Abstract
Comorbid depression is common in patients with type 2 diabetes mellitus and is associated with greater mortality risk and a higher incidence of diabetic complications and decreased quality of life. In an earlier pilot study, we found that treatment with the serotonin norepinephrine reuptake inhibitor antidepressant, milnacipran, significantly improved metabolic parameters in diabetic patients with comorbid depression who had an antidepressant response. We sought to replicate these results in a larger cohort (n = 135). Patients received milnacipran and metformin for 6 months and metabolic parameters and depressive symptoms were measured at baseline and after 3 and 6 months. At the end of the study, 72.6% of patients had an antidepressant response (≥50% reduction of baseline Beck Depression Inventory score). Overall, there was significant improvement in the metabolic and anthropometric parameters measured. The number of patients with glycated hemoglobin > 8% (>63.9 mmol/mol), an indicator of poor metabolic control requiring intensive therapeutic intervention, decreased from 31.9% at baseline to 11.9% during the study. As found in the pilot study, levels of total cholesterol and triglycerides were only significantly decreased in antidepressant responders. Body weight was significantly reduced in both responders and nonresponders but the effect size was significantly greater in the responder group. In contrast to the pilot study, fasting blood glucose and glycated hemoglobin were significantly decreased to a similar extent in both antidepressant-responders and nonresponders. The present study thus replicates some of the original findings. The main difference between the present and the pilot study is that in the larger cohort significant reductions in fasting blood glucose and glycated hemoglobin were found in all patients irrespective of whether or not they responded to antidepressant treatment. The present data underline the importance of diagnosis and treatment of comorbid depression in patients with type 2 diabetes mellitus with milnacipran.
Introduction
Depression is a common comorbid complication in patients with diabetes mellitus.Citation1 The prevalence of major and minor depression in patients with type 2 diabetes is almost twice that of the general population.Citation2 The comorbidity of diabetes and depression is associated with a significantly greater mortality risk than the sum of the risk of the two diseases,Citation3 suggesting a synergistic interaction between diabetes and depression. The presence of depression frequently results in impaired metabolic control, a higher incidence of micro- and macroangiopathic diabetic late complications and decreased quality of life.Citation3–Citation6
There is relatively little data on the effects of antidepressant therapy on metabolic and anthropometric parameters in diabetic patients with comorbid depression.Citation7–Citation10 A few years ago, in a pilot study,Citation11 we investigated the effect of the serotonin norepinephrine reuptake inhibitor antidepressant, milnacipran, on metabolic and psychological parameters in 64 patients with type 2 diabetes and comorbid depression. Patients received milnacipran and metformin in a 6-month open-label study. At the end of the study, 72% of patients had an antidepressant response (≥50% reduction of baseline Beck Depression Inventory score). Fasting blood glucose (FBG), glycated hemoglobin (HbA1c), body mass index (BMI), serum total and low-density lipoprotein (LDL)-cholesterol and triglyceride levels were all significantly decreased in these patients whereas in nonresponders to antidepressant treatment these parameters were not significantly changed.
The present study is a replication of our earlier pilot study using a similar protocol and was performed in the private practices of general practitioners and internists in a larger population.
Patients and methods
All patients had a diagnosis of diabetes mellitus type 2 as defined by the diagnostic criteria of the Austrian Diabetes Association.Citation12 Comorbid depression was diagnosed according to the International Statistical Classification of Disease and Related Health Problems, 10th revision (ICD-10) criteria for depressive episode.Citation13 Exclusion criteria were contraindications for either metformin or milnacipran, or significant suicidal ideation.
ICD-10 criteria recognizes mild, moderate, and severe depressive episodes. However, patients in this study were recruited irrespective of the severity category. The severity of depression was evaluated at baseline and after 3 and 6 months of treatment using the Beck Depression Inventory Manual (BDI-II).Citation14 An antidepressant response was defined as ≥50% reduction in the baseline BDI-II score and remission as a BDI score ≤ 12.
FBG, HbA1c, total cholesterol, LDL-cholesterol, high-density lipoprotein (HDL)-cholesterol, serum triglycerides, blood pressure, and weight were measured at baseline and after 6 months treatment; height was measured at baseline only. Blood samples were taken after an overnight fast of 12 hours. BMI (kg/m2) was calculated using body weight measured to the nearest kg and height measured to the nearest cm. Other predefined metabolic parameters were also measured at 3 months (this data was incomplete for many patients and no analysis was undertaken [data not shown]).
Spontaneously reported adverse events were recorded at each visit
The study was conducted at 50 investigational sites including private practice of general practitioners and internists across Austria in an open longitudinal manner. As a prospective non-interventional study complying with the Directive 2001/20/EC, article 2 and §2a of the Austrian Medical Products Act, ethics committee authorization was not required. Patient recruitment started in March 2010 and the last visit of the last patient occurred in August 2011.
