = 0.3842]).
Glucose-related measures
Rates of glucose-related adverse events were 5.1% (22 of 429) in the chronically ill subjects and 2.8% (6 of 216) in recently diagnosed subjects, with “blood glucose increased” being the most frequent (4.0% versus 1.9%, ). In logistic regression models, age (P < 0.0001; odds ratio [OR] 1.087; 95% CI 1.047–1.129) had a significant association with incidence of glucose-related adverse events (one-year increase in age is associated with an 8.7% increase in odds of experiencing “blood glucose increased”), whereas body mass index (P = 0.0516; OR 1.057; 95% CI 1.000–1.118) and age at diagnosis of schizophrenia (P = 0.0578; OR 1.040; 95% CI 0.999–1.082) showed trends towards significance. Other baseline characteristics had no impact (ie, weight, P = 0.4310; current smoking status, P = 0.6082; and race [black, P = 0.1737; other, P = 0.0810]).
Table 4 Glucose-related events from the first injection through open-label extension phaseTable Footnotea
Mean glucose levels at open-label endpoint were not significantly different between the groups (least squares mean difference −0.1, 95% CI −0.61–0.32, P = 0.5436). Regression models showed that age (P = 0.0126, regression coefficient 0.025 ± 0.010) and age at diagnosis of schizophrenia (P = 0.0021, regression coefficient 0.042 ± 0.014) were associated with changes at endpoint.
Prolactin-related measures
Prolactin levels increased in both sexes in both subgroups. Mean prolactin values were consistently higher in recently diagnosed female subjects compared with chronically ill female subjects (). Potentially prolactin-related adverse events were reported by 7.9% of recently diagnosed subjects compared with 3.5% of chronically ill subjects through endpoint (). The most commonly reported events were amenorrhea (7 [3.2%] and 4 [0.9%], respectively) and galactorrhea (2 [0.9%] and 6 [1.4%], respectively).
Table 5 Mean prolactin levels at transition baseline and open-label endpoint by sex in the recently diagnosed and chronically ill subgroups
Table 6 Potentially prolactin-related events from first injection through open-label extension phaseTable Footnotea
Discussion
The primary objective of this post hoc analysis was to examine the long-term tolerability of once-monthly injectable paliperidone palmitate in subjects with a more recent diagnosis of schizophrenia compared with those with a longer duration of illness. Among the range of events anticipated to occur more commonly in the recently diagnosed subgroup, only events related to prolactin elevation emerged as more likely to be increased. Weight increases, sedation/somnolence, overall extrapyramidal symptoms, and glucose-related events did not occur at a greater rate in recently diagnosed subjects. Strengths of this analysis include the length and size of the study. This multiphase study database provides the longest exposure data currently available with the paliperidone palmitate once-monthly injection in patients early in the course of their illness. Nevertheless, these findings are limited in that this work represents the post hoc analysis of a single study.
Relevant to the tolerability findings, the recently diagnosed and chronically ill subgroups had similar doses, durations of treatment exposure (>300 days in each subgroup), and discontinuation rates. These treatment similarities deserve comment because one might have expected lower doses and/ or shorter exposures to be used in subjects with early illness if they tolerated the drug less well than more chronically ill subjects.Citation5,Citation15,Citation43,Citation56–Citation58 However, it is relevant to note that the paliperidone palmitate doses allowed in this study (39, 78, 117, and 156 mg [25, 50, 75, and 100 mg eq]) did not include the highest available 234 mg (150 mg eq) dose. Further, initiation doses were lower than the currently recommended initiation dosage (234 mg day 1 and 156 mg day 8 [150 mg eq day 1 and 100 mg eq day 8]). Therefore, while similarities were noted between the two subgroups, the overall rates of adverse events reported during the first month may have been lower than what would have been seen with the currently recommended initiation regimen. Pertinent to this, somewhat higher overall adverse event rates were observed in a recently published post hoc analysis of a double-blind, placebo-controlled trial in recently diagnosed subjects receiving the recommended day 1 and day 8 doses. Citation55 During the week following the initial 234 mg (150 mg eq] paliperidone palmitate or placebo injection, 37.6% (41 of 109) and 29.7% (11 of 37), respectively, of subjects reported an adverse event. During the month following the day 8 injection of paliperidone palmitate 156 mg (100 mg eq) or placebo, adverse event rates were 41.0% (16 of 39) and 37.8% (14 of 37), respectively.Citation55 During the first week, a broad range of events reported in one or two paliperidone palmitate subjects contributed to the higher rate; the events reported more often by patients on active treatment than by those on placebo were injection site pain, agitation, and headache. In the month following the second injection, anxiety was the most common event, and was reported more often by patients on active treatment than by those on placebo. These events did not emerge as more likely to occur in the recently diagnosed versus more chronic subjects in the present analysis ().
