Abstract
Background
The purpose of this study was to evaluate the long-term effect of repetitive transcranial magnetic stimulation (rTMS) as adjunctive treatment in patients with partial remission of major depressive disorder.
Methods
This was a 12-month, prospective, open-label study in patients meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria for nonpsychotic major depressive disorder who responded to 8 weeks of medication treatment but did not reach remission. All patients were assigned to receive 10 sessions of rTMS applied at the left dorsolateral prefrontal cortex. During the course of rTMS, the patients were still taking their usual medication. Patients were followed up for 12 months to determine the long-term antidepressant effect.
Results
There were nine patients (seven women and two men) who met the inclusion criteria and agreed to receive rTMS. The mean Hamilton rating scale for depression (HAM-D) score prior to treatment with rTMS was 12.89 ± 2.15. At 12 months after treatment, the mean HAM-D score was 6.45 ± 1.67 using a Friedman test, and in patients with partial remission of major depressive disorder, the HAM-D score significantly decreased after treatment with rTMS at 12 months (P = 0.001). Seven patients (77.78%) had reached the stage of remission (HAM-D < 8) after treating with rTMS at 12 months. There were no serious adverse events. One patient had vertigo after the first session of treatment and one patient felt scalp contractions during treatment, and both fully recovered within half an hour with no medical intervention.
Conclusion
For patients with major depressive disorder in partial remission, high frequency rTMS at the left dorsolateral prefrontal cortex may provide benefits in adjunctive treatment with well tolerability. Also, follow-up findings show a long duration of benefit.
Introduction
Major depression is a severe psychiatric disorder with a lifetime prevalence in excess of 15%,Citation1 and is the fourth leading cause of disability worldwide. The World Health Organization estimates that depression causes 6% of the burden of all diseases in Europe in terms of disability-adjusted life years. Full remission is the goal of treatment, but many patients fail to reach a full remission.Citation2 Partial remission is characterized by the presence of residual depressive symptoms, including low mood, psychic anxiety, sleep disturbance, fatigue, and diminished interest, while major biological symptoms are typically absent.Citation3 Lack of complete remission from an acute episode of major depressive disorder (especially the first one) is associated with a high risk of relapse,Citation3–Citation5 more severe depressive episodes,Citation5 shorter durations between episodes,Citation5 continued impairment in work and relationships,Citation6 increased all-cause mortality,Citation7 and risk of suicide.Citation8 These statistics emphasize the need for alternative treatment strategies to optimize outcomes.
Repetitive transcranial magnetic stimulation (rTMS) allows noninvasive electrical stimulation of the cerebral cortex by means of magnetic fields generated by a coil. During the last decade, numerous studies, including several meta-analyses, have indicated the efficacy of rTMS in acute treatment of major depressive disorder which ultimately led to its approval by the US Food and Drug Administration.Citation9–Citation11 In our previous study, we used ten daily sessions (total of 12,500 magnetic pulses) of rTMS in patients with nonpsychotic major depressive disorder who responded to 8 weeks of medication but still had residual symptoms, ie, a Hamilton rating scale for depression (HAM-D) score of 8–18. At 8 weeks after treatment with adjunctive rTMS, seven of nine patients (78%) reached the stage of remission (HAM-D score < 8) and there was a statistically significant difference in HAM-D score reduction (P < 0.001).Citation12
Nevertheless, investigation of long-term efficacy is lacking. Bortolomasi et al found that at 12 weeks after treatment, patients who received rTMS as adjunctive treatment had better alleviation of their depressive symptoms than a controlled group.Citation13 Demirtas-Tatlidele et al used rTMS without medication in refractory major depressive disorder and found the mean interval before patients had to receive rTMS again was 5 months.Citation14 Fitzgerald et al reported on a series of 19 patients who received rTMS for treatment of up to four episodes of depressive relapse.Citation15 The mean relapse time for each round of treatments ranged between 6.0 and 11.6 months. The authors concluded that rTMS was of value in the treatment of depressive relapse, with a slight reduction in efficacy over time. However, from our current knowledge, we do not know how long the efficacy lasts if we use rTMS as adjunctive treatment in major depressive disorder. This study aimed to examine the long-term efficacy of rTMS as an adjunctive treatment in patients with major depressive disorder in partial remission.