Diabetes therapy was performed according to the Guidelines of the Austrian Diabetes AssociationCitation12 starting with metformin hydrochloride at 500 to 2000 mg/day after lifestyle adjustment failure. Antidepressant treatment with milnacipran was initiated at 25 to 100 mg/day. The initial dose and subsequent dose adjustment of both drugs was at the clinicians’ discretion and based on clinical response and patient tolerance of the drugs.
Statistical analysis
All analyses were based on patients completing the study and for whom a full data set was available at baseline and end- point. Values at baseline and end-point (6 months) for each patient were compared using a paired t-test. All significance values are calculated as two-tailed.
Results
Two hundred forty patients were recruited into the study and had a full baseline examination. Sixty-six patients discontinued metformin and 14 patients discontinued milnacipran and dropped out of study: only nine of these dropouts were because of adverse events: principally agitation (6), insomnia (2), sweating (2), nausea (2), palpitation (1), mild hypertension (1), and dysuria (1). The only serious adverse event was the case of dysuria accompanied by urinary retention. Other reasons included symptom improvement, patient choice, or change of medication or inclusion of additional medication decided by the treating physician. One hundred sixty patients completed the study. Datasets of 25 patients were incomplete and these were thus excluded from the analysis.
shows the baseline demographic and clinical characteristics of the 135 patients who completed the study with a full dataset. One hundred eleven patients (82%) had never taken antidepressant medication and 77 patients (57%) had never taken antidiabetes drugs and had only lifestyle-adjustment therapy. Patients were generally overweight or obese (mean BMI = 30.8) and their glycemic control was moderate or poor ().
Table 1 Characteristics at baseline of patients completing the study
Table 2 Metabolic parameters at baseline and after 6 months treatment with milnacipran of patients completing the study
Metabolic parameters at baseline and after 6 months treatment are shown in . Over the duration of the study, mean values showed statistically significant improvements for FBG levels, HbA1c, body weight, blood pressure, BMI, total cholesterol, and serum triglycerides. The proportion of patients with HbA1c < 7% (53.0 mmol/mol) increased from 26.7% at baseline to 57.8% after 6 months treatment while the proportion of patients with HbA1c > 8% (63.9 mmol/mol) decreased from 31.9% at baseline to 11.9% at endpoint. Mean blood pressure significantly (P < 0.001) decreased from 140/83 at baseline to 134/80 mmHg at the end of the study. A similar decrease was found even in the most hypertensive patients (systolic blood pressure > 150, n = 26) who had a significant decrease (P < 0.001) from 160/89 to 140/81 mmHg at the end of the study.
and show the improvement in BDI scores over the duration of the study. After 3 months of treatment, 38.8% of patients had responded to antidepressant treatment (≥50% reduction of baseline BDI score) and 72.6% after 6 months. There was no difference between responders and nonresponders concerning age, severity of depression, metabolic control, or BMI at baseline. At the end of the study, 78 patients (57.8%) were in remission (BDI score ≤ 12).Citation15
Figure 1 Evolution of Beck Depression Inventory scores and patients responders and remitters during the study.
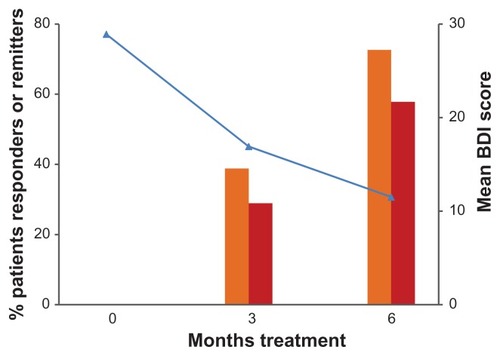
Table 3 Evolution of Beck Depression Inventory scores throughout the study
Mean doses of milnacipran and of metformin administered during the study were similar for responders and nonresponders ().
Table 4 Mean doses of drugs administered throughout the study
As shown in , antidepressant responders and nonresponders had significant and similar improvements in FBG and HbA1c. In contrast, responders had significantly greater reductions in body weight, BMI, total serum cholesterol, and triglycerides compared to non-responders.
Table 5 Change of metabolic and anthropometric parameters in depression responders and nonresponders during milnacipran treatment
Discussion
Diabetic patients with severe depressive symptoms adhere less well to diet and medication regimes than patients with less severe or no depressive symptoms.Citation16,Citation17 Several studies have shown that depression is directly associated with an increased risk of diabetic complications, including retinopathy and micro- and macrovascular complications.Citation5,Citation6
The primary aim of the present study was to evaluate the effects of an antidepressant therapy on metabolic parameters and on depression score in diabetic patients. Our main finding was a significant reduction in fasting blood glucose and HbA1c in all patients, irrespective of whether or not they responded to the antidepressant treatment. Further, we demonstrated a significant decrease in body weight, independent of the response to antidepressant treatment. However, the effect size was significantly greater in the responder group. The decline of serum lipids was associated with response to antidepressants.