Rates of any extrapyramidal symptom-related events, which are of particular concern in patients with early illness, were generally similar or numerically lower in this analysis of recently diagnosed compared with chronically ill subjects. Rates tended to stabilize after month 3, with incremental increases becoming smaller with continued treatment. Findings related to specific types of extrapyramidal symptoms also require consideration. As reported, there was a somewhat higher rate of nonspecifically coded extrapyramidal symptoms in the recently diagnosed subgroup. In the previously mentioned post hoc analysis of recently diagnosed subjects from a double-blind, placebo-controlled trial, movement-related event rates were 10.3% (4 of 39) with paliperidone palmitate (234 mg day 1 and 156 mg day 8 [150 mg eq day 1 and 100 mg eq day 8] and monthly thereafter) and 8.1% (3 of 37) with placebo over 13 weeks (RR 1.3; 95% CI 0.30–5.27; P > 0.05).Citation55 Among the specific types of events, the most common in the recently diagnosed population was parkinsonism (7.7% paliperidone palmitate and 0% placebo). In the current long-term evaluation, parkinsonism was again the most common movement disorder-related event, with the same rate in recently diagnosed and more chronic subjects (5.6%).
Prolactin findings were also anticipated by prior work.Citation55 Levels increased in both subgroups and in both sexes, with higher levels in females with early illness compared with those having chronic illness, and a higher percentage of recently diagnosed versus chronically ill subjects reported prolactin-related adverse events (7.9% versus 3.5%, respectively).
The limitations to these findings include the fact that the original study was not designed to assess long-term tolerability of the drug in patients with recently diagnosed schizophrenia. The 5-year cut point used to define early illness relied on historical information and patient self-reporting, which may have had variable reliability, and many patients may have been ill for some time prior to receiving a formal diagnosis. Nonetheless, it is generally accepted that the first 5–10 years of illness is a critical period for effective intervention.Citation8–Citation12,Citation24 Using this 5-year cut point likely captured a population that was enriched with those at an earlier stage of schizophrenia. Also, although there was no comparison with a placebo group in this analysis, these findings are still relevant to the question at hand regarding the long-term tolerability of paliperidone palmitate in subjects early in the course of their illness compared with those with more chronic illness. An unexpected finding of this safety analysis is the similar or even somewhat lower rate of total adverse events or any extrapyramidal-related events reported by recently diagnosed compared with subjects with chronic illness. This is at variance with other reports in the literature. Also, with few exceptions, baseline phenotypes did not have a significant effect on the results.
In conclusion, these long-term findings complement prior tolerability analyses of the initiation dosing of injectable paliperidone palmitate in subjects with recently diagnosed schizophrenia,Citation55 and may help guide clinicians in the management of these patients.
Disclosure
LA, JKS, D-JF, and CAB are employees of Janssen Scientific Affairs LLC, Titusville, NJ. IT is an employee of Janssen Research and Development LLC, Titusville, NJ. This research was funded by Janssen Scientific Affairs LLC. Writing, editorial, and technical support services were provided by Susan Ruffalo of MedWrite Inc, Newport Coast, CA. Some of these data were presented at the 164th annual meeting of the American Psychiatric Association, held on May 14–18, 2011, Honolulu, HI, and at the 13th International Congress on Schizophrenia Research, April 2–6, 2011, Colorado Springs, CO.