Materials and methods
Patients
Patient s who had a major depressive disorder without psychotic features (the diagnosis was made by an experienced psychiatrist using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria), had received treatment with antidepressants for more than 8 weeks, and had responded to treatment with only partial remission were eligible for inclusion in this study. Response to treatment was defined using the Clinical Global Impression-Improvement (CGI-I) with at least moderate improvement. Partial remission was defined using the Thai version of the 17-item HAM-D with a score between 7 and 18. All subjects were right-handed. Patients with a history of head trauma, epilepsy, or head surgery were excluded. Subjects did not have other axis I disorders or an implant that could be disturbed magnetically. The study protocol was approved by the local ethics committee and all patients were required to sign an informed consent form before their enrollment in the trial.
Therapies
All patients received antidepressant medication for at least 8 weeks before enrollment. While continuing their medication, rTMS was given as an adjunctive treatment using the Magstim Super Rapid stimulator (Magstim Co, Whitland, UK) with a figure of eight coil. Stimulations were given at 100% of the motor threshold to the left dorsolateral prefrontal cortex, deemed to be located 5 cm anterior to the abductor pollicis brevis in the parasagittal plane. Each session entailed 25 trains at 10 Hz for 5 seconds (1250 stimuli per day). A full course comprised ten daily sessions (total of 12,500 magnetic pulses) administered on week days, beginning on Monday.
Assessment and outcomes
The efficacy of rTMS was determined by a decrease in HAM-D score. Baseline assessments were performed before starting rTMS. The outcome measures were repeated at day 6 of treatment, 2 months after treatment (to determine remission), and every 2 months after that until 12 months. Additional baseline data, obtained by interview and review of hospital records, included age, gender, duration of the current depressive episode, history of depression, and current psychotropic medications. The primary outcome measure was the 12-month score on the Thai version of the 17-item HAM-D and the rate of remission, which was defined as a HAM-D score of ≤7. All subjects were monitored for potential side effects of rTMS (eg, seizure induction, scalp discomfort, hearing loss) and any unpredictable adverse events with every treatment and visit. All subjects were assessed using a Young Mania Rating Scale (Thai version) at follow-up visits.
Statistical analyses
The Thai HAM-D scores at baseline and every 2 months after treatment for 12 months were analyzed using the Friedman test. The mean ± standard deviation was calculated for each visit. The number of patients in remission at 12 months was reported as a percentage. Each subscale of the Thai HAM-D was also assessed using the Friedman test. Statistical significance was defined as a P value <0.05.
Results
Nine patients (seven women and two men) met the inclusion criteria and agreed to receive rTMS. The characteristics of the patients are shown in . The mean HAM-D scores decreased from 12.89 ± 2.15 at baseline to 6.45 ± 1.67 at 12 months. Twelve months after a course of rTMS, seven of nine patients (77.78%) were in remission (). There was no change in medication during the 12 months of follow-up. Using the Friedman test, the HAM-D score in patients with partial remission of major depressive disorder decreased significantly after treatment with rTMS (P = 0.001, χ2 19.81, df 4).
Table 1 Baseline characteristics and Thai HAM-D scores on follow-up visits for up to 12 months
Symptoms of depression in the HAM-D score that improved significantly (P < 0.05) were insomnia (early, middle), work and activity, psychomotor retardation, psychological anxiety, somatic anxiety, and general somatic symptoms (). The mean scores of the HAM-D subscales that were and were not significantly changed during the 12 months of follow-up are plotted in and , respectively.
Figure 1 Mean score of HAM-D subscales that were significantly decreased during the 12-month follow-up.
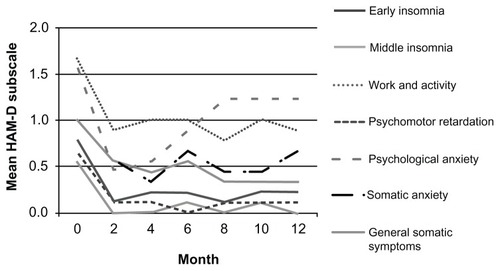
Figure 2 Mean score of HAM-D subscales that were not significantly changed during the 12-month follow-up.
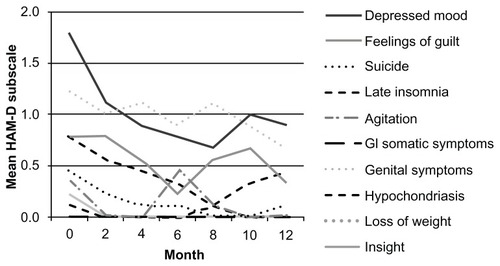
Table 2 HAM-D subscale evaluated every 2 months for 12 months using Friedman test
No serious adverse events were observed. One patient had vertigo after their first session of treatment and another felt scalp contractions during treatment but recovered fully within half an hour without medical intervention. After the course of treatment, there were no adverse events until 12 months. No manic symptoms were documented.