Studies analyzing the effects of antidepressant therapy on metabolic control have shown variable results.Citation7,Citation8,Citation18,Citation19 In a study with sertraline, HbA1c levels were reduced during treatment, but did not differ between the sertraline and placebo group.Citation7 Other studies have not found significant reductions in HbA1c levels in patients treated with fluoxetine or paroxetine although depressive symptoms were significantly improved.Citation8,Citation18 Similarly, treatment with escitalopram resulted in a significant reduction of depression ratings but only a modest, nonsignificant reduction in FBG levels and HbA1c levels.Citation19 With bupropion, BMI and HbA1c levels decreased significantly over an acute treatment phase with the reduction of depression severity associated with lower HbA1c levels.Citation20
An integrated therapeutic approach, considering biochemical parameters such as HbA1c and blood glucose on the one hand, and psychiatric disturbances on the other should be introduced into routine diabetes care. A recent study demonstrated significant improvement in HbA1c and depression scores by implementation of integrated management of type 2 diabetes mellitus.Citation8
In many cases, diabetes is diagnosed when the disease has been present for many years and the patient is already showing signs of micro and/or macrovascular complications. The choice of antidepressant medication is thus confounded by the presence of these complications and the concomitant diabetes therapy. Ideally, an antidepressant for diabetic patients should not cause weight gain and should have a low risk of interactions with the numerous medications the patient is taking. It should be devoid of any appreciable risk of hepatotoxicity and cardiovascular or blood pressure effects. It should not cause sedation or sexual dysfunction.
For this reason, we decided to use the antidepressant, milnacipran, in the initial pilot study.Citation11 In addition to a good overall tolerability,Citation21 it has been demonstrated to be weight neutral, to be free of cytochrome P450 drug interactions, to be devoid of cardiotoxicity, and to produce minimal sexual dysfunction.
The reduction in depressive symptoms throughout the present study was similar to that seen with milnacipran in the pilot studyCitation11 and other studies of milnacipran in major depression,Citation22–Citation24 which showed response rates of over 60% and remission rates in excess of 35%.
It is interesting to note that treatment with metformin and milnacipran resulted in a significant lowering of blood pressure even in the most hypertensive patients. This suggests that concerns about potential increases in blood pressure resulting from noradrenergic stimulation with milnacipran are possibly exaggerated.
In contrast to the earlier study, significant reductions in body weight and BMI were also found in patients not responding to antidepressant treatment. These reductions were however significantly smaller than those found in patients who had responded to antidepressant treatment.
The main difference between the present study and the pilot study is that in the larger cohort, significant reductions in FBG and HbA1c were found in all patients irrespective of whether they responded to antidepressant treatment. The different finding in the pilot study was probably due to the small size of the nonresponder population (n = 12).
The present results are thus consistent with other data that suggest that successful treatment of depression results in a parallel improvement of at least some metabolic parameters.Citation7,Citation8,Citation18–Citation20 Diabetes is extremely psychologically and behaviorally demanding since about 90% of diabetes management is conducted by the patient him/herself. The presence of comorbid depression can reduce motivation for self-care resulting in an unfavorable or even potentially fatal course of diabetes.Citation25 This underlines the need for diagnosis and treatment of comorbid depression. Recent evidence supports the utility for both psychosocial and pharmacological interventions for patients with diabetes and comorbid depression.Citation26
This replication study was performed in a larger cohort than the original pilot study. Nevertheless, it has several methodological weaknesses. It was not a randomized, doubleblind design. The noninterventional nature of the study led to a large number of patients of the original cohort who were not available for analysis.
In addition, the population was heterogeneous in terms of treatment history since both patients with a history of diabetes and/or depression treatment and patients naïve to these treatments were included. Separate analysis of patients naïve to both treatments (n = 64) shows results similar to the full cohort. Because of the small numbers, however, many of the differences are not significant (data not shown).
Long-term treatment of type 2 diabetic patients with comorbid depression with metformin and milnacipran results in a clear overall improvement in both depressive symptoms and metabolic parameters. Furthermore, patients who did not respond to the antidepressant therapy did not show significant improvement in certain metabolic parameters such as total cholesterol and triglycerides despite receiving diabetes treatment as recommended by national guidelines. Weight loss was significantly greater in patients who responded to the antidepressant treatment.
The present results strongly suggest that an improvement of depressive symptoms results in a greater overall improvement of metabolic parameters, which is in agreement with earlier findingsCitation18–Citation20 including our pilot study.Citation11 It is therefore important that all diabetic patients should be screened for depression. Patients found to be suffering from comorbid depression should be treated, in addition to classical diabetes treatment, with an effective antidepressant drug such as milnacipran.