References
- MarshallMRathboneJEarly intervention for psychosisCochrane Database Syst Rev20064CD00471817054213
- BartzokisGLuPHAmarCPLong acting injection versus oral risperidone in first-episode schizophrenia: Differential impact on white matter myelination trajectorySchizophr Res2011132354121767934
- KevashanMSAmirsadriAEarly intervention in schizophrenia: current and future perspectivesCurr Psychiatry Rep2007932532817880865
- LiebermanJAIs schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspectiveBiol Psychiatry19994672973910494440
- LiebermanJAPerkinsDBelgerAThe early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approachesBiol Psychiatry20015088489711743943
- ChakosMHSchobelSAGuHDuration of illness and treatment effects on hippocampal volume in male patients with schizophreniaBr J Psychiatry2005186263115630120
- DavidsonLMcGalshanTHThe varied outcomes of schizophreniaCan J Psychiatry19974234439040921
- KellyDLConleyRRCarpenterWTFirst-episode schizophrenia: a focus on pharmacological treatment and safety considerationsDrugs2005651113113815907146
- HarriganSMMcGorryPDKrstevHDoes treatment delay in first-episode psychosis really matter?Psychol Med2003339711012537041
- MarshallMLewisSLockwoodADrakeRJonesPCroudaceTAssociation between duration of untreated psychosis and in cohorts of first-episode outcome patients – a systematic reviewArch Gen Psychiatry20056297598316143729
- McGorryPDKillackeyEYungAREarly intervention in psychosis: concepts, evidence and future directionsWorld Psychiatry2008714815618836582
- McGorryPDKillackeyEYungAREarly intervention in psychotic disorders: detection and treatment of the first episode and the critical early stagesMed J Aust2007187Suppl 7S8S1017908033
- BarnesTRLeesonVCMutsatsaSHWattHCHuttonSBJoyceEMDuration of untreated psychosis and social function: 1-year follow-up study of first-episode schizophreniaBr J Psychiatry200819320320918757977
- PerkinsDOGuHBotevaKLibermanJARelationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysisAm J Psychiatry20051621785180416199825
- SchoolerNRabinowitzJDavidsonMRisperidone and haloperidol in first-episode psychosis: a long-term randomized trialAm J Psychiatry200516294795315863797
- WyattRJEarly intervention with neuroleptics may decrease the long-term morbidity of schizophreniaSchizophr Res199152012021684719
- WeidenPJSchoolerNRWeedonJCElmouchtariASunakawaAGoldfingerSMA randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcomeJ Clin Psychiatry2009701397140619906343
- CahnWvan HarenEMHulshoffHEBrain volume changes in the first year of illness and 5-year outcomes of schizophreniaBr J Psychiatry200618938138217012664
- TiihonenJHaukkaJTaylorMHaddadPMPatelMXKorhonenPA nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophreniaAm J Psychiatry201116860360921362741
- MacfaddenWBossieCATurkozIHaskinsJTRisperidone longacting therapy in stable patients with recently diagnosed schizophreniaInt Clin Psychopharmacol201025758220101185
- CanusoCMBossieCAAmatniekJTurkozIPandinaGCornblattBPaliperidone extended-release tablets in patients with recently diagnosed schizophreniaEarly Interv Psychiatry20104647820199482
- AllisonDBCaseyDEAntipsychotic-induced weight gain: a review of the literatureJ Clin Psychiatry200162Suppl 7223111346192
- Alvarez-JimenezMGonzalez-BlanchCCrespo-FacorroBAntipsychotic-induced weight gain in chronic and first-episode psychotic disorders: a systematic critical reappraisalCNS Drugs20082254756218547125
- FranceySMNelsonBThompsonAParkerAGKerrMMacNeilCWho needs antipsychotic medication in the earliest stages of psychosis? A reconsideration of benefits, risks, neurology and ethics in the era of early interventionSchizophr Res201011911020347270
- LlorcaPMChereauIBayleFJLanconCTardive dyskinesias and antipsychotics: a reviewEur Psychiatry20021712913812052573
- McEvoyJPLiebermanJAPerkinsDOEfficacy and tolerability of olanzapine, quetiapine and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparisonAm J Psychiatry20071641050106017606657
- MuenchJCareyMDiabetes mellitus associated with atypical antipsychotic medications: new case report and review of the literatureJ Am Board Fam Pract20011427828211458971
- TschonerAEngJLaimerMMetabolic side effects of antipsychotic medicationInt J Clin Pract2007611356137017627711
- SalimiKJarskogLFLiebermanJAAntipsychotic drugs for first-episode schizophrenia: a comparative reviewCNS Drugs20092383785519739694
- SangerTMLiebermanJATohenMGrundySBeasleyCJrTollefsonGDOlanzapine versus haloperidol treatment in first-episode psychosisAm J Psychiatry199915679879892301
- WoodsSWMartinASpectorSGMcGlashanTHEffects of development on olanzapine-associated adverse eventsJ Am Acad Child Adolesc Psychiatry200241439446