Discussion
This open-label study provides evidence that high-frequency rTMS at the left dorsolateral prefrontal cortex is not only effective in the adjunctive treatment of major depressive disorder, but also has long-term efficacy in helping patients move from partial remission to full remission. To our knowledge, this is the longest prospective study that has followed up the effect of rTMS as adjunctive treatment. The patients showed improvement after ten sessions of rTMS. The HAM-D score indicated remission of depressive symptoms at 2 months as well as one year after the course of rTMS. Our results are in line with the finding of Fitzgerald et al that the mean time to relapse after first rTMS treatment in nonpsychotic patients with major depressive disorder was 10 ± 6.6 months.Citation15 However, the results differ from those of Demirtas-Tatlidede et al who showed the mean time to recurrence after remission following rTMS was only 5 months.Citation15 This difference might be due to the fact that the previous study used rTMS alone without medication.
Our findings demonstrate that the adjunctive antidepressant effect of rTMS improved common residual symptoms of depression, such as psychic anxiety, sleep disturbance, and fatigue. Moreover, this long-term study confirms good tolerability of rTMS, which is in line with the findings of previous studies.
Some limitations should be taken into account when interpreting findings from this study. First, like many rTMS studies, the sample size was very small. However, this limitation would not explain our positive findings. Second, the particular sample characteristics from a university hospital in Thailand might reduce generalization of the findings to other patients with major depressive disorder in different settings.
Conclusion
This study shows that rTMS may have long-term efficacy and be well tolerated adjunctive treatment for major depressive disorder in partial remission. The lack of controlled subjects suggests that further double-blind, randomized, controlled studies are needed before rTMS can be considered as an adjunctive treatment for major depressive disorder. The small sample size and lack of a controlled group limit the findings of this study.
Acknowledgment
The research was funded by the Faculty of Medicine at Chiang Mai University.
Disclosure
The authors report no conflicts of interest in this work.
References
- KesslerRCBurglundPBDemlerOThe epidemiology of major depressive disorderJAMA20032893095310512813115
- SinyorMSchafferALevittAThe sequenced treatment alternatives to relieve depression (STAR*D) trial: a reviewCan J Psychiatry20105512613520370962
- McClintockSMHusainMMWisniewskiSRResidual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medicationJ Clin Psychopharmacol20113118018621346613
- PaykelESAchieving gains beyond responseActa Psychiatr Scand Suppl2002415121712492768
- JuddLLPaulusMJSchettlerPJDoes incomplete recovery from first lifetime major depressive episode herald a chronic course of illness?Am J Psychiatry20001571501150410964869
- MillerIWKeitnerGISchatzbergAFThe treatment of chronic depression, part 3: psychosocial functioning before and after treatment with sertraline or imipramineJ Clin Psychiatry1998596086199862607
- MurphyJMMonsonRROlivierDCSobolAMLeightoneAHAffective disorders and mortality: a general population studyArch Gen Psychiatry1987444734803555383
- JuddLLAkiskalHSPaulusMPThe role and clinical significance of subsyndromal depressive symptoms (SSD) in unipolar major depressive disorderJ Affect Disord1997455189268771
- Pascual-LeoneATormosJMKeenanJStudy and modulation of human cortical excitability with transcranial magnetic stimulationJ Clin Neurophysiol1998153333439736467
- LamRWChanPWilkins-HoMYathamLNRepetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysisCan J Psychiatry20085362163118801225
- RumiDOGattazWFRigonattiSPTranscranial magnetic stimulation accelerates the antidepressant effect of amitriptyline in severe depression: a double-blind placebo-controlled studyBiol Psychiatry20055716216615652875
- CharnsilCSuttajitSBoonyanarutheeVLeelarphatSAn open-label study of adjunctive repetitive transcranial magnetic stimulation (rTMS) for partial remission in major depressive disorderInt J Psychiatry Clin Pract2012169810222339175
- BortolomasiMMinelliAFuggettaGLong-lasting effect of high frequency repetitive transcranial magnetic stimulation in major depressed patientsPsychiatry Res200715018118617303249
- Demirtas-TalidedeAMechanic-HamiltonDPressDZAn open-label, prospective study of repetitive transcranial magnetic stimulation (rTMS) in long-term treatment of refractory depression: reproducibility and duration of the antidepressant effect in medication-free patientsJ Clin Psychiatry20086993093418505308
- FitzgeraldPBBenitezJde CastellaARBrownTLDaskalakisZJKulkarniJNaturalistic study of the use of transcranial magnetic stimulation in the treatment of depressive relapseAust N Z J Psychiatry20064076476816911751