The findings of this second open-label study, which essentially replicates the earlier study, are still tentative and a randomized controlled trial needs to be undertaken.
Acknowledgments
We gratefully acknowledge the comments and suggestions of Dr Mike Briley in the preparation of this manuscript, of Dr Martin Kacetl and Dr Karl Nekrep for supporting the planning and performance of this study and last but not least the late Professor Dr Hans Georg Zapotoczky for his generous support of the underlying interdisciplinary therapeutic concept.
Disclosure
The authors report no conflicts of interest in this work.
References
- KatonWJThe comorbidity of diabetes mellitus and depressionAm J Med200812111 Suppl 2S81518954592
- AliSStoneMAPetersJLThe prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysisDiabet Med2006231165117317054590
- EgedeLENietertPJZhengDDepression and all-cause and coronary heart disease mortality among adults with and without diabetesDiabetes Care2005281339134515920049
- EgedeLEEllisCThe effects of depression on diabetes knowledge, diabetes self-management, and perceived control in indigent patients with type 2 diabetesDiabetes Technol Ther20081021321918473696
- KatonWJRussoJEVon KorffMLong-term effects on medical costs of improving depression outcomes in patients with depression and diabetesDiabetes Care2008311155115918332158
- deGrootMAndersonRFreedlandKEAssociation of depression and diabetes complications: A meta-analysisPsychosom Med20016361963011485116
- LustmannPJClouseRENixBDSertraline for prevention of depression recurrence in diabetes mellitusArch Gen Psychiatry20066352152916651509
- GülserenLGülserenSHekimsoyZComparison of fluoxetine and paroxetine in type 2 diabetes mellitus patientsArch Med Res20053615916515847950
- BognerHRMoralesKHde VriesHFCappolaARIntegrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trialAnn Fam Med201210152222230826
- FilipcicIMargeticBSimunovicIJakovljevicMDepression treatment and its impact upon the quality of life in patients with diabetes type 2 – the Croatian studyPsychiatria Danubina20102223123520562752
- AbrahamianHHofmannPPragerRToplakHDiabetes mellitus and co-morbid depression: treatment with milnacipran results in significant improvement of both diseases (results from the Austrian MDDM study group)Neuropsychiatr Dis Treat2009526126619557120
- Osterreichische Diabetes GesellschaftDiabetes mellitus – guidelines for the practice. Revised and expanded 2007 editionWien Klin Wochenschr2009121Suppl 5S187 Article in German
- WhooleyMAAvinsALMirandaJCase-finding instrument for depressionJ Gen Intern Med1997124394459229283
- BeckATBeamesderferAAssessment of depression: the depression inventoryMod Probl Pharmacopsychiatry197471511694412100
- RiedelMMöllerHJObermeierMResponse and remission criteria in major depression – a validation of current practiceJ Psychiatr Res2010441063106820447651
- RushWAWhitebirdRRRushMRDepression in patients with diabetes: does it impact clinical goals?J Am Board Fam Med20082139239718772293
- GonzalezJSSafrenStACaglieroEDepression, self-care and medication adherence in type 2 diabetesDiabetes Care2007302222222717536067
- LustmannPJGriffitiLSFreedlandKEFluoxetine for depression in diabetesDiabetes Care20002361862310834419
- AmsterdamJDShultsJRutherfordNSafety and efficacy of s-citalopram in patients with comorbid major depression and diabetes mellitusNeuropsychobiology20075420821417337914
- LustmannPJWilliamsMWSayurGSFactors influencing glycemic control in type 2 diabetes during acute- and maintenance-phase treatment of major depressive disorder with bupropionDiabetes Care20073045946617327305
- MontgomerySATolerability of serotonin norepinephrine reuptake inhibitor antidepressantsCNS Spectr200813:7Suppl 11273318622372
- Lopez-IborJGuelfiJDPletanYMilnacipran and selective serotonin reuptake inhibitors in major depressionInt Clin Psychophamacol199611Suppl 44146
- ClercGMilnacipran/Fluvoxamine Study GroupAntidepressant eff icacy and tolerability of milnacipran, a dual serotonin and noradrenaline reuptake inhibitor: a comparison with fluvoxamineInt Clin Psychopharmacol20011614515111354236
- SechterDVandelPWeillerEA comparative study of milnacipran and paroxetine in outpatients with major depressionJ Affect Disord20048323323615555719
- GonzalezJSPeyrotMMcCarlLADepression and diabetes treatment non-adherence: A meta-analysisDiabetes Care2008312398240319033420
- MarkowitzSGonzalezJSWilkinsonJLSafrenSAA review of treating depression in diabetes: emerging findingsPsychosomatics20115211821300190