- WudarskyMNicolsonRHambergerSDElevated prolactin in pediatric patients on typical and atypical antipsychoticsJ Child Adolesc Psychopharmacol1999923924510630453
- MerloMCGHoferHGekleWRisperidone, 2 mg/day vs 4 mg/day, in first-episode, acutely psychotic patients: treatment efficacy and effects on fine motor functioningJ Clin Psychiatry20026388599112416598
- MasiGCosenzaAMucciMProlactin levels in young children with pervasive developmental disorders during risperidone treatmentJ Child Adolesc Psychopharmacol20011138939411838821
- GuptaSFrankBMadhusodananSRisperidone-associated galactorrhea in a male teenagerJ Am Acad Child Adolesc Psychiatry20014050450511349691
- BobesJGilbertJCiudadASafety and effectiveness of olanzapine versus conventional antipsychotics in the acute treatment of first-episode schizophrenic inpatientsProg Neuropsychopharmacol Biol Psychiatry20032747348112691783
- KaneJMGarcia-RiberaCClinical guideline recommendations for antipsychotic long-acting injectionsBr J Psychiatry20091956367
- KozmaCMSlatonTDiraniRGopalSFastenauJHoughDChanges in schizophrenia-related health-care resource utilization among patients receiving paliperidone palmitate: results of a 52-week clinical trialCurr Med Res Opin2011271603161121696265
- SchoolerNRRelapse and rehospitalization: comparing oral and depot antipsychoticsJ Clin Psychiatry200364Suppl 16141714680414
- SheehanJFuDJRemmerieBSliwaJKAlphsLSamtaniMNThe management of antipsychotic treatment discontinuation and interruptions using model-based simulationsPoster presented at the 51st Annual New Clinical Drug Evaluation Unit New Research Approaches for Mental Health Interventions MeetingJune 13–16, 2011Boca Raton, FL.
- ZhuBAscher-SvanumHShiLBTime to discontinuation of depot and oral first-generation antipsychotics in the usual care of schizophreniaPsychiatr Serv20085931531718308914
- ParelladaEAndrezinaRMilanovaVPatients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectableJ Psychopharmacol20051951416144781
- EmsleyRMedoriRKoenLOosthuizenPPNiehausDRabinowitzJLong-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary studyJ Clin Psychopharmacol20082821021318344732
- NapryeyenkoOBurbaBMartinezGRisperidone long-acting injectable in recent-onset schizophrenia examined with clinician and patient self-report measuresJ Clin Psychopharmacol20103020020220520297
- DuboisVMegensJMertensCLong-acting risperidone in early-episode schizophreniaActa Psychiatric Belg2011111921
- KimBLeeSHChoiTKEffectiveness of risperidone long-acting injection in first-episode schizophrenia: in naturalistic settingProg Neuropsychopharmacol Biol Psychiatry2008321231123518442879
- Invega® Sustenna® (paliperidone palmitate, full prescribing information)Titusville, NJJanssen Pharmaceuticals Inc92011
- GopalSHoughDWXuHLullJMGassmann-MayerCRemmerieBMEfficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized double-blind, placebo-controlled, dose-response studyInt Clin Psychopharmacol20102524725620389255
- HoughDLindenmayerJPGopalSSafety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophreniaProg Neuropsychopharmacol Biol Psychiatry2009331022103119481579
- NasrallahHGopalSGassmann-MayerCA controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophreniaNeuropsychopharmacol20103520722082
- PandinaGLindenmayerJPLullJA randomized, placebo-controlled study to assess the efficacy and safety of three doses of paliperidone palmitate in adults with acutely exacerbated schizophreniaJ Clin Psychopharmacol20103023524420473057
- HoughDGopalSVijapurkarULimPMorozovaMEerdekensMPaliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled studySchizophr Res201011610711719959339
- CoppolaDLiuYGopalSLong-term safety, tolerability and pharmacokinetics of the highest available dose of paliperidone palmitate: a one-year open-label study in patients with schizophreniaPoster presented at the American Society of Clinical Pharmacology and Therapeutics annual meetingMarch 17–21, 2010Atlanta, GA
- GopalSVijapurkarULimPMorozovaMEerdekensMHoughDA 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophreniaJ Psychopharmacol20112568569720615933
- BossieCAFuDJSliwaJKMaYWAlphsLTolerability of initiation doses of once-monthly paliperidone palmitate in subjects with recently diagnosed schizophrenia in an acute treatment trialTher Adv Psychopharmacol20111111124
- LasserRABossieCAZhuYLocklearJCKaneJMLong-acting risperidone in young adults with early schizophrenia or schizoaffective illnessAnn Clin Psychiatry200719657117612845
- International Early Psychosis Association Writing GroupInternational clinical practice guidelines for early psychosisBr J Psychiatry2005187Suppl 48S120S124
- WeidenPJBuckleyPFGrodyMUnderstanding and treating “first-episode” schizophreniaPsychiatr Clin North Am20073048151017720033